Abstract
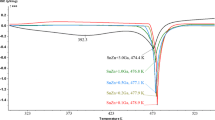
Low melting point alloy is a potential high-temperature heat transfer medium because of the high thermal conductivity, low solidus temperature and wide range of use temperature. Consequently, we investigated the possibility of using Sn–Bi–Zn–Ga alloys as heat storage and heat transfer material. Moreover, we investigated the microstructure and phase compositions by electron probe micro-analysis (EPMA) and X-ray diffusion (XRD). Results show that the new structures and phases are formed in the alloy matrix with Ga additions, which lead to the improvement of the thermal properties. An extensive thermophysical characterization of the Sn–Bi–Zn–Ga alloys has been performed by differential scanning calorimeter (DSC) analysis. The addition of Ga lowers the peak temperature and increases the heat capacity of the alloys. The thermal expansion of the test alloys increases with increasing temperature and the densities decreases with Ga additions. As the density, specific heat capacity and thermal diffusivity change with temperature and physical state, the thermal conductivity of the alloys first decreases and then increases. These results demonstrate the feasibility of using Sn–Bi–Zn–Ga alloys as the high-temperature heat transfer fluid.
Similar content being viewed by others
References
Reilly J, Paltsev S, Felzer B, et al. Global Economic Effects of Changes in Crops, Pasture, and Forests Due to Changing Climate, Carbon Dioxide, and Ozone[J]. Energy Policy, 2007, 35(11): 5 370–5 383
Hadjieva M M, Bozukov M, Gutzov I. Next Generation Phase Change Materials as Multifunctional Watery Suspension for Heat Transport and Heat Storage[J]. Mrs Online Proceedings Library Archive, 2009, 1 188: 627
Chen Y Y, Zhao C Y. Thermophysical Properties of Ca(NO3)2-NaNO3-KNO3 Mixtures for Heat Transfer and Thermal Storage[J]. Solar Energy, 2017, 172–179
Zhu C, Cheng X, Li Y, et al. Influence of Heat Treatment on Solidus Temperature of NaNO3-KNO3 Molten Salt[J]. Solar Energy, 2015, 118: 303–312
Lopez J, Acem Z, Barrio E P D. KNO/NaNO-Graphite Materials for Thermal Energy Storage at High Temperature: Part II.-Phase Transition Properties[J]. Applied Thermal Engineering, 2010, 30(13): 1 586–1 593
Li Y, Xu X, Wang X, et al. Survey and Evaluation of Equations for Thermophysical Properties of Binary/Ternary Eutectic Salts from NaCl, KCl, MgCl2, CaCl2, ZnCl2, for Heat Transfer and Thermal Storage Fluids in CSP[J]. Solar Energy, 2017, 152: 57–79
Lorenzin N, Abánades A. A Review on the Application of Liquid Metals as Heat Transfer Fluid in Concentrated Solar Power Technologies[J]. International Journal of Hydrogen Energy, 2016, 41(17): 6 990–6 995
Frazer D, Stergar E, Cionea C, et al. Liquid Metal as a Heat Transport Fluid for Thermal Solar Power Applications[J]. Energy Procedia, 2014, 49: 627–636
Venkitaraj K P, Suresh S. Experimental Study on Thermal and Chemical Stability of Pentaerythritol Blended with Low Melting Alloy as Possible PCM for Latent Heat Storage[J]. Experimental Thermal & Fluid Science, 2017: 73–87
Wang S H, Chin T S, Yang C F, et al. Pb-free Solder-alloy Based on Sn-Zn-Bi with the Addition of Germanium[J]. Journal of Alloys & Compounds, 2010, 497(1): 428–431
Mahmudi R, Geranmayeh A R, Noori H, et al. Effect of Isothermal Aging on Room Temperature Impression Creep of Lead Free Sn-9Zn and Sn-8Zn-3Bi Solders[J]. Materials Science & Engineering A, 2008, 26(8): 1 001–1 007
Gain A K, Zhang L. Microstructure, Thermal Analysis and Damping Properties of Ag and Ni Nano-particles Doped Sn-8Zn-3Bi Solder on OSP-Cu Substrate[J]. Journal of Alloys & Compounds, 2014, 617: 779–786
Shen J, Pu Y, Yin H, et al. Effects of Minor Cu and Zn Additions on the Thermal, Microstructure and Tensile Properties of Sn-Bi-Based Solder Alloys[J]. Journal of Alloys & Compounds, 2014, 614(10): 63–70
Zhang Y, Li P. Minimum System Entropy Production as the FOM of High Temperature Heat Transfer Fluids for CSP Systems[J]. Solar Energy, 2017, 152: 80–90
Kaya H, Engin S, Aker A, et al. Microstructural, Mechanical, Electrical, and Thermal Properties of the Bi-Sn-Ag Ternary Eutectic Alloy[J]. Journal of Wuhan University of Technology-Materials Science Edition, 2017, 32(1): 147–154
Sezen Aksöz, Yavuz Ocak, Necmettin Maraşli, et al. Determination of Thermal Conductivity and Interfacial Energy of Solid Zn Solution in the Zn-Al-Bi Eutectic System[J]. Experimental Thermal & Fluid Science, 2011, 35(2): 395–404
Yeh C H, Chang L S, Straumal B B. Wetting Transition of Grain Boundaries in the Sn-rich Part of the Sn-Bi Phase Diagram[J]. Journal of Materials Science, 2011, 46(5): 1 557–1 562
Andraka C E, Kruizenga A M, B A Hernandez-Sanchez, et al. Metallic Phase Change Material Thermal Storage for Dish Stirling[J]. Energy Procedia, 2015, 69: 726–736
Blancorodriguez P, Rodriguezaseguinolaza J, Risueño E, et al. Thermophysical Characterization of Mg-51%Zn Eutectic Metal Alloy: A Phase Change Material for Thermal Energy Storage in Direct Steam Generation Applications[J]. Energy, 2014, 72: 414–420
Fang D, Sun Z, Li Y, et al. Preparation, Microstructure and Thermal Properties of Mg, Bi Alloys as Phase Change Materials for Thermal Energy Storage[J]. Applied Thermal Engineering, 2016, 92: 187–193
Zhang J, Li N. Review of the Studies on Fundamental Issues in LBE Corrosion[J]. Journal of Nuclear Materials, 2008, 373(1): 351–377
Yuan J, Zhang K, Zhang X, et al. Thermal Characteristics of Mg-Zn-Mn Alloys with High Specific Strength and High Thermal Conductivity[J]. Journal of Alloys & Compounds, 2013, 578(6): 32–36
Barin I, Knacke O, Kubaschewski O. Thermochemical Properties of Inorganic Substances[M]. Berlin: Springer-Verlag, 1977
Fazio C, Sobolev V P, Aerts A, et al. Handbook on Lead-bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-hydraulics and Technologies-2015 Edition[R]. Organisation for Economic Co-Operation and Development, 2015
Vizdal J, Braga M H, Kroupa A, et al. Thermodynamic Assessment of the Bi-Sn-Zn System[J]. Calphad-computer Coupling of Phase Diagrams & Thermochemistry, 2007, 31(4): 438–448
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by National Key Technology Research & Development Program of China (No. 2012BAA05B05), Key Technology Research & Development Program of Hubei (No. 2015BAA111), Science and Technology Department of Hubei Province and the Fundamental Research Funds for the Central Universities (No. WUT: 2017 II 23GX)
Rights and permissions
About this article
Cite this article
Wang, Q., Cheng, X., Li, Y. et al. Microstructures and Thermal Properties of Sn–Bi–Zn–Ga Alloys as Heat Transfer and Heat Storage Materials. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 34, 676–683 (2019). https://doi.org/10.1007/s11595-019-2103-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-019-2103-1