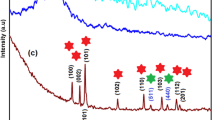
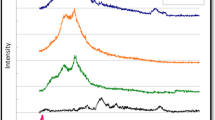
Abstract
Within the scope of this study, triple composites consisting of chlorine-doped graphene oxide (Cl-GO)/sulfur-doped graphene oxide (S-GO)/polyaniline (PANI) in different ratios were produced to use as electrode materials of asymmetric type supercapacitors for the first time in the literature. Cl-GO and S-GO were produced by using chronoamperometric method in one step and room temperature. PANI was also prepared by using chemical synthesis route. Produced conductive polymer and heteroatom-doped graphene oxides were characterized by using of spectroscopic and microscopic techniques. XPS and FT-IR analyses showed that heteroatom-doped graphene oxide was successfully synthesized. The FT-IR spectrum of PANI supported that polyaniline production was achieved. SEM images of all synthesized components show that the materials were successfully produced in accordance with the literature. Capacitive behavior of the produced supercapacitors was characterized by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) methods. The PANI/S-GO/Cl-GO-5 electrode reached to highest areal capacitance as 62.45 mF.cm−2 at 10 mV.s−1 scan rate. This triple composite has significant potential for industrial supercapacitor applications, thanks to its high pseudo-capacitive behavior. Also, capacitive behavior of this electrode was tested during 1000 cycles. Capacitance retention of the system was more than 100% at the end of 1000 cycle.







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Gencten M, Sahin Y (2020) A critical review on progress of the electrode materials of vanadium redox flow battery. Int J Energy Res 44:7903–7923. https://doi.org/10.1002/er.5487
Arvas MB, Gürsu H, Gencten M, Sahin Y (2021) Preparation of different heteroatom doped graphene oxide based electrodes by electrochemical method and their supercapacitor applications. J Energy Storage 35:102328. https://doi.org/10.1016/j.est.2021.102328
Ok B, Gencten M, Arvas MB, Sahin Y (2022) Preparation of copper doped conducting polymers and their supercapacitor applications. ECS J Solid State Sci Technol 11:033004. https://doi.org/10.1149/2162-8777/ac57f5
Gursu H, Guner Y, Arvas MB et al (2021) Production of chlorine-containing functional group doped graphene powders using Yucel’s method as anode materials for Li-ion batteries. RSC Adv 11:40059–40071. https://doi.org/10.1039/D1RA07653A
Arvas MB, Karatepe N, Gencten M, Sahin Y (2022) One-step synthesis of nitrogen-doped graphene powders and application of them as high-performance symmetrical coin cell supercapacitors in different aqueous electrolyte. Int J Energy Res 46:7348–7373. https://doi.org/10.1002/er.7642
Arvas MB, Gürsu H, Gencten M, Sahin Y (2022) Supercapacitor applications of novel phosphorus doped graphene-based electrodes. J Energy Storage 55:105766. https://doi.org/10.1016/j.est.2022.105766
Basili T, Yildirim Kalyon H, Gencten M et al (2023) High performance supercapacitive behaviors of oximes in a poly(aniline- co -pyrrole) based conducting polymer structure. New J Chem 47:691–707. https://doi.org/10.1039/D2NJ05212A
Aydin O, Birol B, Gencten M (2023) Production of ZnS based supercapacitor electrode material from ferrochrome ash waste. Ionics (Kiel) 29:3335–3352. https://doi.org/10.1007/s11581-023-05018-7
Besir Arvas M, Gencten M, Sahin Y (2022) Investigation of supercapacitor properties of chlorine-containing functional groups doped graphene electrodes. J Electroanal Chem 918:116438. https://doi.org/10.1016/j.jelechem.2022.116438
Arvas MB, Gencten M, Sahin Y (2021) One-step synthesized N-doped graphene-based electrode materials for supercapacitor applications. Ionics (Kiel) 27:2241–2256. https://doi.org/10.1007/s11581-021-03986-2
Pal M, Subhedar KM (2023) CNT yarn based solid state linear supercapacitor with multi-featured capabilities for wearable and implantable devices. Energy Storage Mater 57:136–170. https://doi.org/10.1016/j.ensm.2023.01.051
Habib H, Wani IS, Husain S (2022) High performance nanostructured symmetric reduced graphene oxide/polyaniline supercapacitor electrode: effect of polyaniline morphology. J Energy Storage 55:105732. https://doi.org/10.1016/j.est.2022.105732
Wang Y, Xie Y (2022) Sandwich-structured polypyrrole layer/KCl-polyacrylamide-gelatin hydrogel/polypyrrole layer as all-in-one polymer self-healing supercapacitor. Electrochim Acta 435:141371. https://doi.org/10.1016/j.electacta.2022.141371
Yazar S, Arvas MB, Sahin Y (2022) S, N and Cl separately doped graphene oxide/polyaniline composites for hybrid supercapacitor electrode. J Electrochem Soc 169:120536. https://doi.org/10.1149/1945-7111/acadb1
Arvas MB, Gürsu H, Gencten M, Sahin Y (2022) New approach synthesis of S, N co-doped graphenes for high-performance supercapacitors. ChemistrySelect 7:e202200360. https://doi.org/10.1002/slct.202200360
Rosli NHA, Lau KS, Winie T et al (2021) Synergistic effect of sulfur-doped reduced graphene oxide created via microwave-assisted synthesis for supercapacitor applications. Diam Relat Mater 120:108696. https://doi.org/10.1016/j.diamond.2021.108696
Kakaei K, Hamidi M, Husseindoost S (2016) Chlorine-doped reduced graphene oxide nanosheets as an efficient and stable electrode for supercapacitor in acidic medium. J Colloid Interface Sci 479:121–126. https://doi.org/10.1016/j.jcis.2016.06.058
Paul A, Ghosh S, Kolya H et al (2022) Synthesis of nickel-tin oxide/nitrogen-doped reduced graphene oxide composite for asymmetric supercapacitor device. Chem Eng J 443:136453. https://doi.org/10.1016/j.cej.2022.136453
Wang Q, Wang J, Zhao Y et al (2022) NiO/NiFe2O4@N-doped reduced graphene oxide aerogel towards the wideband electromagnetic wave absorption: experimental and theoretical study. Chem Eng J 430:132814. https://doi.org/10.1016/j.cej.2021.132814
Arvas MB, Karatepe N, Gencten M, Sahin Y (2021) Fabrication of high-performance symmetrical coin cell supercapacitors by using one step and green synthesis sulfur doped graphene powders. New J Chem 45:6928–6939. https://doi.org/10.1039/D0NJ06061E
Mansuroglu A, Gencten M, Arvas MB et al (2021) A novel electrolyte additive for gel type valve regulated lead acid batteries: sulfur doped graphene oxide. Int J Energy Res 45:21390–21402. https://doi.org/10.1002/er.7189
Yasa S, Kumbasi O, Arvas MB et al (2023) S-doped graphene oxide/N-doped graphene oxide/PANI: a triple composite for high-performance supercapacitor applications. ECS J Solid State Sci Technol 12:051002. https://doi.org/10.1149/2162-8777/acd3af
Arvas MB, Karatepe N, Gencten M, Sahin Y (2023) Fabrication of S, N co-doped graphene powders for symmetrical supercapacitors in different aqueous electrolytes. J Mater Sci Mater Electron 34:1068. https://doi.org/10.1007/s10854-023-10441-7
Mansuroglu A, Arvas MB, Kiraz C et al (2021) N-doped graphene oxide as additive for fumed silica based gel electrolyte of valve regulated lead acid batteries. J Electrochem Soc 168:060512. https://doi.org/10.1149/1945-7111/ac0555
Yazar S, Arvas MB, Yilmaz SM, Sahin Y (2022) Effects of pyridinic N of carboxylic acid on the polymerization of polyaniline and its supercapacitor performances. J Energy Storage 55:105740. https://doi.org/10.1016/j.est.2022.105740
Yazar S, Arvas MB, Sahin Y (2022) Hydrothermal synthesis of flexible Fe-doped polyaniline/dye-functionalized carbon felt electrode for supercapacitor applications. ChemistrySelect 7:e202200016. https://doi.org/10.1002/slct.202200016
Dhandapani E, Thangarasu S, Ramesh S et al (2022) Recent development and prospective of carbonaceous material, conducting polymer and their composite electrode materials for supercapacitor — a review. J Energy Storage 52:104937. https://doi.org/10.1016/j.est.2022.104937
Arya A, Iqbal M, Tanwar S et al (2022) Mesoporous carbon/titanium dioxide composite as an electrode for symmetric/asymmetric solid-state supercapacitors. Mater Sci Eng B 285:115972. https://doi.org/10.1016/j.mseb.2022.115972
Pandey VK, Verma S, Verma B (2022) Polyaniline/activated carbon/copper ferrite (PANI/AC/CuF) based ternary composite as an efficient electrode material for supercapacitor. Chem Phys Lett 802:139780. https://doi.org/10.1016/j.cplett.2022.139780
Huang C, Hao C, Zheng W et al (2020) Synthesis of polyaniline/nickel oxide/sulfonated graphene ternary composite for all-solid-state asymmetric supercapacitor. Appl Surf Sci 505:144589. https://doi.org/10.1016/j.apsusc.2019.144589
Das T, Verma B (2020) Polyaniline-acetylene black-copper cobaltite based ternary hybrid material with enhanced electrochemical properties and its use in supercapacitor electrodes. Int J Energy Res 44:934–949. https://doi.org/10.1002/er.4951
Heydari H, Abdouss M, Mazinani S et al (2021) Electrochemical study of ternary polyaniline/MoS2−MnO2 for supercapacitor applications. J Energy Storage 40:102738. https://doi.org/10.1016/j.est.2021.102738
Hong X, Wang X, Li Y et al (2022) Potassium citrate-derived carbon nanosheets/carbon nanotubes/polyaniline ternary composite for supercapacitor electrodes. Electrochim Acta 403:139571. https://doi.org/10.1016/j.electacta.2021.139571
Gürsu H, Güner Y, Dermenci KB et al (2020) A novel green and one-step electrochemical method for production of sulfur-doped graphene powders and their performance as an anode in Li-ion battery. Ionics (Kiel) 26:4909–4919. https://doi.org/10.1007/s11581-020-03671-w
John A, Mahadeva SK, Kim J (2010) The preparation, characterization and actuation behavior of polyaniline and cellulose blended electro-active paper. Smart Mater Struct 19:045011. https://doi.org/10.1088/0964-1726/19/4/045011
Xu J-C, Liu W-M, Li H-L (2005) Titanium dioxide doped polyaniline. Mater Sci Eng C 25:444–447. https://doi.org/10.1016/j.msec.2004.11.003
Robati D, Mirza B, Rajabi M et al (2016) Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697. https://doi.org/10.1016/j.cej.2015.08.131
García-Picazo FJ, Pérez-Sicairos S, Fimbres-Weihs GA et al (2021) Preparation of thin-film composite nanofiltration membranes doped with N- and Cl-functionalized graphene oxide for water desalination. Polymers (Basel) 13:1637. https://doi.org/10.3390/polym13101637
Thota C, Modigunta JKR, Reddeppa M et al (2022) Light stimulated room-temperature H2S gas sensing ability of Cl-doped carbon quantum dots supported Ag nanoparticles. Carbon N Y 196:337–346. https://doi.org/10.1016/j.carbon.2022.05.008
Lee J, Noh S, Pham ND, Shim JH (2019) Top-down synthesis of S-doped graphene nanosheets by electrochemical exfoliation of graphite: metal-free bifunctional catalysts for oxygen reduction and evolution reactions. Electrochim Acta 313:1–9. https://doi.org/10.1016/j.electacta.2019.05.015
Krishnamoorthy K, Veerapandian M, Yun K, Kim S-J (2013) The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon N Y 53:38–49. https://doi.org/10.1016/j.carbon.2012.10.013
Razmjooei F, Singh KP, Song MY, Yu JS (2014) Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: a comprehensive study. Carbon N Y 78:257–267. https://doi.org/10.1016/j.carbon.2014.07.002
Wang X, Li G, Seo MH et al (2015) Sulfur atoms bridging few-layered MoS 2 with S-doped graphene enable highly robust anode for lithium-ion batteries. Adv Energy Mater 5:1501106. https://doi.org/10.1002/aenm.201501106
Siddiqui AS, Hayat A, Nawaz MH et al (2020) Effect of sulfur doping on graphene oxide towards amplified fluorescence quenching based ultrasensitive detection of hydrogen peroxide. Appl Surf Sci 509:144695. https://doi.org/10.1016/j.apsusc.2019.144695
Madhan Kumar A, Suresh Babu R, Obot IB, Gasem ZM (2015) Fabrication of nitrogen doped graphene oxide coatings: experimental and theoretical approach for surface protection. RSC Adv 5:19264–19272. https://doi.org/10.1039/C4RA13470B
Peng XY, Liu XX, Diamond D, Lau KT (2011) Synthesis of electrochemically-reduced graphene oxide film with controllable size and thickness and its use in supercapacitor. Carbon N Y 49:3488–3496. https://doi.org/10.1016/j.carbon.2011.04.047
Lu J, Yang J, Wang J et al (2009) One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 3:2367–2375. https://doi.org/10.1021/nn900546b
Li B, Zhou L, Wu D, et al (2011) Photochemical chlorination of graphene. In: ACS Nano. pp 5957–5961
Wu Y, Lin X, Shen X et al (2015) Exceptional dielectric properties of chlorine-doped graphene oxide/poly (vinylidene fluoride) nanocomposites. Carbon N Y 89:102–112. https://doi.org/10.1016/j.carbon.2015.02.074
Varodi C, Pogăcean F, Cioriță A et al (2021) Nitrogen and sulfur co-doped graphene as efficient electrode material for L-cysteine detection. Chemosensors 9:146. https://doi.org/10.3390/chemosensors9060146
Tang Y, Jing F, Xu Z et al (2017) Highly crumpled hybrids of nitrogen/sulfur dual-doped graphene and Co 9 S 8 nanoplates as efficient bifunctional oxygen electrocatalysts. ACS Appl Mater Interfaces 9:12340–12347. https://doi.org/10.1021/acsami.6b15461
Ersozoglu MG, Gursu H, Gencten M et al (2021) A green approach to fabricate binder-free S-doped graphene oxide electrodes for vanadium redox battery. Int J Energy Res 45:2126–2137. https://doi.org/10.1002/er.5906
Arvas MB, Gençten M, Sahin Y (2020) A two-dimensional material for high capacity supercapacitors: S-doped graphene. Int J Energy Res 44:1624–1635. https://doi.org/10.1002/er.4973
Ghasem Hosseini M, Shahryari E (2017) A novel high-performance supercapacitor based on chitosan/graphene oxide-MWCNT/polyaniline. J Colloid Interface Sci 496:371–381. https://doi.org/10.1016/j.jcis.2017.02.027
Etman AE-S, Ibrahim AM, Darwish FA-ZM, Qasim KF (2023) A 10 years-developmental study on conducting polymers composites for supercapacitors electrodes: a review for extensive data interpretation. J Ind Eng Chem 122:27–45. https://doi.org/10.1016/j.jiec.2023.03.008
Yao B, Yuan L, Xiao X et al (2013) Paper-based solid-state supercapacitors with pencil-drawing graphite/polyaniline networks hybrid electrodes. Nano Energy 2:1071–1078. https://doi.org/10.1016/j.nanoen.2013.09.002
Lu X, Zheng D, Zhai T et al (2011) Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ Sci 4:2915–2921. https://doi.org/10.1039/c1ee01338f
Zhu JIA, Xiang LEI, Xi D et al (2018) One-step hydrothermal synthesis of flower-like CoS hierarchitectures for application in supercapacitors. Bull Mater Sci 41:1–6. https://doi.org/10.1007/s12034-018-1570-x
Mao M, Mei L, Wu L et al (2014) Facile synthesis of cobalt sulfide/carbon nanotube shell/core composites for high performance supercapacitors. RSC Adv 4:12050. https://doi.org/10.1039/c4ra00485j
Meng X, Deng J, Zhu J, et al (2016) Cobalt sulfide / graphene composite hydrogel as electrode for high- performance pseudocapacitors. Nat Publ Gr 1–9. https://doi.org/10.1038/srep21717
Zhu J, Zhou W, Zhou Y et al (2019) Cobalt sulfide/reduced graphene oxide nanocomposite with enhanced performance for supercapacitors. J Electron Mater 48:1531–1539. https://doi.org/10.1007/s11664-018-06910-z
Gençten M, Gürsu H, Şahin Y (2016) Electrochemical investigation of the effects of V(V) and sulfuric acid concentrations on positive electrolyte for vanadium redox flow battery. Int J Hydrogen Energy 41:9868–9875. https://doi.org/10.1016/j.ijhydene.2016.03.200
Yildirim Kalyon H, Gencten M, Gorduk S, Sahin Y (2022) Novel composite materials consisting of polypyrrole and metal organic frameworks for supercapacitor applications. J Energy Storage 48:103699. https://doi.org/10.1016/j.est.2021.103699
Hosseini MG, Shahryari E (2017) Fabrication of novel solid-state supercapacitor using a Nafion polymer membrane with graphene oxide/multiwalled carbon nanotube/polyaniline. J Solid State Electrochem 21:2833–2848. https://doi.org/10.1007/s10008-017-3606-3
Li S, Cheng P, Luo J et al (2017) High-performance flexible asymmetric supercapacitor based on CoAl-LDH and rGO electrodes. Nano-Micro Lett 9:31. https://doi.org/10.1007/s40820-017-0134-8
Qasim KF, Mousa MA (2023) Physicochemical properties of oriented crystalline assembled polyaniline/metal doped Li4Ti5O12 composites for Li-ion storage. J Inorg Organomet Polym Mater 33:2601–2617. https://doi.org/10.1007/s10904-023-02720-x
Jiang H, Yang L, Li C et al (2011) High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires. Energy Environ Sci 4:1813. https://doi.org/10.1039/c1ee01032h
Yan J, Wang Q, Wei T, Fan Z (2014) Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv Energy Mater 4:1300816. https://doi.org/10.1002/aenm.201300816
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/C1CS15060J
Khalid M, Tumelero MA, Pasa AA (2015) Asymmetric and symmetric solid-state supercapacitors based on 3D interconnected polyaniline-carbon nanotube framework. RSC Adv 5:62033–62039. https://doi.org/10.1039/c5ra11256g
Gholami Laelabadi K, Moradian R, Manouchehri I (2020) One-step fabrication of flexible, cost/time effective, and high energy storage reduced graphene oxide@PANI supercapacitor. ACS Appl Energy Mater 3:5301–5312. https://doi.org/10.1021/acsaem.0c00317
Cho W-H, Cheng I-C, Chen J-Z (2023) Performance comparison of reduced graphene oxide (rGO)-polyaniline (PANI) supercapacitors with LiCl, Li 2 SO 4, and H 2 SO 4 electrolytes. J Electrochem Soc 170:010532. https://doi.org/10.1149/1945-7111/acb38b
Funding
This work was supported by the Yildiz Technical University under the contract number of FBA-2021–4711.
Author information
Authors and Affiliations
Contributions
All authors contributed to the concept and design of this study. SY and OK took part in the production and characterization of the materials. MBA carried out the characterization of the produced materials. MG served as the supervisor of the study and the manager of the project. MG and SY took part in the writing of the manuscript, and MG, MS, and YS took part in the revision and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yasa, S., Kumbasi, O., Arvas, M.B. et al. Construction of a ternary composite of S-doped GO, Cl-doped GO, and PANI for coin cell-type asymmetric supercapacitor. Ionics 30, 3021–3031 (2024). https://doi.org/10.1007/s11581-024-05482-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05482-9