Abstract
The electrical conductances of very dilute solutions of the ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate [emim][BF4] and 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4] in the low-permittivity solvent dichloromethane have been measured in the temperature range from 278.15 to 303.15 K at 5 K intervals. The data was analyzed assuming the possible presence of contact (CIP) and solvent-separated (SSIP) ion pairs in the solution on the basis of lcCM model to obtain ionic association constants, K A, and the limiting molar conductivities, Λo, of these electrolytes. The examined ionic liquids are strongly associated in dichloromethane over the whole temperature range. From the temperature dependence of the limiting molar conductivities, the Eyring’s activation enthalpy of charge transport was determined. The thermodynamic functions such as Gibbs energy, entropy, and enthalpy of the process of ion pair formation were calculated from the temperature dependence of the association constants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creating sets of data on physical and chemical properties of ionic liquids (ILs) are of essential significance for both pure scientific and industrial purposes. The transport properties of the mixtures of ionic liquids (conductance, viscosity, and transference numbers) are important because the values provide useful and sensitive information about ion–solvent interaction, ionic association, and solvent structure. A survey of literature indicates that most studies report only the specific conductivity data for pure ionic liquids or binary and ternary mixtures of ILs with various solvents at 298.15 K. The conductance studies of dilute solutions of ionic liquids in a wide temperature range allow the determination of the values of association constants and thermodynamic functions of association, which consequently allows the better understanding of the behavior of ILs in various solvents [1–3]. This paper is both a continuation and an extension of our and other authors’ works on association of imidazolium-based ionic liquids, i.e., [emim][BF4] and [bmim][BF4] in water [4], 1-propanol [5], N,N-dimethylformamide [6], acetonitrile [7, 8], dimethylsulfoxide [9], and methanol [9, 10]. Moderate ionic association of ILs occurs in N,N-dimethylformamide, acetonitrile, and methanol, slight in dimethylsulfoxide, whereas it becomes significant in 1-propanol. Water promotes significantly dissociation of the ionic liquids.
According to our knowledge, conductometric data for solutions of [emim][BF4] and [bmim][BF4] in dichloromethane (DCM) at various temperatures have not yet been reported. The only paper is by Katsuda et al. [11], who investigated the conductance of [bmim][BF4] in DCM exclusively at 298.15 K. Aiming to cover this topic, the present work deals with the precise conductivity measurements, which have been carried out in the concentration range c = 0.4 to 4 · 10−4 mol · dm−3 of [emim][BF4] and [bmim][BF4] in DCM at temperatures range 278.15–303.15 K at atmospheric pressure. Imidazolium-based ionic liquids were chosen because of their thermal and chemical stability and the insignificant degree of susceptibility to air and moisture. The obtained data were used to calculate the values of the limiting molar conductivities, Λo, and the association constants, K A on the basis of lcCM model. The Eyring activation enthalpy of charge transport, ΔH ‡λ , as well as the Gibbs energy, ΔG oA , enthalpy, ΔH oA , and entropy, ΔS oA , of ion pair formation, for the electrolytes have been evaluated.
It should be stressed that measuring the conductance in the selected systems is a difficult task, since the experiments require high accuracy. It results from the fact that the relative permittivity of dichloromethane is low, its ionization properties are relatively weak, and electrolytes dissolved in DCM are strongly associated [11–15]. Therefore, it is necessary to use the extremely low concentrations of the electrolyte. For concentrations below the limit given by Fuoss [16], c max = 3.2 · 10−7 · ε r 3 mol · dm−3 (in DCM c max = 2.3 · 10−4 mol · dm−3), it can be expected that only the free ions and ion pairs are present in the solution [17].
Experimental
Materials
[Emim][BF4] of 99 % purity and [bmim][BF4] of 98.5 % purity were purchased from Fluka and were used as received. The water content of the studied chemicals was determined by Karl–Fischer titration. The final water mass fraction was less than 0.015 % in [emim][BF4] and 0.05 % in [bmim][BF4], respectively. Dichloromethane (minimum 99.8 %, water content <0.02 %) was received from POCH Gliwice (Poland) and was used without further purification. The actual purity of DCM was estimated to be 99.97 % by gas chromatography. The specific conductance, к, of the solvent was in the range of 2–2.5 · 10−9 S · cm−1 at 278.15–303.15 K, which is in good agreement with available data [11, 15].
Methods
All the solutions were prepared by mass using an analytical balance (Sartorius RC 210D) with a precision of ±1 · 10−5 g.
The measurement procedure was based on the method described by Bešter-Rogač et al. [13, 18] and used by us in our previous works [4–6, 19, 20]. Conductivity measurements were performed with a three-electrode cell with the use of a Precision Component Analyzer 6430B (Wayne-Kerr, UK) under argon atmosphere and at the different frequencies, ν, (0.2, 0.5, 1, 2, 3, 5, 10, and 20) kHz. The temperature was kept constant within 0.003 K (Calibration Thermostat UB 20F with Through-flow cooler DLK 25, Lauda, Germany). The details of the experimental procedure for conductometric measurements were described in our previous paper [6]. The estimated uncertainty of the measured values of conductivity was 0.20 %. The estimation of this uncertainty takes into account the high degree of difficulty associated with the conductivity measurements of electrolyte solutions in DCM. These difficulties, among others, are related to the use of very low concentrations of electrolytes and high ionic association.
Densities were measured with an Anton Paar DMA 5000M oscillating U-tube densimeter equipped with a thermostat with a temperature stability within ±0.001 K. The densimeter was calibrated with extra pure water, previously degassed ultrasonically. The estimated uncertainty of the density is ±2· 10−4 g · cm−3.
Results and discussion
The physical properties of dichloromethane are given in Table 1.
The measured density of DCM agreed well with those values published in the literature [13, 21, 22].
To convert molonity, \( \tilde{m} \) (moles of electrolyte per kilogram of solution) into molarity, c, the values of density gradients, b, have been determined independently and used in the equation
where ρ o is the density of the solvent. Molar concentrations, c, were necessary to use the conductivity equation. The density gradients and the molar conductivities of the ILs in solution, Λ, as a function of IL molality, m (moles of electrolyte per kilogram of solvent) and temperature are presented in Table 2. The relationship among m, m̃, and c is the following
where M is the molar mass of electrolyte.
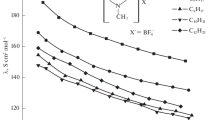
The plot of molar conductivity, Λ, versus the square root of the molar concentration, c 1/2, for the investigated systems monotonically decreases as shown in Figs. 1 and 2, respectively, over the temperature range from 273.15 to 303.15 K. The values of Λ for ionic liquids in DCM are smaller than in DMF [6], water [4], and 1-propanol [5]. With a change of temperature, they change very slightly, in contrast to the above-mentioned solvents.
The conductivity data were analyzed in the framework of the low concentration Chemical Model (lcCM) [23]. This approach uses the following set of equations
and
In these equations, Λo is the limiting molar conductivity, α is the dissociation degree of an electrolyte, K A is the ionic association constant, R is the distance parameter of ions, y ± is the activity coefficient of ions on the molar scale, and A and B are the Debye–Hückel equation coefficients. The analytical form of the parameters S, E, J 1, and J 2 was presented previously [23].
In all previous papers [4–6, 19, 20], the values of Λo, K A, and R were obtained using the well-known procedure given by Fuoss [24]. However, in this case, we were unable to optimize the values of R in a sufficiently reliable manner. Therefore, we calculated the values of R independently, assuming the possible existence of contact (CIP) and solvent-separated (SSIP) ion pairs in the solution. For this purpose, the distance of closest approach (contact distance) of cation and anion, R = a = a + + a −, we calculated from the ionic radius of BF4 −, a − = 0.227 and a + = 0.331 nm for [bmim]+ and a + = 0.310 nm for [emim]+ [11]. The latest value was extrapolated by us from the radii of ions [bmim]+, [hmim]+, and [omim]+[11]. In the case of solvent-separated ion pair, R = a + s, where s is the length of an orientated solvent molecule, we assumed according to [13, 25] s = 0.177 nm. The results obtained by a two-parameter fit (Λo and K A) are collected in Table 3. For the last three concentrations from Table 2, the calculated values of Λ were lower than the experimental ones. Moreover, the differences between calculated and experimental values of Λ increase with an increase of concentration. This suggests that in solution form higher aggregates such as triple ion formation. Therefore, we did not use the last three concentrations for calculations, and we used a range of concentrations (c = 0.4 to 4 · 10−4 mol · dm−3) consistent with the Fuoss condition [16].
As seen from Table 3, the limiting molar conductivities increase as the temperature increases since the mobility of free ions is higher. The values of Λo for [emim][BF4] are higher compared to those values for [bmim][BF4] because the Λo values increase with decreasing alkyl chain length of the ILs. Furthermore, the differences between the Λo values for both ionic liquids increase with increasing temperature. The limiting molar conductivities for [emim][BF4] and [bmim][BF4] presented in Table 3 are much higher than corresponding values determined in 1-propanol, DMF, and water [4–6]. However, one should pay attention to the fact that the determining factor that affects the Λo value, a macroscopic viscosity of the solvent, in the case of dichloromethane, is very small (see Table 1). The value of Λo for [bmim][BF4] at 298.15 K is in good agreement with this reported by Katsuta et al. [11] (Λo = 162.5 S cm2 mol−1). Obtained values of standard deviations, σ(Λ), may be considered to be high, but should be noted that the conductometric measurements of analyzed systems are very difficult to be performed.
From data collected in Table 4 for investigated ILs in various solvents results that the values of limiting molar conductivities, Λo, follow the order: acetonitrile > dichloromethane > methanol > water > N,N-dimethylformamide > dimethyl sulfoxide > 1-propanol. As mentioned above, the conductivity increase is related to the decrease in solvent viscosity. However, for the series given above, this relationship is not entirely fulfilled. But if we consider the protic and aprotic character of solvents, the conductivity decreases in the order: MeOH > Water > 1-PrOH, and for aprotic: ACN > DCM > DMF > DMSO, which correlates very well with viscosity increase.
It is observed from Table 3 that both ionic liquids are very highly associated in DCM. This observation stays in agreement with literature data for various imidazolium ILs in DCM (KA ≈ 1 · 104–38 · 104 dm3 mol−1) [11, 13]. The value of K A = 384300 dm3 mol−1 for [bmim][BF4] in DCM at 298.15 K presented in paper [11] is slightly lower than ours. This can be explained by different procedure used for experimental data analysis and the different measuring procedure. As we can see from the Table 3, K A values depend significantly on the adopted model of ion pairs. In the case of solvent-separated (SSIP) ion pairs, the K A and Λo values are higher. The data collected in Table 3 also show that the association constants increase with increasing temperature, and the effect is much more pronounced in the case of [emim][BF4]. This can be due to decrease in ILs-DCM interactions with increasing temperature.
In the case of [emim][BF4] and bmim][BF4] solutions in dimethyl sulfoxide [9] and N,N-dimethylformamide [6], the association constants are practically negligible; in methanol and acetonitrile [7–10], ion association is rather weak. In turn, in water [4], both ionic liquids are practically fully dissociated, whereas in 1-propanol [5] and especially in dichloromethane, they were definitely associated. The data are consistent with the classical ionic association theory of electrolytes [28]. Similar behavior of other ionic liquids in various solvents was also observed [1, 3, 29–34]. In turn, in very low-permittivity tetrahydrofuran (ε r = 7.58), ionic liquid (1-butyl-2,3-dimethylimidazolium tetrafluoroborate [bmmim][BF4]) form not only ion pairs but also triple ions [29]. It means that ions are more associated in the solvent possessing a low-relative permittivity. However, not only the relative permittivity of the solvent plays an essential role in the ionic association process, but also the participation of the ion–solvent interactions and the alkyl chain length of the ILs should be considered too.
From the temperature dependence of Λo, the Eyring activation enthalpy of charge transport, ΔH ‡λ , was obtained by using Eq. 5
where D is an empirical constant. From the slope of the linear dependencies of ln Λo + 2/3 ln ρ o versus the inverse of the temperature (1/T), which are shown in Fig. 3(a, b), we obtained the following ΔH ‡λ values 6,138 (CIP) and 6,142 (SSIP) for [emim][BF4] and 5,257 (CIP) and 5,271 (SSIP) for [bmim][BF4] (all in J · mol−1), respectively. For [emim][BF4], the value of ΔH ‡λ is thus higher than [bmim][BF4] by 881 (CIP) and 871 (SSIP) units. It is somewhat surprising, considering the fact that in other solvents [4–7, 9], the values of ΔH ‡λ are higher for [bmim][BF4] than [emim][BF4]. It is the result of the presence of a larger substituent in the [bmim]+ cation compared to [emim]+. In the case of dichloromethane, it may suggest that the effective ionic radius of [emim]+ is larger than the [bmim]+. Taking into account the values of the Eyring activation enthalpy of charge transport for other solvents, we can conclude that ΔH ‡λ depends mainly on the solvent. In the case of protic solvents (water and 1-propanol) values are very similar (about 16,000 J · mol−1) and almost twice or more higher than those for DMF, DCM, and ACN. It can be concluded that the value of ΔH ‡λ depends on the formation of hydrogen bonds between molecules of the solvent and its structure.
The temperature dependence of the association constant was used to calculation of Gibbs-free energy of ion formation, ΔG oA
ΔG oA (T) can also be expressed by the polynomial
The values of parameters A, B, and C of Eq. (7) and correlation coefficients, r 2, are summarized in Table 5.
The entropy and enthalpy of ion association are defined as
The thermodynamic functions of the ion pair formation (ΔG oA , ΔS oA , ΔH oA ) at different temperatures are presented in Table 6 and in Figs. 4, 5, and 6, respectively.
The values of ΔG oA presented in Table 6 and Fig. 4 indicate that the spontaneity of the ion pair formation in the case of both ionic liquids is comparable. The increase of temperature leads to more negative ΔG oA values, which means shifting the equilibrium toward the formation of ion pairs due to reduction in preferential solvation of ions by temperature (interactions between IL and DCM become weaker with increasing temperature).
The data collected in Table 4 show that the values of Gibbs-free energy of the studied ILs in other solvents are also negative (and become more negative as temperature increases).
As can be seen in Figs. 5 and 6, both the values of entropy and enthalpy of association are positive and greater for [emim][BF4]. Moreover, the values of ΔS oA and ΔH oA increase with increasing temperature for both tested electrolytes. Positive values of entropy prove that the transition from the free solvated ions into the ion pairs causes that system becomes less ordered. This is related to the partial desolvation of ions prior to the formation of ion pair. The positive values of ΔH oA indicate that the ion pair-forming processes are endothermic, particularly in the case of [emim][BF4]. From Eq. (10)
it follows that entropic effects seem to dominate over the enthalpic effects because the Gibbs-free energy, ΔG oA , is negative, and thus the ion pair formation is spontaneous in both cases. Furthermore, earlier studies [4–7, 19] confirm that in the case of other solvents, spontaneity of ionic association process results mainly from changes of the entropy.
Conclusions
Molar conductivities of very dilute solutions of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate in dichloromethane have been reported for the first time at T = 278.15 to 303.15 K. Conductivity data were analyzed using the Barthel’s low concentration Chemical Model (lcCM). The examined ionic liquids act like very weak electrolytes (K A ≈ 60 · 104 mol−1 dm3 for [emim][BF4] and ≈ 48 · 104 mol−1 dm3 for [bmim][BF4], respectively) in the low-permittivity solvent DCM at used temperature range. With increasing temperature, the tendency to form the ion pairs increases. [Emim][BF4] is more associated electrolyte than [bmim][BF4]. K A values depend significantly on the adopted model of ion pairs, (CIP) or (SSIP). The values of Λ for ionic liquids in DCM are smaller than in DMF [6], water [4], and 1-propanol [5] and change very slightly with a change of temperature. In turn, Λo values are much higher than corresponding values determined in 1-propanol, DMF, and water. The evaluated values of thermodynamic functions of association suggest the spontaneity of the association process. The values of ΔH oA are positive and suggest that the ion-pairing process is endothermic. Because the Gibbs-free energy is negative, entropic effects seem to dominate over the enthalpic effects, and thus, the ion pair formation of ionic liquids in DCM is spontaneous in both cases.
References
Luo J, Hu J, Saak W, Beckhaus R, Wittstock G, Vankelecom IFJ, Agert C, Conrad O (2011) Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes. J Mater Chem 21:10426–10436
Luo J, Tan TV, Conrad O, Vankelecom IF (2012) 1H-1,2,4-Triazole as solvent for imidazolium methanesulfonate. Phys Chem Chem Phys 14:11441–11447
Luo J, Conrad O, Vankelecom IFJ (2013) Imidazolium methanesulfonate as a high temperature proton conductor. J Mater Chem A 1:2238–2247
Boruń A, Fernandez C, Bald A (2015) Conductance studies of aqueous ionic liquids solutions [emim][BF4] and [bmim][BF4] at temperatures from (283.15 to 318.15) K. Int J Electrochem Sci 10:2120–2129
Boruń A, Bald A (2014) Conductometric studies of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate in 1-propanol at temperatures from (283.15 to 318.15) K. Int J Electrochem Sci 9:2790–2804
Boruń A, Bald A (2012) Conductometric studies of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate in N, N-dimethylformamide at temperatures from (283.15 to 318.15) K. J Chem Eng Data 57:475–481
Bešter-Rogač M, Stoppa A, Buchner R (2014) Ion association of imidazolium ionic liquids in acetonitrile. Phys Chem B 118:1426–1435
Kalugin ON, Voroshylova IV, Riabchunova AV, Lukinova EV, Chaban VV (2013) Conductometric study of binary systems based on ionic liquids and acetonitrile in a wide concentration range. Electrochim Acta 105:188–199
Bešter-Rogač M, Hunger J, Stoppa A, Buchner R (2010) Molar conductivities and associationc of 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium tetrafluoroborate in methanol and DMSO. J Chem Eng Data 55:1799–1803
Voroshylova IV, Smaga SR, Lukinova EV, Chaban VV, Kalugin ON (2015) Conductivity and association of imidazolium and pyridinium based ionic liquids in methanol. J Mol Liq 203:7–15
Katsuta S, Imai K, Kudo Y, Takeda Y, Seki H, Nakakoshi M (2008) Ion pair formation of alkylimidazolium ionic liquids in dichloromethane. J Chem Eng Data 53:1528–1532
Hunger J, Stoppa A, Buchner R (2008) From ionic liquid to electrolyte solution: dynamics of 1-N-butyl-3-N-methylimidazolium tetrafluoroborate/dichloromethane mixtures. J Phys Chem B 112:12913–12919
Bešter-Rogač M, Hunger J, Stoppa A, Buchner R (2011) 1-Ethyl-3-methylimidazolium ethylsulfate in water, acetonitrile, and dichloromethane: molar conductivities and association constants. J Chem Eng Data 56:1261–1267
Katsuta S, Shiozawa Y, Imai K, Kudo Y, Takeda Y (2010) Stability of ion pairs of bis(trifluoromethanesulfonyl)amide-based ionic liquids in dichloromethane. J Chem Eng Data 55:1588–1593
Bait S, Chattel GD, Kieviet WD, Tieleman A (1978) Ion association and solvation in dichloromethane of tetrachloro- and tetrabromoferrates(III) compared with simple halides. Z Naturforsch 33:745–749
Fuoss RM (1975) Conductance-concentration function for associated symmetrical electrolytes. J Phys Chem 79:525–540
Gestblom B, Songstad J (1987) Solvent properties of dichloromethane. 6. Dielectric properties of electrolytes in dichloromethane. Acta Chem Scand 41:396–409
Bešter-Rogač M, Habe D (2006) Modern advances in electrical conductivity measurements of solutions. Acta Chim Slov 53:391–395
Boruń A, Bald A (2012) Conductometric studies of sodium tetraphenylborate, tetrabutylammonium bromide, and sodium tetrafluoroborate in N, N-dimethylformamide at temperatures from (283.15 to 318.15) K. J Chem Eng Data 57:2037–2043
Boruń A, Trzcińska I, Bald A (2014) Conductometric studies of sodium iodide, sodium tetraphenylborate, tetrabutylammonium iodide, and sodium tetrafluoroborate in 1-propanol at temperatures from (283.15 to 318.15) K. Int J Electrochem Sci 9:7805–7818
Tôrres RB, Hoga HE (2008) Volumetric properties of binary mixtures of dichloromethane and amines at several temperatures and p = 0.1 MPa. J Mol Liq 143:17–22
Su L, Wang H (2009) Volumetric properties of dichloromethane with aniline or nitrobenzene at different temperatures: a theoretical and experimental study. J Chem Thermodyn 41:315–322
Barthel MG, Krienke H, Kunz W (1998) Physical chemistry of electrolyte solutions: modern aspects. Springer, New York
Fuoss RM (1978) Conductance-concentration function for the paired ion model. J Phys Chem 82:2427–2440
Wang Y, Tremmel J, Smedt JD, Alsenoy CV, Geise HJ, Veken BV (1997) Ab initio determination of the force field of dichloromethane, verified by gas-phase infrared frequencies and intensities and applied to a combined electron diffraction and microwave investigation of geometry. J Phys Chem A 101:5919–5925
Riddick JA, Bunger WB, Sakano TK (1986) Organic solvents. Wiley, New York
Krestov GA, Afanas’ev VN, Efremova LS (1988) Fizikokhimicheskie svoistva binarnykh rastvoritelei (Physicochemical properties of binary solvents). Khimiya, Leningrad
Marcus Y, Hefter G (2006) Ion pairing. Chem Rev 106:4585–4621
Roy MN, Ray T, Roy MC, Datta B (2014) Subsistence of ion-pair and triple-ion origination of an ionic liquid, ([bmmim][BF4]) predominant in solvent systems. RSC Adv 4:62244–62254
Bešter-Rogač M, Stoppa A, Hunger J, Hefter G, Buchner R (2011) Association of ionic liquids in solution: a combined dielectric and conductivity study of [bmim][Cl] in water and in acetonitrile. Phys Chem Chem Phys 13:17588–17598
Gupta S, Chatterjee A, Das S, Basu B, Das B (2013) Electrical conductances of 1-butyl-3-propylimidazolium bromide and 1-butyl-3-propylbenzimidazolium bromide in water, methanol, and acetonitrile at (308, 313, and 318) K at 0.1 MPa. J Chem Eng Data 58:1–6
Sadeghi R, Ebrahimi N (2011) Ionic association and solvation of the ionic liquid 1-hexyl-3-methylimidazolium chloride in molecular solvents revealed by vapor pressure osmometry, conductometry, volumetry, and acoustic measurements. J Phys Chem B 115:13227–13240
Roy MC, Roy MN (2014) Conductometric investigation of ion–solvent interactions of an ionic liquid {[emim]CH3SO3} in pure n-alkanols. J Mol Liq 195:87–91
Majdan-Cegincara R, Zafarani-Moattar MT, Shekaari H (2015) The study of solute–solvent interactions in 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate+acetonitrile from solvent activity, density, speed of sound, viscosity, electrical conductivity and refractive index measurements. J Mol Liq 203:198–203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boruń, A., Bald, A. Ionic association and conductance of ionic liquids in dichloromethane at temperatures from 278.15 to 303.15 K. Ionics 22, 859–867 (2016). https://doi.org/10.1007/s11581-015-1613-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1613-x




 278.15 K,
278.15 K,  283.15 K,
283.15 K,  288.15 K,
288.15 K,  293.15 K,
293.15 K,  298.15 K,
298.15 K,  303.15 K
303.15 K
 278.15 K,
278.15 K,  283.15 K,
283.15 K,  288.15 K,
288.15 K,  293.15 K,
293.15 K,  298.15 K,
298.15 K,  303.15 K
303.15 K
 ), CIP, and (
), CIP, and ( ), SSIP
), SSIP
 ), SSIP, (
), SSIP, ( ), CIP, and [bmim][BF4] in DCM, (
), CIP, and [bmim][BF4] in DCM, ( ), SSIP, (
), SSIP, ( ), CIP
), CIP
 ), SSIP, (
), SSIP, ( ), CIP, and [bmim][BF4] in DCM, (
), CIP, and [bmim][BF4] in DCM, ( ), SSIP, (
), SSIP, ( ) CIP
) CIP
 ), SSIP, (
), SSIP, ( ), CIP and [bmim][BF4] in DCM, (
), CIP and [bmim][BF4] in DCM, ( ), SSIP, (
), SSIP, ( ) CIP
) CIP