Abstract
The very hot summers of recent years have led to an increase in the number of large forest fires in Europe. We investigated four large fire sites in Brandenburg and Saxony (Germany) up to 4 years after the fires with a focus on studying the post-fire fungal communities. In this context, we documented two species of Agaricomycetes associated with mosses, which are common but particularly emerge on burnt areas, i.e., Arrhenia bryophthora sp. nov. and Bryopistillaria clavarioides sp. nov. The former is an agaric with omphalinoid habit that causes the dieback of the common moss Ceratodon purpureus; the latter is a clavarioid fungus associated with either Ceratodon purpureus or another common moss, Funaria hygrometrica. Both fungal species appear to be restricted to recently burnt areas and have otherwise not been observed on or in close vicinity of these mosses. Herein, we describe these fungi macro- and micromorphologically as well as on a molecular basis and discuss their taxonomic position and potential lifestyles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burnt areas represent special, spatially and temporally limited habitats that are colonized by a specialized fungal community (Dix and Webster 1995). These species are generally considered to be highly adapted but weak in competition (El-Abyad and Webster 1968b). After a fire, the topsoil in particular is poor in microorganisms and alkalinized to a certain extent (El-Abyad and Webster 1968a). The rapid colonization mechanisms of fire sites by phoenicoid fungi have been intensely documented and discussed since the second half of the twentieth century and four general hypotheses have so far been proposed: dispersal hypothesis, dormancy hypothesis, heat tolerance hypothesis, and body snatchers hypothesis (Matheny et al. 2018). However, none of these hypotheses has been sufficiently verified, and it seems plausible that fungi can pursue different strategies (Matheny et al. 2018; Filialuna and Cripps 2021).
In the absence of freshly burnt areas, certain species of the genera Sphaerosporella, Wilcoxinia, Geopyxis, and Peziza s.l. are endophytically associated and/or form mycorrhizas with different tree species and may only fruit after their host is damaged by wildfires (Hughes et al. 2020; Pulido-Chavez et al. 2021). The rather common species Pholiota carbonaria and Plicaria carbonaria follow a typical saprotrophic lifestyle as decomposers of dead plant biomass (Matheny et al. 2018; Kouki and Salo 2020). However, it was shown that the range of trophic lifestyles is broader than originally assumed (Raudabaugh et al. 2020). In addition to saprotrophy, P. carbonaria can interact with Polytrichum commune, a moss that also occurs on burnt areas (Raudabaugh et al. 2021). In the case of Plicaria spp., it has recently been reported that they have endophytic phases in mosses (Raudabaugh et al. 2020). Moreover, members of the genera Anthracobia, Geopyxis, Peziza, Tricharina, Psathyrella, and Cotylidia seem to be endophytically associated with various bryophytes as well (Raudabaugh et al. 2020; Korotkin et al. 2018).
Thus, many groups of fungi are dependent on post-fire mosses quickly colonizing burnt areas. Due to the perfect light and nutrient situation on such areas, these bryophytes can reach high abundancies of individuals, for example, Funaria hygrometrica and Ceratodon purpureus (Esposito et al. 1999; Sim-Sim et al. 2004).
In addition to phoenicoid fungi, bryophilic associations are not uncommon in some fungal families. For instance, the Hygrophoraceae (Agaricales) show a broad spectrum of interactions with phototrophic partners. They range from species that form lichens (Dictyonema, Lichenomphalia) to ectomycorrhizal species that live in mutualistic symbiosis with trees (Hygrophorus); others are endophytic biotrophs in grasslands (Hygrocybe s. l.) or develop strong associations with bryophytes as in the case of Arrhenia (Halbwachs et al. 2018; Redhead et al. 2002b; Lodge et al. 2014; Lawrey et al. 2009). The Rickenellaceae (Hymenochaetales) consist of various genera that are strictly associated with mosses (as parasites, commensals, or symbionts), e.g., Bryopistillaria, Muscinupta, Sphagnomphalia, Rickenella, and Loreleia (Redhead et al. 2002a; Korotkin et al. 2018).
Until the recent major forest fires in the Northern Hemisphere, such exceptional habitats could only rarely be studied, especially in Europe (Moser 1949; Butin and Kappich 1980). One reason for this is the strict forest fire policy of European countries, which leads to the removal of dead wood and prohibits the development of smaller fire areas in the wild. For this reason, phoenicoid fungi have only rarely been found in European countries (Knudsen and Vesterholt 2012; Dämmrich et al. 2016). On the other hand, after the great fires of the last years, it can be assumed that still undiscovered species live in these specific and (so far) underrepresented habitats. Against this background, we have mycologically examined four large forest fire areas in Germany (Brandenburg and Saxony) over the last 6 years. Among the collected fungi, two members of the Agaricomycetes, Hygrophoraceae and Rickenellaceae, are described herein.
Material and methods
Morphological study
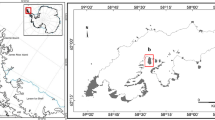
The basis of this study is the investigation of large forest fire events in Saxony and Brandenburg, Germany (for details see the examined samples below). The fires took place in the years 2018, 2019, and 2022 (Brandenburg) and 2022 (Saxony). Sampling was carried out from 2019 on in periods of higher precipitation and in the winter half-year, when the weather was favorable for fungal fruiting.
Fresh specimens were photographed in the field, collected, morphologically examined and documented, and finally, dried and stored. In addition, the surrounding plants and mosses were recorded. The microscopic examination and documentation of the morphological characteristics of fresh and dried specimens were done with a Zeiss AXio1 light microscope with × 100, × 400, and × 1000 magnification in combination with a Canon EOS 60D, digital camera, or a Bresser microscope equipped with a Toupcam SPCMOS05000KPA. The microscopic measurement was done manually with a measuring eyepiece (Zeiss, Germany) or digitally in CorelDRAW2019 or in ToupView. Microscopic drawings are based on real microscopic photographs traced in CorelDRAW2019. Type specimen are deposited at Herbarium Senckenbergianum Görlitz (GLM).
Molecular genetic study and phylogenetic reconstruction
DNA of the samples was extracted using the E.Z.N.A.® Plant DNA Kit or the REDExtract-N-Amp Kit (Sigma-Aldrich) in 1:5 dilution. The primer pairs ITS1F/ITS4, ITS1F/ITS4B, and LR6/LR0R (White et al. 1990; GARDES and BRUNS 1993) and the DreamTaq Green PCR Master Mix (2x, Thermo Scientific™) or YourTaqPolymerase (in case of Touchdown PCR) were used to amplify and sequence the partial 28 s rDNA (large subunit, LSU) and internal transcribed spacer (ITS) barcoding region. The polymerase chain reaction was setup in an Eppendorf 5341 Mastercycler. The reaction involved initial denaturation at 95 °C for 2 min, followed by 35 cycles in series of denaturation at 95 °C for 0.5 min, annealing at 52 °C for 0.5 min, and extension at 72 °C for 1 min, with a final step of one cycle at 72 °C for 10 min to final extension. Subsequently, the PCR products were purified using the PCR Purification Kit-Column Kit (Jena BioScience) or sent directly to Eurofins (Germany) for further processing. The purified PCR products were sequenced in both directions at LGC Genomics GmbH (Berlin) or at Eurofins Genomics. The resulting chromatograms were quality-checked and edited in Geneious Prime 2023.2 (Kearse et al. 2012).
The newly generated sequences (ITS and LSU) were compared and identified with entries in GenBank using a blastn search (Altschul et al. 1990). Selected sequences of related species from GenBank and UNITE (Nilsson et al. 2019) and own generated sequences were concatenated and aligned using Clustal Omega 1.2.2 (Sievers and Higgins 2021) implemented in Geneious 2023.2. The alignments were carefully checked and edited manually; ambiguous regions were masked and not used for phylogenetic analysis. All newly gathered sequences were submitted to GenBank under accession numbers OR912523–OR912545 (or listed in Table 1).
Maximum Likelihood (ML) phylogenetic analysis was performed using RAxML 8.2.11 (Stamatakis 2014) implemented in Geneious 2023.2, applying the rapid bootstrapping algorithm with 1000 replicates. Four nucleotide partitions (ITS1, 5.8S, ITS2, 28S) were set, and the GTR GAMMA I substitution model was applied, in addition to one binary partition (indel characters) that was set to default. The Bayesian Inference (BI) phylogeny was carried out using MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001) implemented in Geneious 2023.2. Four gamma categories were used with the GTR substitution model and the invgamma rate variation. The Markov Chain Monte Carlo were set to 1,000,000 generations, sampling every 1000th generation. The first 20% of the trees was discarded as burn-in. For the remaining trees, a 50% majority rule consensus phylogram with posterior probabilities as nodal supports was computed. Clades with bootstrap support above 95% for ML and 0.95 for BI are considered highly supportive. Phylogenetic trees were edited in Geneious 2023.2 and in Corel Draw (v21.0.0.593 Corel Corporation).
Results
Phylogenetic analysis
Result of the phylogenetic analysis based on sequences of the ITS and 28S rDNA (LSU) region from A. bryophthora and members of the Hygrophoraceae Lotsy selected from GenBank and UNITE is depicted as RAxML tree in Fig. 1. Serpulomyces borealis and Amylocorticium cebennense were selected as outgroup.
RAxML tree combining selected ITS and LSU-sequences from the Hygrophorineae Aime, Dentinger & Gaya including A. bryophthora BP and BI values less than 50 and 0.75, respectively, are omitted. Bold lettering indicates species’ sequences generated in this study, and red font indicates the newly described species. Pictograms mark presence or absence of clamps
The aligned matrix contained 1814 unique nucleotide position features (262 ITS1, 157 5.8S, 353 ITS2, and 1041 28S) for the entire dataset of 93 specimens. Generated phylogenetic ML and BI trees had similar topological structures (compare Fig. 1 and S1).
The genera of Hygrophorineae, i.e., Cantharocybe Chromosera, Chrysomphalina, Haasiella, and Lichenomphalia, each form statistically well-validated clades. Sequences of Arrhenia bryophthora cluster in a statistically well-validated clade (BP/BI: 100/1) that is basally located in Arrhenia s.l., indicating that this species belongs to that monophyletic clade. However, this clade is only moderately supported statistically (BP/BI: 51/0.96).
Arrhenia s.l. comprises species with different ecological affiliations. These are categorized as follows: The gerardiana/bigelowii clade and the telmatiaea/philonotis clade include facultative or obligate sphagnophilic species and one lignicolous species. The obatra/velutipes clade (plus two unknown and possibly undescribed species), the rustica clade, and the obscurata/acerosa clade include species with unknown but possibly biotrophic ecology. Bryotrophic species are represented by the bryophthora clade and species within Arrhenia sensu stricto (including the type species of the genus A. auriscalpium).
A RaxML tree based on ITS and LSU sequences from B. clavarioides and allied sequences of representatives from the Rickenellaceae in GenBank and UNITE is depicted in Fig. 2. The aligned matrix contained 2322 unique nucleotide position features (267 ITS1, 163 5.8S, 247 ITS2, and 1645 28 s) for the entire dataset of 86 specimens. Generated phylogenetic ML and BI trees had similar topological structures (compare Fig. 2 and S2).
RAxML tree combining selected ITS and LSU-sequences of the Rickenellaceae Vizzini (Hymenochaetales) related to B. clavarioides. BP and BI values less than 75 and 0.75, respectively, are omitted. Bold lettering indicates species’ sequences generated in this study, and red font indicates the newly described species
Sequences of Bryopistillaria clavarioides generated in this study and three additional sequences of environmental samples form a distinct and well-supported clade that is closely related to specimen-sequences of Bryopistillaria sagittiformis from Olariaga et al. (2020) displaying sequence similarities in ITS sequences from 89.25 to 90.42%. Sequences of both species form a well-supported clade in the Rickenellaceae RaxML tree (BP/I: 90/1).
The RAxML tree contains six monospecific genera, i.e., Globulicium, Canthrarellopsis, Sphagnomphalia, Muscinupta, Blasiphalisa, and Ginnsia. Together with the genera Contumyces and Alloclavaria, all these clades are well supported (BP/BI:100/1). Samples of the genus Cotylidia form two separate clades, each being well supported (BP/BI: 100/1). One includes C. undulata, the genus’ type species, and hence is accounted as Cotylidia sensu stricto. And the second phylogenetically separate clade groups around Cotylidia diaphana. These results indicate that Cotylidia is polyphyletic and should be divided. However, this is beyond the scope of this work.
Moss-associated species of the genus Rickenella form a well-supported clade. R. minuta, that was recently discovered to have an ectomycorrhizal relation to Notofagaceae in Argentina builds a sister clade to those Rickenella with strict bryophilic relation (Korotkin et al. 2018). The entire Rickenella clade is only moderately well supported (BP/BI:75/1).
Taxonomy
Arrhenia bryophthora Karich, Jarling, R. Ullrich, sp. nov.
Mycobank number: 851246
Type: GLM-F137751 – Germany, Zeithain, Gohrischheide, Plot 3, 13,33206 E 51,43189 N, 95 m asl., fire site in forest with Pinus sylvestris and Betula pendula, on and in between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica and causing their dieback, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
Etymology: From ancient Greek βρύον (brúon, “moss”) and φθορά (phthorá, “destruction”).
Diagnosis: Small brown agaric with omphalinoid habit growing on old fire sites in association with Ceratodon purpureus and causing its dieback. Being distinct from other similar species in Arrhenia s.l. by the absence of clamp connections.
Macromorphology (Fig. 3a–d): Basidiocarps small, omphalinoid. Cap 4–10 mm broad, distinctly translucently striate when fresh, striae and in the center dark, chestnut to coffee brown (RAL 040 40 30 and RAL 040 30 20, respectively), between striae lighter, maple syrup brown (RAL 060 60 40), strongly hygrophanous when drying, glabrous to slightly scaly when young (scales hygrophanous), margin crenulate. Lamellae falcate-decurrent, distant, L = 14–18 with 1–2 lamellulae, maple syrup brown. Stem 6–12 × 1–1.5 mm, of cap color, finely pruinose in fresh specimen, turning glabrous at age, basally with whitish mycelium when young. No specific smell and taste. Spore print white.
Micromorphology (Fig. 4a, b): Basidia solely four-spored to predominantly two-spored, 25–30 × 6–8 µm, clampless. Basidiospores heteromorphic, cylindrical, dacryoid to subglobose, with distinct apiculus, (6)6.5–8(9) × 3.5–5(5.5) µm, ave. 7.1–8.1 × 3.7–4.1 µm, Q = 1.4–2.4 ave. 1.8–2.0 an exceptional specimen bearing sublobose to globose spores with deviating measurements, 7–9 × 4.5–7.5(8) µm ave. 7.9 × 5.7 µm, Q = 1.1–1.7(1.8) ave. 1.4 (IHI-23Arr1-KS2). Trama and cutis in pileus and stipe composed of 3–9 µm broad hyphae with partially fine to distinct incrusting pigmentation. Lamellar trama irregular, hyphae 2–9 µm broad, frequently distinctly incrusted and often branched. No cystidia observed. No structures amyloid or dextrinoid. No clamps observed.
Habitat (Fig. 5a–e): Growing on old fire sites (0.5 to 4 years postfire) solitarily or in clusters in between the dioicous moss Ceratodon purpureus, also observed near Funaria hygrometrica and Marchantia polymorpha, causing the dieback of the surrounding C. purpureus, being evident by brownish, circular, about 15 cm diameter large spots on the moss surface (compare Fig. 5). Fruit body formation was observed from September to May, mainly in November and March.
Distribution: So far, only known from four localities in Brandenburg and Saxony, Germany.
Additional specimens examined:
20201007-V312 – Germany, west of Luckenwalde, Felgentreu, Plot V3, 13.041819 E, 52.087368 N, 65 m asl., fire site on former Pinus sylvestris forest with few Betula pendula, on and between post fire moss Ceratodon purpureus causing its dieback, 7th of October 2020, leg. J. Neuendorf, T. Pilz-Hunter.
20201125-U913 – Germany, west of Luckenwalde, Felgentreu, Plot U9, 13.039827 E, 52.089232 N, 65 m asl., fire site on former Pinus sylvestris forest with few Betula pendula, on and between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica causing their dieback, 25th of November 2020, leg. W. Linder, A. Schaepe, S. Hutter.
20210310-J209 – Germany, east of Treuenbrietzen, Frohnsdorf, Plot J2, 12.936418 E, 52.043814 N, 95 m asl., Populus-Betula succession on fire site on former Pinus sylvestris forest, cleared, plowed, and reafforested with Pinus sylvestris, on and between post fire moss Ceratodon purpureus, causing its dieback, and healthy Polytrichum piliferum, 10th of March 2021, leg. W. Linder, A. Schaepe.
20210311-F607 – Germany, east of Treuenbrietzen, Frohnsdorf, Plot F6, 12.918541 E, 52.042959 N, 95 m asl., Populus-Betula succession on fire site on former Pinus sylvestris forest, partly cleared and reafforested with Quercus petraea and Pinus sylvestris, on and between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica causing their dieback, 11th of March 2021, leg. J. Ehrich, J. Seelbach.
20210317-D614 – Germany, east of Treuenbrietzen, Frohnsdorf, Plot D6, 12.915415 E, 52.039248 N, 95 m asl., Populus-Betula succession on fire site on former Pinus sylvestris forest, partly cleared, on and between post fire moss Ceratodon purpureus causing its dieback, 17th of March 2021, leg. J. Ehrich, T. Röthling.
20210512-V903 – Germany, west of Luckenwalde, Felgentreu, Plot V9, 13.041126 E, 52.088145 N, 65 m asl., fire site on former Pinus sylvestris forest with few Betula pendula, on and between post fire mosses Ceratodon purpureus, Funaria hygrometrica, and Marchantia polymorpha, possibly causing their dieback, 12th of May 2021, leg. J. Ehrich, J. Seelbach.
IHI-23Arr1-KS2 – Germany, Nationalpark Sächsische Schweiz, Richterschlüchte—Krinitzgrab, fire site in forest with Picea abies, Larix decidua, Pinus sylvestris, and Fagus sylvatica, 14.27467 E, 50.89607 N, 450 m asl. on and in between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica and causing their dieback, 4th of April 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
IHI-23Arr2-KS48 – Germany, Zeithain, Gohrischheide, fire site in forest with Pinus sylvestris and Betula pendula (fire in summer 2022), Plot 1, 13.33253 E, 51.41907 N, 95 m asl., on and in between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica and causing their dieback, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
IHI-23Arr3-KS49 – Germany, Zeithain, Gohrischheide, fire site in forest with Pinus sylvestris and Betula pendula (fire in summer 2022), Plot 4, 13.33253 E, 51.41907 N, 95 m asl., on and in between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica and causing their dieback, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
IHI-23Arr4-KS52 – Germany, Zeithain, Gohrischheide, fire site in forest with Pinus sylvestris and Betula pendula (fire in summer 2022), Plot 4, 13.33148 E, 51.4124 N, 95 m asl., on and in between post fire moss Ceratodon purpureus and possibly Funaria hygrometrica and causing their dieback, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
Bryopistillaria clavarioides Karich, Jarling & R. Ullrich, sp. nov.
= Clavaria tenuipes ss. Kajan & Grauwinkel 1987
Non Clavaria tenuipes Berk. & Broome 1848
Mycobank number: 851247
Type: GLM-F137752 – Germany, Zeithain, Gohrischheide, Plot 2, 13,33372 E 51,42659 N, 95 m asl., fire site in forest with Pinus sylvestris and Betula pendula (fire in summer 2022), on and in between post fire mosses Funaria hygrometrica and Ceratodon purpureus, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
Etymology: Referring to the macromorphological similarity with species of the genus Clavaria L.
Diagnosis: Small to medium sized club shaped fruit body with whitish to cream color. Occurring on old fire sites in association with Funaria hygrometrica and Ceratodon purpureus. Distinct from other clavarioid fungi by its phylogenetical association to Bryopistillaria sagittiformis. Distinct from the later by its larger size and different habit and lifestyle.
Macromorphology (Fig. 6a–f): Basidiocarps clavarioid (club shaped) 10–25(30) × 1–3 mm, whitish to yellowish-crème (RAL 9003 to RAL 1012) to almost pale yolk-yellow (RAL 1002). Sterile stem about 1/5 to 1/4 (1/3) of entire club, glabrous and with somewhat glassy surface, demarcated from fertile head which is broadened, glabrous or wrinkled with age and with a dull surface, tips tan when drying. No specific smell and taste. Spore print white.
Micromorphology (Fig. 7a, b): Basidia 16–26 × 5–6 µm, four or two (one) spored, clampless. Basidiospores subglobose, ellipsoid to oblong dacryoid, often adhering to each other in packages of two or four, with distinct apiculus, inamyloid, (4.5)5.0–6.0(6.5) × 2.5–3.5 µm, ave. 5.6 × 3.0 µm, Q = (1.4)1.7–2.2 ave. 1.90 in typical predominantly four spored specimens (GLM-F13775/IHI-23RClav2-KS29), and 7.5–9.0(9.5) × (2.5)3.0–3.5(4) µm, ave. 8.1 × 3.3 µm, Q = 2.1–3.2 ave. 2.5 µm in predominantly two spored specimens (IHI-23RClav1-KS2). Stipiticutis consisting of 4–7 µm broad cylindric and parallel directed hyphae. Trama in stipe and hymenium consisting of 5–20 µm wide, subirregularly directed hyphae, often constricted at the septa. No cystidia observed. All structures without iodine reactions. All septa clampless.
Habitat: Growing on up to two years old fire sites in between Funaria hygrometrica and Ceratodon purpureus. Sometimes, the mosses concomitant dieback was observed. Fruiting observed in spring and autumn. Attempts to obtain cultures from shed spores and fresh basidiocarps unsuccessful.
Distribution: Known from Europe and two soil samples from North America.
Additional collections examined:
IHI-23RClav1-KS2 – Germany, Nationalpark Sächsische Schweiz, Richterschlüchte—Krinitzgrab, 14,27467 E 50,89607 N, 450 m asl., fire site in forest with Picea abies, Larix decidua, Pinus sylvestris, and Fagus silvatica (fire in summer 2022), on and in between post fire mosses Funaria hygrometrica and Ceratodon purpureus, 4th of April 2023, leg. A. Karich, R. Ullrich, F. Salzsieder and R. Erlacher.
IHI-23RClav2-KS29 – Germany, Zeithain, Gohrischheide, Plot 1, 13,33253 E 51,41907 N, 95 m asl., fire site in forest with Pinus sylvestris and Betula pendula (fire in summer 2022), on and in between post fire mosses Funaria hygrometrica and Ceratodon purpureus, 23rd of March 2023, leg. A. Karich, R. Ullrich and R. Erlacher.
20190906–002 – Germany, east of Treuenbrietzen, Frohnsdorf, 12.905322 E, 52.037015 N, 95 m asl. Populus-Betula succession on fire site on former Pinus sylvestris forest (fire in summer of 2018), on and between post fire moss Ceratodon purpureus causing its dieback, September 6th 2019, leg. R. Jarling, J. Ehrich.
20201118-F618 – Germany, east of Treuenbrietzen, Frohnsdorf, Plot F6, 12.918541 E, 52.042959 N, 95 m asl., Quercus-plantation with Populus-succession on fire site (fire in summer of 2018) on former Pinus sylvestris forest, on and between post fire moss Ceratodon purpureus causing its dieback, 18th of November 2020, leg. A. Schaepe, W. Linder.
20201118-FF39 – Germany, east of Treuenbrietzen, Frohnsdorf, Site F, 12.917358 E, 52.042550 N, 95 m asl., Quercus-plantation with Populus-succession on fire site (fire in summer of 2018) on former Pinus sylvestris forest, on and between post fire mosses Ceratodon purpureus and Funaria hygrometrica causing their dieback, 18th of November 2020, leg. A. Schaepe, W. Linder.
20201119-D126 – Germany, east of Treuenbrietzen, Frohnsdorf, Plot D1, 12.916197 E, 52.040119 N, 95 m asl., Populus-succession on fire site (fire in summer of 2018) on former Pinus sylvestris forest, on and between post fire moss Ceratodon purpureus causing its dieback, 19th of November 2020, leg. P. Mohr, N. Scharnofski.
20230328-KF27 – Germany, east of Treuenbrietzen, Frohnsdorf, 12.914158 E, 52.037606 N, 95 m asl., Populus-succession on fire site (after two fires, 2018 & 2022) on former Pinus sylvestris forest, on and between post fire mosses Ceratodon purpureus and Funaria hygrometrica causing their dieback, 28th of March 2023, leg. R. Jarling, J. Ehrich.
Discussion
Arrhenia-species are either obligatory bryophiles, e.g., A. retiruga and A. lobata, others are lichenophiles, e.g., A. peltigerina, still others are not strictly moss-bound at all, such as species of the acerosa-complex (Voitk et al. 2020, 2022). Recently, Voitk et al. (2022) have shown that the species diversity of sphagnophilous Arrhenia is much greater than originally thought and that some are obligate and others facultative sphagnophiles, i.e., A. bigelowii, A. gerardiana, A. telmatiaea, and A. philonotis, respectively. A. bryophthora appears to be closely associated with Ceratodon purpureus and possibly Funaria hygrometrica, both dioicous mosses common on recent to old burnt areas. However, unlike the sphagnophilous and most other moss-dwelling Arrhenia, A. bryophthora causes a necrosis on its host and must therefore be considered a true parasite (Voitk et al. 2022). To our knowledge, a similarly destructive lifestyle is only known from the lichenophilic A. peltigerina (Diederich et al. 2022), which is macroscopically similar, but microscopically distinct by the presence of clamp connections and phylogenetically related to species of the auriscalpium/spathulata complex (Voitk et al. 2020).
Morphologically similar applies to A. velutipes, but this species has a faint odor of pelargonium, abundant clamp connections in all tissues and also differs in its molecular data (Bas et al. 1995; Vesterholt 2012). Arrhenia obatra and Arrhenia obscura can be macroscopically confused with A. bryophthora, as well, but both bare clamp connections (Bas et al. 1995; Vesterholt 2012).
Specimens of Arrhenia bryophthora were so abundant in late autumn, winter and early spring after the fire in all the areas studied (sometimes with hundreds of fruiting bodies on a few square meters) that it seems incredible to us that this species is not found in the literature (Moser 1983; Bas et al. 1995; Ludwig 2001; Vesterholt 2012). Ludwig (2001) mentioned in his comment on Omphalina velutina (= Lichenomphalia velutina) that it can occur after fires. Unfortunately, it is not clear where this information comes from. However, that species belongs to another linage of omphalinoid agarics that are lichenized (Redhead et al. 2002b). Vesterholt (2012) remarks on A. velutipes that it can also occur on old fire places. It is noteworthy that these two species can have a distinct pubescent stipe, as we have seen in many young specimens of A. bryophthora. Therefore, we believe that confusion with A. velutipes or L. velutina may have occurred in the past.
In the phylogenetic tree based on ITS and LSU sequences (Fig. 1), all specimens of A. bryophthora group in the same moderately supported clade that is the most basal one of all sequences assigned to Arrhenia specimens. Considering the phylogenetic results presented in Fig. 1 and in the literature (Voitk et al. 2020, 2022; Zhang et al. 2022) and the fact that the current understanding of the genus Arrhenia includes species with facultative and obligate bryophilous, lichenophilous, and saprotrophic lifestyles as well as cyphelloid, pleurotoid, and omphalinoid habitus; it is plausible that this taxonomic group should be revised and studied in more detail in the future. This includes careful morphological and phylogenetic studies based on multi-locus sequencing and comprehensive sampling. Thus, we believe that Arrhenia s.l. will be split into further genera and consequently, A. bryophthora will be recombined into another, perhaps new, genus. However, in view of the critical discussions on the establishment of new genera (Vellinga et al. 2015), we consider it best not to establish a new monospecific genus for A. bryophthora at this point, but to include it in the current understanding of Arrhenia s.l.
Bryopistillaria clavarioides has been confused with Clavaria tenuipes Berk. & Broome in the past. That fact is caused by a description of C. tenuipes by Rea (1922), which was later accepted by Corner (1950). Rea was the first who included old charcoal heaps in the substrate range of C. tenuipes. This obviously wrong information was later adopted in keys to the genus Clavaria, even until recent times (Pilát 1958; Jülich 1984; Knudsen et al. 2012;). Schild (1981) described clavarioid specimens growing on recently burnt sites, but was convinced that these were related to another collection that came from a flowerpot in the garden of the Geobotanical Institute of ETH Zurich (Switzerland). Therefore, he concurred with Corner’s opinion on C. tenuipes and grouped all these specimens under this taxon. Kajan (1986) and Kajan and Grauwinkel (1987) were the first to recognize that C. tenuipes Berk. & Broome and clavarioid specimens growing on recently burnt sites are not conspecific. They, however, incorrectly applied the taxon C. tenuipes to specimens growing exclusively on burnt sites (= B. clavarioides), and in turn established a new taxon, namely, C. krieglsteineri, for non-phoenicoid specimens.
Apart from the divergent ecology, the macroscopic description of C. tenuipes Berk. & Broome does not seem to correspond to B. clavarioides. Features like the pale clay-color and the up to 7 mm swollen head rather suggest a relation to C. daulnoyae Quel., or alike. In view of this and the fact that the type specimen is in poor condition (Schild 1981), we leave the clarification of what is to be understood as C. tenuipes to further studies.
All specimens of B. clavarioides discussed in this publication grew near or among the common fire mosses Funaria hygrometrica or Ceratodon purpureus. The same applies to the specimens presented in Kajan and Grauwinkel (1987). According to these authors, B. clavarioides is not uncommon on burnt areas in the spring and autumn after a fire up to 2.5 years. At this point, it must be noted that although the clubs grew along and between Funaria, the moss always appeared healthy and fresh, without any necrosis (which neither we nor other authors have observed before). However, we could observe the dieback of Ceratodon purpureus growing in the vicinity of B. clavarioides. Whether this was actually caused by the Bryopistillaria is not clear to us.
Bryophily is common among members of the Rickenellaceae. Muscinupta laevis and Bryopistillaria sagittiformis grow biotrophically on mosses, but it is still controversial whether they also live facultatively saprotrophically (Olariaga et al. 2020). Rickenella spp., Loreleia spp., and Blasiphalia pseudogrisella (= R. pseudogrisella) and Cantharellopsis prescotii are strictly associated with various mosses and therefore cannot be found separately from their partner. Dentinger and McLaughlin (2006) who erected Alloclavaria as the first clavarioid taxon within the HymenochaetaceaeFootnote 1 suggested to test whether A. purpurea is bryophilic, because it is frequently found among mosses. However, later results suggested that it represents a new linage of ectomycorrhizal fungi within the Hymenochaetales (Korotkin et al. 2018). The authors of the latter study also pointed out that L. marchantiae or Blasiphalia pseudogrisella, Muscinputa laevis, and Rickenella swartzii belong to the same ectomycorrhizal (ECM) cluster and can thus be classified as ECM or rather ECM-like. They specifically ruled out a parasitic lifestyle, but mentioned that a possible commensalism should not be dismissed unless the benefit to the fitness of their bryophytic partners will have been proven (Korotkin et al. 2018). Especially on our plots studied, we can exclude true ECM because most of the plant partners, i.e., Pinus sylvestris and Betula pendula, died in the fires, and other potential partners were missing. Therefore, a commensal (bryophilic) or even bryotrophic lifestyle of B. clavarioides is plausible.
Some representatives of the Rickenellaceae have hymenial cystidia or setae (Rickenella/Alloclavaria etc.); others do not (Muscinupta laevis, B. sagittiformis). Thus, it is not surprising that B. clavarioides has no cystidia, although it is assigned to the Hymenochaetales.
It is interesting that the spores of B. clavarioides often stick together in clusters (tetrads or more), which was also observed for Bryopistillaria sagittiformis (Olariaga et al. 2020) and Rickenella spp. (Ludwig 2001). Furthermore, it is remarkable that the latter author mentioned a high variability in spore size of R. swartzii, a fact that also applies to B. clavarioides.
Looking at the macro-morphology of B. clavarioides and B. sagittiformis, it is strange at first to place them in the same genus. While the club-shaped basidiocarps of B. sagittiformis are only up to 1.2 mm in size (Olariaga et al. 2020), B. clavarioides can form clubs that are up to 30 times larger. However, despite some little differences, their microscopic features match well; the spores are about of same size and lack an iodine reaction in both species (Olariaga et al. 2020). The RaxML-tree based on ITS and LSU loci (Fig. 2), the sequences of B. clavarioides and B. sagittiformis form a strongly supported clade. B. sagittiformis is biotrophic in/on various mosses and additionally can follow a saprotrophic lifestyle (Olariaga et al. 2020). The fact that B. clavarioides seems specific to its hosts, on the other hand, argues for a separate genus. Nevertheless, based on the available data, we do not consider it advisable to establish a new genus in this case and therefore follow the recommendation of Vellinga et al. (2015).
Finally, one question remains to be answered regarding the ecology of the two fungal species discussed here. Both are obviously associated with the mosses Ceratodon purpureus and/or Funaria hygrometrica. The mosses, for their part, prefer to grow on older burnt areas, but can also thrive outside former forest fire sites (fire-disturbed habitats). However, it has never been observed that the fungi were associated with the mosses when were no such sites. This phenomenon is remarkable and should be studied in more detail in the future.
Data Availability
Alignements available at figshare https://doi.org/10.6084/m9.figshare.24763770 and https://doi.org/10.6084/m9.figshare.25249969.
Notes
Note: later proposed by Vizzini (2010) to be placed in the Rickenellaceae.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Bas C, Kuyper TW, Noordeloos ME, Vellinga EC (1995) Flora agaricina neerlandica - Tricholomataceae (2), vol 3. A. A. Balkema Rotterdam
Binder M, Larsson K-H, Matheny PB, Hibbett DS (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102(4):865–880. https://doi.org/10.3852/09-288
Butin H, Kappich I (1980) Untersuchungen zur Neubesiedlung von verbrannten Waldböden durch Pilze und Moose. Forstwiss. Cent.bl. 99(1):283–296. https://doi.org/10.1007/BF02770950
Corner EJH (1950) A monograph of Clavaria and allied genera, vol 1. Annals of botany memoirs. Oxford University Press, London
Dämmrich F, Lotz-Winter H, Schmidt M, Scholler M, Schurig B, Winterhoff W, Gminder A, Hardtke H, Hirsch G, Karasch P, Lüderitz M, Schmidt-Stohn G, Siepe K, Täglich U, Wöldecke K (2016) Rote Liste der Großpilze und vorläufige Gesamtartenliste der Ständer- und Schlauchpilze (Basidiomycota und Ascomycota) Deutschlands mit Ausnahme der Flechten und der phytoparasitischen Kleinpilze., vol 8. Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Pilze (Teil 1) – Großpilze. Naturschutz und Biologische Vielfalt vol 70. Landwirtschaftsverlag Münster, Münster, Germany
Dentinger BTM, McLaughlin DJ (2006) Reconstructing the Clavariaceae using nuclear large subunit rDNA sequences and a new genus segregated from Clavaria. Mycologia 98(5):746–762. https://doi.org/10.1080/15572536.2006.11832646
Diederich P, Millanes AM, Wedin M, Lawrey JD (2022) Flora of lichenicolous fungi, vol 1. National Museum of Natural History, Luxembourg
Dix NJ, Webster J (1995) Fungal Ecol Springer. Dordrecht. https://doi.org/10.1007/978-94-011-0693-1
El-Abyad MSH, Webster J (1968a) Studies on pyrophilous discomycetes: I. Comparative physiological studies. Trans Br Mycol Soc 51(3):353–367. https://doi.org/10.1016/S0007-1536(68)80002-6
El-Abyad MSH, Webster J (1968b) Studies on pyrophilous discomycetes: II. Competition Trans Br Mycol Soc 51(3):369–375. https://doi.org/10.1016/S0007-1536(68)80003-8
Esposito A, Mazzoleni S, Strumia S (1999) Post-fire bryophyte dynamics in mediterranean vegetation. J Veg Sci 10(2):261–268. https://doi.org/10.2307/3237147
Esteve-Raventós F, Alvarado P, Reyes JD, Manjón JL (2011) Nuevos datos sobre la identidad de Pleurotus dryinus var. luteosaturatus (Agaricales) sobre la base de estudios morfológicos y moleculares. Bol Soc Micol Madrid 35:77–83
Filialuna O, Cripps C (2021) Evidence that pyrophilous fungi aggregate soil after forest fire. For Ecol Manag 498:119579. https://doi.org/10.1016/j.foreco.2021.119579
Gao C, Montoya L, Xu L, Madera M, Hollingsworth J, Purdom E, Singan V, Vogel J, Hutmacher RB, Dahlberg JA, Coleman-Derr D, Lemaux PG, Taylor JW (2020) Fungal community assembly in drought-stressed sorghum shows stochasticity, selection, and universal ecological dynamics. Nat Commun 11(1):34. https://doi.org/10.1038/s41467-019-13913-9
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Geml J, Timling I, Robinson CH, Lennon N, Nusbaum HC, Brochmann C, Noordeloos ME, Taylor DL (2012) An arctic community of symbiotic fungi assembled by long-distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J Biogeogr 39(1):74–88. https://doi.org/10.1111/j.1365-2699.2011.02588.x
Hahn PG, Bullington L, Larkin B, LaFlamme K, Maron JL, Lekberg Y (2018) Effects of short- and long-term variation in resource conditions on soil fungal communities and plant responses to soil biota. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01605
Halbwachs H, Easton GL, Bol R, Hobbie EA, Garnett MH, Peršoh D, Dixon L, Ostle N, Karasch P, Griffith GW (2018) Isotopic evidence of biotrophy and unusual nitrogen nutrition in soil-dwelling Hygrophoraceae. Environ Microbiol 20(10):3573–3588. https://doi.org/10.1111/1462-2920.14327
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinform 17(8):754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Hughes KW, Case A, Matheny PB, Kivlin S, Petersen RH, Miller AN, Iturriaga T (2020) Secret lifestyles of pyrophilous fungi in the genus Sphaerosporella. Am J Bot 107(6):876–885. https://doi.org/10.1002/ajb2.1482
Jülich W (1984) Die Nichtblätterpilze, Gallertpilze und Bauchpilze, vol IIb/1. Kleine Kryptogamenflora, vol IIb/1. Gustav Fischer Verlag, Stuttgart, New York
Kajan E (1986) Clavaria tenuipes ss. str. und Clavaria krieglsteineri nom. nov. APN Mitteilungsblatt Niederrhein 4(2):96–104
Kajan E, Grauwinkel B (1987) Neues über Clavaria tenuipes ss. restr. und Clavaria krieglsteineri nov. spec. Beiträge Zur Kenntnis Der Pilze Mitteleuropas 3:355–358
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Knudsen H, Vesterholt J (2012) Funga nordica - agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera, vol 2. Nordsvamp, Copenhagen, Denmark
Knudsen H, Shiryaev AG, Kautmanová I (2012) Clavaria L.: Fr. In: Knudsen H, Vesterholt J (eds) Funga nordica, agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera, vol 1. 2 edn. Nordsvamp, Copenhagen, pp 244–246
Korotkin HB, Swenie RA, Miettinen O, Budke JM, Chen K-H, Lutzoni F, Smith ME, Matheny PB (2018) Stable isotope analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. Am J Bot 105(11):1869–1887. https://doi.org/10.1002/ajb2.1183
Kouki J, Salo K (2020) Forest disturbances affect functional groups of macrofungi in young successional forests – harvests and fire lead to different fungal assemblages. For Ecol Manag 463:118039. https://doi.org/10.1016/j.foreco.2020.118039
Larsson K-H (2007) Re-thinking the classification of corticioid fungi. Mycol Res 111(9):1040–1063. https://doi.org/10.1016/j.mycres.2007.08.001
Larsson K-H, Parmasto E, Fischer M, Langer E, Nakasone KK, Redhead SA (2006) Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98(6):926–936. https://doi.org/10.1080/15572536.2006.11832622
Latha KPD, Raj KNA, Paramban R, Manimohan P (2015) Two new bryophilous agarics from India. Mycoscience 56(1):75–80. https://doi.org/10.1016/j.myc.2014.03.004
Lawrey JD, Lücking R, Sipman HJM, Chaves JL, Redhead SA, Bungartz F, Sikaroodi M, Gillevet PM (2009) High concentration of basidiolichens in a single family of agaricoid mushrooms (Basidiomycota: Agaricales: Hygrophoraceae). Myc Res 113(10):1154–1171. https://doi.org/10.1016/j.mycres.2009.07.016
Liu D, Goffinet B, Wang X-Y, Hur J-S, Shi H-X, Zhang Y-Y, Yang M-X, Li L-J, Yin A-C, Wang L-S (2018) Another lineage of basidiolichen in China, the genera Dictyonema and Lichenomphalia (Agaricales: Hygrophoraceae). Mycosystema 37(7):849–864. https://doi.org/10.13346/j.mycosystema.170246
Lodge DJ, Padamsee M, Matheny PB, Aime MC, Cantrell SA, Boertmann D, Kovalenko A, Vizzini A, Dentinger BTM, Kirk PM, Ainsworth AM, Moncalvo J-M, Vilgalys R, Larsson E, Lücking R, Griffith GW, Smith ME, Norvell LL, Desjardin DE, Redhead SA, Ovrebo CL, Lickey EB, Ercole E, Hughes KW, Courtecuisse R, Young A, Binder M, Minnis AM, Lindner DL, Ortiz-Santana B, Haight J, Læssøe T, Baroni TJ, Geml J, Hattori T (2014) Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Div 64(1):1–99. https://doi.org/10.1007/s13225-013-0259-0
Lücking R, Thorn RG, Saar I, Piercey-Normore MD, Moncada B, Doering J, Mann H, Lebeuf R, Voitk M, Voitk A (2017) A hidden basidiolichen rediscovered: Omphalina oreades is a separate species in the genus Lichenomphalia (Basidiomycota: Agaricales: Hygrophoraceae). Lichenologist 49(5):467–481. https://doi.org/10.1017/S0024282917000378
Ludwig E (2001) Pilzkompendium - Beschreibungen, vol 1. IHW-Verlag, Eching
Lutzoni FM (1997) Phylogeny of lichen- and non-lichen-forming omphalinoid mushrooms and the utility of testing for combinability among multiple data sets. Syst Biol 46(3):373–406. https://doi.org/10.1093/sysbio/46.3.373
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung G-H, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91(10):1446–1480. https://doi.org/10.3732/ajb.91.10.1446
Marčiulynienė D, Marčiulynas A, Lynikienė J, Vaičiukynė M, Gedminas A, Menkis A (2021) DNA-metabarcoding of belowground fungal communities in bare-root forest nurseries: focus on different tree species. Microorganisms 9(1). https://doi.org/10.3390/microorganisms9010150
Marthinsen G, Rui S, Timdal E (2019) OLICH: a reference library of DNA barcodes for Nordic lichens. Biodivers Data J 7:e36252
Masumoto H, Degawa Y (2020) Multiclavula petricola sp. nov. (Cantharellales, Basidiomycota), a new clavarioid and lichenized fungus growing on rocks. Mycoscience 61(4):155–159. https://doi.org/10.1016/j.myc.2020.03.004
Matheny PB, Wang Z, Binder M, Curtis JM, Lim YW, Nilsson RH, Hughes KW, Hofstetter V, Ammirati JF, Schoch CL, Langer E, Langer G, McLaughlin DJ, Wilson AW, Frøslev T, Ge ZW, Kerrigan RW, Slot JC, Yang ZL, Baroni TJ, Fischer M, Hosaka K, Matsuura K, Seidl MT, Vauras J, Hibbett DS (2007) Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol Phylogenet Evol 43(2):430–451. https://doi.org/10.1016/j.ympev.2006.08.024
Matheny PB, Swenie RA, Miller AN, Petersen RH, Hughes KW (2018) Revision of pyrophilous taxa of Pholiota described from North America reveals four species—P. brunnescens, P. castanea, P. highlandensis, and P. molesta. Mycologia 110(6):997–1016. https://doi.org/10.1080/00275514.2018.1516960
Moncalvo J-M, Vilgalys R, Redhead SA, Johnson JE, James TY, Catherine Aime M, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Greg Thorn R, Jacobsson S, Clémençon H, Miller OK (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23(3):357–400. https://doi.org/10.1016/S1055-7903(02)00027-1
Moser M (1949) Über das Massenauftreten von Formen der Gattung Morchella auf Waldbrandflächen. Sydowia 3:174–195
Moser M (1983) Die Röhrlinge und Blätterpilze (Polyporales, Boletales, Agaricales, Russulales). Band IIb/2 - Basidiomyceten - 2. Teil, 5th edn. VEB Gustav Fischer Verlag Jena
Nagy J, Németh C, Dima B, Papp V (2020) Lichenomphalia meridionalis, an agaricoid basidiolichen species new to Central Europe. Herzogia 33(1):25–33, 29
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucl Acid Res 47(D1):D259–D264. https://doi.org/10.1093/nar/gky1022
Olariaga I, Huhtinen S, Læssøe T, Petersen JH, Hansen K (2020) Phylogenetic origins and family classification of typhuloid fungi, with emphasis on Ceratellopsis, Macrotyphula and Typhula (Basidiomycota). Stud Mycol 96:155–184. https://doi.org/10.1016/j.simyco.2020.05.003
Parnmen S, Nooron N, Leudang S, Sikaphan S, Polputpisatkul D, Rangsiruji A (2019) Phylogenetic evidence revealed Cantharocybe virosa (Agaricales, Hygrophoraceae) as a new clinical record for gastrointestinal mushroom poisoning in Thailand. Toxicol Res 36(3):239–248. https://doi.org/10.1007/s43188-019-00024-2
Pilát A (1958) Übersicht der europäischen Clavariaceen unter besonderer Berücksichtigung der tschechoslowakischen Arten, vol XIV. Acta Mus Nat Pragae Ser B Hist Nat 14(3–4):129–255
Pulido-Chavez MF, Alvarado EC, DeLuca TH, Edmonds RL, Glassman SI (2021) High-severity wildfire reduces richness and alters composition of ectomycorrhizal fungi in low-severity adapted ponderosa pine forests. For Ecol Manag 485:118923. https://doi.org/10.1016/j.foreco.2021.118923
Raudabaugh DB, Matheny PB, Hughes KW, Iturriaga T, Sargent M, Miller AN (2020) Where are they hiding? Testing the body snatchers hypothesis in pyrophilous fungi. Fungal Ecol 43:100870. https://doi.org/10.1016/j.funeco.2019.100870
Raudabaugh DB, Wells DG, Matheny PB, Hughes KW, Sargent M, Iturriaga T, Miller AN (2021) In vitro observations of the interactions between Pholiota carbonaria and Polytrichum commune and its potential environmental relevance. Life 11(6):518
Rea C (1922) British Basidiomycetae: a handbook to the larger British fungi. Univesity press, Cambridge
Redhead S, Moncalvo J-M, Vilgalys R, Lutzoni F (2002a) Phylogeny of agarics: partial systematics solutions for bryophilous omphalinoid agarics outside of the Agaricales. Mycotaxon 82:151–168
Redhead SA, Lutzoni F, Moncalvo JM, Vilgalys R (2002b) Phylogeny of agarics: partial systematics solutions for core omphalinoid genera in the Agaricales (Euagarics). Mycotaxon 83:19–57
Schild E (1981) Was ist Clavaria tenuipes Berk. & Broome? Zeitschrift für Mykologie Z Mykol 47(2):215–219
Sievers F, Higgins DG (2021) The clustal omega multiple alignment package. In: Katoh K (ed) Multiple sequence alignment: methods and protocols. Springer US, New York, pp 3–16. https://doi.org/10.1007/978-1-0716-1036-7_1
Sim-Sim M, Carvalho P, Sérgio C, Garcia C, Rego F (2004) Recolonisation and changes in bryophyte and lichen biodiversity in burned areas from the Serra da Estrela (Portugal). Cryptogam Bryol 25(4):367–384
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinform 30(9):1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Varga T, Krizsán K, Földi C, Dima B, Sánchez-García M, Sánchez-Ramírez S, Szöllősi GJ, Szarkándi JG, Papp V, Albert L, Andreopoulos W, Angelini C, Antonín V, Barry KW, Bougher NL, Buchanan P, Buyck B, Bense V, Catcheside P, Chovatia M, Cooper J, Dämon W, Desjardin D, Finy P, Geml J, Haridas S, Hughes K, Justo A, Karasiński D, Kautmanova I, Kiss B, Kocsubé S, Kotiranta H, LaButti KM, Lechner BE, Liimatainen K, Lipzen A, Lukács Z, Mihaltcheva S, Morgado LN, Niskanen T, Noordeloos ME, Ohm RA, Ortiz-Santana B, Ovrebo C, Rácz N, Riley R, Savchenko A, Shiryaev A, Soop K, Spirin V, Szebenyi C, Tomšovský M, Tulloss RE, Uehling J, Grigoriev IV, Vágvölgyi C, Papp T, Martin FM, Miettinen O, Hibbett DS, Nagy LG (2019) Megaphylogeny resolves global patterns of mushroom evolution. Nat Ecol Evol 3(4):668–678. https://doi.org/10.1038/s41559-019-0834-1
Vellinga EC, Kuyper TW, Ammirati J, Desjardin DE, Halling RE, Justo A, Læssøe T, Lebel T, Lodge DJ, Matheny PB, Methven AS, Moreau P-A, Mueller GM, Noordeloos ME, Nuytinck J, Ovrebo CL, Verbeken A (2015) Six simple guidelines for introducing new genera of fungi. IMA Fungus 6(2):A65–A68. https://doi.org/10.1007/BF03449356
Vesterholt J (2012) Arrhenia Fr. In:Knudsen H, Vesterholt J (eds) Funga Nordica, agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera, vol 1. 2 edn. Nordsvamp, Copenhagen, pp 252–260
Viner I, Spirin V, Larsson K-H, Miettinen O (2023) Systematic placement of Lagarobasidium cymosum and description of two new species. Mycologia 115(1):122–134. https://doi.org/10.1080/00275514.2022.2146978
Vizzini A (2010) Segnalazioni di Muscinupta laevis (Basidiomycota, Agaricomycetes) per il Nord Italia. Micol Veg Medit 25:141–148
Vizzini A, Consiglio G, Setti L, Ercole E (2012) The phylogenetic position of Haasiella (Basidiomycota, Agaricomycetes) and the relationships between H. venustissima and H. splendidissima. Mycologia 104(3):777–784. https://doi.org/10.3852/11-334
Voitk A, Saar I, Lücking R, Moreau P-A, Corriol G, Krisai-Greilhuber I, Thorn RG, Hay CRJ, Moncada B, Gulden G (2020) Surprising morphological, ecological and ITS sequence diversity in the Arrhenia acerosa complex (Basidiomycota: Agaricales: Hygrophoraceae). Sydowia 73:133–162. https://doi.org/10.12905/0380.sydowia73-2020-0133
Voitk A, Saar I, Moncada B, Lickey EB (2022) Circumscription and typification of sphagnicolous omphalinoid species of Arrhenia (Hygrophoraceae) in Newfoundland and Labrador: three obligate and one facultative species. Mycol Prog 21(6):57. https://doi.org/10.1007/s11557-022-01806-z
Voyron S, Ercole E, Ghignone S, Perotto S, Girlanda M (2017) Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytol 213(3):1428–1439. https://doi.org/10.1111/nph.14286
Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2018.05.001
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, (Eds) PCR Protocols: A Guide to Methods and Applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Wu S-H, Nilsson HR, Chen C-T, Yu S-Y, Hallenberg N (2010) The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of the Polyporales (Basidiomycota). Fungal Div 42(1):107–118. https://doi.org/10.1007/s13225-010-0031-7
Wu S-H, Wei C-L, Chen Y-P, Chen C-C, Chen S-Z (2021) Schizocorticium gen. nov. (Hymenochaetales, Basidiomycota) with three new species. Mycol Prog 20(6):769–779. https://doi.org/10.1007/s11557-021-01670-3
Zhang M, Li T-H, Chen F (2018) Rickenella danxiashanensis, a new bryophilous agaric from China. Phytotaxa 350(3):283–290. https://doi.org/10.11646/phytotaxa.350.3.7
Zhang T, Zhu X, Vizzini A, Li B, Cao Z, Guo W, Qi S, Wei X, Zhao R (2022) New insights into lichenization in Agaricomycetes based on an unusual new basidiolichen species of Omphalina s. str. J Fung 8 (10). https://doi.org/10.3390/jof8101033
Zoller S, Lutzoni F (2003) Slow algae, fast fungi: exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Mol Phylogenetics Evol 29(3):629–640. https://doi.org/10.1016/S1055-7903(03)00215-X
Acknowledgements
We would like to thank Dr. Annika Busse, Dr. Torsten Bittner, and Stefan Müller for their support on site and permission to investigate the burnt areas in the Saxon Switzerland National Park and the Gohrischheide nature reserve. Furthermore, we thank the Stiftung Naturlandschaften Brandenburg and the Badenitzer Forstgemeinschaft for their permission to investigate the areas close to Jüterbog and Treuenbrietzen. Especially the volunteers, as mention above, are warmly thanked for their help with the field work and Madeleine Schäfer and Ian Volkmann for their lab assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work of R.J. and D.D. is funded by the Fachagentur Nachwachsende Rohstoffe (FNR, grant number: 2219WK50E4) within the framework of the PYROPHOB project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alexander Karich, René Ullrich, René Jarling, and Daniela Demski. The first draft of the manuscript was written by Alexander Karich, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Zhu-Liang Yang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karich, A., Jarling, R., Ullrich, R. et al. Two new Agaricomycetes related to post-fire mosses. Mycol Progress 23, 28 (2024). https://doi.org/10.1007/s11557-024-01965-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01965-1