Abstract
The genus Ceratocystiopsis (Ophiostomatales, Ascomycota) includes 21 species, which can be found mainly in association with bark beetles in the Northern Hemisphere. A survey of Ceratocystiopsis species associated with bark beetles infesting Picea abies and Pinus sylvestris in Norway yielded 126 isolates, representing Ceratocystiopsis neglecta and Ceratocystiopsis rollhanseniana, and four species described herein as Ceratocystiopsis chalcographii, Ceratocystiopsis debeeria, Ceratocystiopsis norroenii and Ceratocystiopsis troendelagii. The new taxa were morphologically characterised and phylogenetically analysed on the basis of sequence data of multiple loci (ITS, LSU, beta-tubulin (TUB2), calmodulin (CAL) and translation elongation factor 1-alpha (TEF1) genes). Ceratocystiopsis norroenii and C. rollhanseniana were the most frequently isolated species, and the latter species had the wider vector range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ceratocystiopsis (Upadhyay and Kendrick (1975) (Ascomycota, Ophiostomatales) is based on Ophiostoma minutum Siemaszko (1939), which was described based on perithecia found on the wood of Picea abies infested with Ips typographus in the Białowieża Forest, Poland (Siemaszko 1939). Conidial states were not described.
Ceratocystiopsis taxonomy has a turbulent history, and species of ceratocystiopsis-like fungi have been accommodated in various genera including Ceratocystiopsis (Upadhyay 1981; Marmolejo and Butin 1990; Hsiau and Harrington 1997; Zipfel et al. 2006; Strzałka et al. 2020; Nel et al. 2021), Ceratocystis (Griffin 1968; Olchowecki and Reid 1974), Cornuvesica (Viljoen et al. 2000) and Ophiostoma (Siemaszko 1939; Hausner et al. 1993, 2003; Kirschner and Oberwinkler 1999). Important historical developments in the taxonomy of Ceratocystiopsis included erecting Ceratocystiopsis for species that have short-necked perithecia and falcate-shaped ascospores with gelatinous sheaths and a Hyalorhinocladiella anamorph (Upadhyay and Kendrick 1975); and reducing the genus to synonymy with Ophiostoma, based on partial SSU and LSU rDNA sequences by Hausner et al. in 1993. Ceratocystiopsis was later on re-instated based on morphological characters and the analysis of combined partial nuclear LSU and β-tubulin gene sequences (Zipfel et al. 2006). Currently, Ceratocystiopsis is one of nine more or less clearly defined genera in the Ophiostomataceae (De Beer and Wingfield 2013; De Beer et al. 2013a, b, 2016; Hyde et al. 2020). It is also worth mentioning that Ceratocystiopsis may also produce cucullate ascospores (De Beer and Wingfield 2013) and occasionally sporothrix-like asexual morphs (Bridges and Perry 1987; Marmolejo and Butin 1990).
As currently circumscribed, Ceratocystiopsis accommodates 21 species (De Beer et al. 2013b; Strzałka et al. 2020; Nel et al. 2021; Chang et al. 2021; Wang et al. 2021), that have a wide geographic distribution including records from Africa (Nel et al. 2021), Asia (e.g. Yamaoka et al. 1997; Wang et al. 2020; Chang et al. 2021; Wang et al. 2021), Europe (e.g. Hausner et al. 1993; Kirisits 2004, Jankowiak 2005; Linnakoski et al. 2012) and North America (Olchowecki and Reid 1974; Bridges and Perry 1987; Marmolejo and Butin 1990; Hsiau and Harrington 1997; Hausner et al. 2003). However, they are mostly known and abundant in the Northern Hemisphere conifer ecosystems of Europe and North America.
Ceratocystiopsis minuta is the most frequently reported and widespread species. However, Plattner et al. (2009), based on molecular phylogenetic analyses, showed that European and Asian strains of C. minuta clustered in at least three well defined, closely related lineages (C, D, and E), whereas ‘C. minuta-like’ strains from North America were unrelated. Hence, C. minuta (clades C, D and E sensu Plattner et al. 2009) is a species complex that probably includes several cryptic yet undescribed species. No type material of C. minuta was originally designated (Siemaszko 1939). Reid and Hausner (2010) proposed an epitypification based on UAMH 11218 (= a dried culture of UM1532 = WIN(M)1532), originally isolated from P. abies infested by I. typographus in the Biebrzański National Park (Poland). This strain belongs to clade D of Plattner et al. (2009) that included also five other strains from Poland and one from Japan. The Polish strains were collected from P. abies infested by I. typographus while the Japanese strain was obtained from an adult beetle of I. typographus japonicus on Picea jezoensis. The two Polish strains of C. minuta in clade E had the same origin as Polish strains in clade D. In turn, clade C contained eight strains that originated from Austria, Scotland and Japan and these were found in association with different Ips species on Larix spp. and Picea spp. (Plattner et al. 2009).
Ceratocystiopsis species occupy a relatively narrow range of habitats. Most species have been recorded on conifers in association with bark and wood boring beetles (e.g. Olchowecki and Reid 1974; Hsiau and Harrington 1997; Yamaoka et al. 1997; Kirisits 2004; Linnakoski et al. 2012; Nel et al. 2021; Wang et al. 2021). Other species have been described from hardwood trees (Olchowecki and Reid 1974; Li et al. 2018, Strzałka et al. 2020).
Very little is known about the distribution of Ceratocystiopsis species in Norway. To date, only C. minuta and C. rollhanseniana have been reported from Norway either, from P. abies infested by I. typographus (Solheim 1986, 1992, 1993) or galleries created by unknown bark beetle species on Pinus sylvestris (Hausner et al. 2003).
The aim of this study was to characterize the morphology, phylogenetic affinities and taxonomy of Ceratocystiopsis isolates found in Norway during a survey of bark beetles and their galleries on conifers from October 2014 to September 2015.
Materials and methods
Fungal isolates
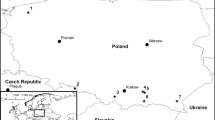
Twenty-two species of bark beetles were collected from several locations in Norway between October 2014 and September 2015 (Table 1, Fig. 1). The species list of beetles collected from all sampling sites is shown in Table 1. The bark beetles were collected by hand from Picea abies and Pinus sylvestris. Bark beetle adults (alive) were excised from the bark and galleries with sterilised tweezers and stored individually in sterile 1.5 ml microcentrifuge tubes for subsequent fungal isolations. Each beetle was removed from its storage microtube with sterilised tweezers and was morphologically identified with taxonomical keys (Lekander et al. 1977; Ehnström and Axelsson 2002). Later, each bark beetle was divided into three parts, elytra, head and the rest, before placing the parts in three separate Petri dishes on malt extract agar [MEA; 6.25 g malt Bacto™ agar powder (Beckton, Dickinson, Sparks, USA), 10 g agar (Bacto™ agar powder from VWR International, Leuven, Belgium), 0.5 L deionized water] without antibiotics. Emerging cultures were purified by transferring small pieces of mycelium or spore masses from individual colonies to fresh MEA. Fungi were incubated at 20 °C, then subcultured onto 2% MEA (20 g Bacto™ malt extract, 20 g agar Bacto™ agar powder (Becton Dickinson and Company, Franklin Lakes, USA) in 1 L deionized water) and stored at 4 °C. After 2 weeks of incubation at 20 °C, the purified fungal cultures were grouped according to their morphological characteristics. Depending on the number of isolates belonging to the same morphotype, 2–19 isolates per morphotype were chosen for molecular identification (Table 2). The frequency of occurrence was calculated according to the following formula: F = (NS/NTs) × 100, where F is the frequency of occurrence (%) of the fungus, NS is the number of beetles from which a particular fungus was isolated and NTs is the total number of beetles.
The cultures are maintained in the culture collection of the Norwegian Institute of Bioeconomy (NIBIO), Norway. Ex-type isolates of new species described in this study were deposited in the collection of the Westerdijk Fungal Biodiversity Institute (CBS), Utrecht, The Netherlands, and in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa. Type specimens were deposited in the Mycological Herbarium (O), Natural History Museum, University of Oslo, Norway. The ex-type strains of C. neglecta and C. rollhanseniana were sourced from the culture collection of the Westerdijk Fungal Biodiversity Institute (CBS), and from the culture collection of the University of Manitoba (WIN) in Canada (Table 2). Taxonomic descriptions and nomenclatural data were registered in MycoBank (www.MycoBank.org) (Robert et al. 2013).
Microscopy and growth studies
Isolates selected for molecular analyses and as herbarium specimens were morphologically characterised. Isolates were grown on 2% MEA. In attempts to induce the formation of ascomata, autoclaved twigs of host trees including bark were placed at the centres of agar plates containing 2% MEA. Fungal cultures were derived from single conidia. To promote the production of ascomata, all isolates of each taxon were crossed among themselves in all possible combinations, following the technique described by Grobbelaar et al. (2010). These cultures were incubated at 25 °C and monitored regularly for the appearance of fruiting structures.
Morphological features were examined by mounting fungal tissue in 80% lactic acid on glass slides and observed using a Nikon Eclipse 50i microscope (Nikon® Corporation, Tokyo, Japan) with an Invenio 5S digital camera (DeltaPix®, Maalov, Denmark) to capture photographic images. Colour designations were based on the colour charts of Kornerup and Wanscher (1978).
For each taxonomically relevant structure, fifty measurements were made, when possible, using the Coolview 1.6.0 software (Precoptic®, Warsaw, Poland). Averages, ranges and standard deviations were calculated for the measurements, and these are presented in the format ‘(min–)(mean–SD)–(mean + SD)(–max)’.
Growth characteristics for the novel species were determined by analysing the radial growth for two isolates (two for each species). Agar disks (5 mm diam.) were cut from the actively growing margins of fungal colonies and these disks were placed at the centres of plates containing 2% MEA. Four replicate plates for each of the six putative new species were incubated at temperatures between 5 and 35 °C at 5 °C intervals. The radial growth (two measurements perpendicular to each other per plate) was determined 14 days after inoculation, and growth rates were calculated as mm/day.
PCR, sequencing and phylogenetic analyses
Morphotypes were initially identified by sequencing the internal transcribed spacer 1 and 2 (ITS), 28S ribosomal large subunit (LSU) or partial β-tubulin (TUB2) gene region of representative isolates (Table 2). DNA extractions were performed as described by Aas et al. (2018). For more detailed analysis, two additional loci were also amplified and sequenced and subjected to phylogenetic analyses: partial calmodulin (CAL) and partial translation elongation factor 1-alpha (TEF1) sequences. The primers used for PCR and sequencing of the various loci were as follows: ITS1-F (Gardes and Bruns 1993) and ITS4 (White et al. 1990) for the ITS region; LR0R and LR5 (Vilgalys R; Vilgalys and Hester 1990) for the LSU region; T10 (O’Donnell and Cigelnik 1997) or Bt2a together with Bt2b (Glass and Donaldson 1995) for TUB2; F-728F (Carbone and Kohn 1999) and EF2 (O’Donnell et al. 1998) were used for TEF1; CL1 and CL2a (O'Donnell et al. 2000) or CL3F and CL3R (De Beer et al. 2016) were used for CAL. Protocols for PCR amplification and DNA sequencing were as described previously (Aas et al. 2018).
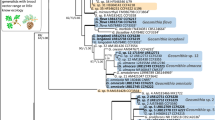
All phylogenetic analyses were performed independently for each locus (Figs. 2, 3, 4, 5 and 6). Resulting trees were visually compared for topological incongruence. Loci showing no topological incongruences (i.e. ITS+LSU+TUB2) were combined and analysed as a concatenated construct (Fig. 7).
Phylogram obtained from Maximum Likelihood (ML) analyses of the ITS data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and Ophiostoma quercus represent the outgroup
Phylogram obtained from maximum likelihood (ML) analyses of the LSU data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and O. quercus represents the outgroup in analyses of LSU data
Phylogram obtained from Maximum Likelihood (ML) analyses of TUB2 data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and O. quercus represents the outgroup in analyses of the TUB2 data
Phylogram obtained from Maximum Likelihood (ML) analyses of TEF1 data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and O. quercus represents the outgroup in analyses of the TEF1 data
Phylogram obtained from Maximum Likelihood (ML) analyses of CAL data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and O. quercus represents the outgroup in analyses of the CAL data
Phylogram obtained from Maximum Likelihood (ML) analyses of the combined datasets of ITS+LSU+TUB2 data for the Ceratocystiopsis spp. Sequences obtained during this study are presented in bold type. Bootstrap values ≥ 75% for ML and Maximum Parsimony (MP) analyses are presented at nodes as follows: ML/MP. Bold branches indicate posterior probabilities values ≥ 0.95 obtained from Bayesian Inference (BI) analyses. *Bootstrap values < 75%. The tree is drawn to scale (see bar) with branch length measured in the number of substitutions per site. Ophiostoma karelicum and O. quercus represents the outgroup in analyses of the combined datasets of ITS+LSU+TUB2
For phylogenetic analyses, sequence alignments were performed using the online version of MAFFT v7 (Katoh and Standley 2013). The ITS, LSU, TUB2, CAL and TEF1 datasets were aligned using the E-INS-i strategy with a 200PAM/κ=2 scoring matrix, a gap opening penalty of 1.53 and an offset value of 0.00. The alignments were checked manually with BioEdit v.2.7.5 (Hall 1999). The resulting alignments and trees were deposited into TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S28950).
Phylogenetic trees were inferred for each of the datasets using three different methods: maximum likelihood (ML), maximum parsimony (MP) and Bayesian inference (BI). For ML and BI analyses, the best-fit substitution models for each aligned dataset were established using the corrected Akaike information criterion (AICc) in jModelTest 2.1.10 (Guindon and Gascuel 2003; Darriba et al. 2012). ML analyses were carried out with PhyML 3.0 (Guindon et al. 2010), utilizing the Montpelier online server (http://www.atgc-montpellier.fr/phyml/). The ML analysis was combined with bootstrapping (1000 bootstrap pseudoreplicates) to assess node support values and the overall reliability of the tree topology. The best evolutionary substitution models were as follows: GTR+I+G for ITS (-lnL = 4817.94), LSU (-lnL = 1452.82) and CAL (-lnL = 3350.13); GTR+G for TUB2 (-lnL = 4368.71) and ITS+LSU+TUB2 (-lnL = 9793.62); and HKY+I+G for TEF1 (-lnL = 5374.80).
MP analyses were performed using PAUP* 4.0b10 (Swofford 2003). Gaps were treated as a fifth state. Bootstrap analysis (1000 bootstrap replicates) was conducted to determine the levels of confidence for the nodes within the inferred tree topologies. Tree bisection and reconnection (TBR) was selected as the branch swapping option. The tree length (TL), Consistency Index (CI), Retention Index (RI), Homoplasy Index (HI) and Rescaled Consistency Index (RC) were recorded for each analysed dataset after the trees were generated.
BI analyses using Markov Chain Monte Carlo (MCMC) methods were carried out with MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003). Four MCMC chains were run in parallel for 10 million generations applying the best-fit model for each dataset. Trees were sampled every 100th generations, resulting in 100,000 trees. Tracer v1.4.1 (Rambaut and Drummond 2007) was utilized to determine the burn-in value for each dataset. The remaining trees were utilized to generate a 50% majority rule consensus tree, which allowed calculating posterior probability values for nodes.
Results
Collections of bark beetles and fungi
Altogether, 459 beetles, representing 22 species, were collected from Norway during this study (Table 1). Among the bark beetles carrying Ceratocystiopsis species, only Pityogenes chalcographus occurred on both pine and spruce. Five bark beetle species with Ceratocystiopsis species were found on pine, four on spruce. The isolations from bark beetles yielded a total of 126 isolates tentatively identified as Ceratocystiopsis spp. (Table 3). Fifty isolates were collected from Ips acuminantus, 21 isolates from Orthtomicus proximus, 15 isolates from P. chalcographus and 12 isolates from Crypturgus cinereus. The additional twenty-eight isolates were collected from C. pusillus, Hylurgops palliatus, Pityogenes bidentatus, P. saalasi, P. quadridens and Polygraphus subopacus (Table 3). No Ceratocystiopsis species were detected in samples from Cryphalus saltuarius, Dryocoetes autographus, D. hectographus, Hylastes brunneus, H. cunicularis, Ips sexdentatus, Orthotomicus laricis, O. suturalis, Pityophthorus micrographus, Polygraphus poligraphus, Tomicus piniperda and Trypodendrum lineatum (Table 1).
Based on preliminary morphological observations, the isolates could be arranged into six taxa. The frequencies of occurrence of Ceratocystiopsis spp. varied between ten bark beetle species (Table 3). Taxon 6, represented by 55 isolates, was isolated from six different bark beetle species with frequencies of occurrence ranging from 11 to 72% from the respective beetle species. It was the dominant species associated with P. bidentatus (72% of specimens carried the fungus) and O. proximus (70%), and taxon 6 also occurred at a relatively high frequency on C. cineraeus (40%). Fifty isolates belonged to taxon 2. This taxon was found only in high frequency on I. acuminatus (54%). The remaining 16 isolates represented four Ceratocystiopsis taxa and were collected from four beetle species at a frequency ≤ 11% (Table 3).
Phylogenetic analyses
Alignments for the ITS data set contained 738 characters; for the LSU data 496 characters; for the CAL data 613 characters; for the TEF1 data 785 characters; for the TUB2 data 526 characters for the ITS+LSU+TUB2 data 1839 characters (including gaps). The exon/intron arrangement of the CAL data included intron 3, exon 4, intron 4, exon 5, intron 5, exon 6 and intron 6. The exon/intron arrangement of the TEF1 data included intron 3, exon 4, intron 4 and exon 5. The aligned TUB2 gene region consisted of exons 3 to 6, interrupted by introns 3, 4 and 5.
DNA sequence data were obtained for 45 isolates representing all six identified morphological groups (Table 2). BLAST analyses of the ribosomal DNA sequences confirmed their affinities with Ceratocystiopsis. Sequences from our 45 representative isolates where combined with those of other members of Ceratocystiopsis. They formed six independent, well-supported terminal clades representing six phylogenetic species (Taxa 1–6) in the ITS, LSU, TUB2, CAL and TEF1 phylogenetic inferences (Figs. 2, 3, 4, 5 and 6). Based on the phylogenetic analyses of the ITS and LSU data sets (Figs. 2 and 3), the isolates emerged as two known and four undescribed taxa.
Four isolates recovered from P. abies and P. sylvestris infested by P. chalcographus (i.e. taxon 1) in the ITS and LSU trees grouped in the ‘C. minuta’ clade C (Plattner et al. 2009). This clade C includes European and Japanese strains of Ceratocystiopsis minuta obtained from conifer-infesting bark beetles (Plattner et al. 2009). In the TUB2 tree, eight isolates of taxon 1 clustered into a distinct clade next to a Ceratocystiopsis strain (CMW4352) from Austria and Japanese isolates of Ceratocystiopsis spp. (YCC330 and YCC329) that belonged to the ‘C. minuta’ clade C according to Plattner et al. (2009) (Fig. 4). Although only poorly supported, this clade appears to share a common node with strains assigned to C. minuta s. str. (the ‘C. minuta’ clade D including UM1532) and Ceratocystiopsis weihaiensis (Fig. 4).
Nine isolates obtained from P. sylvestris infested by I. acuminatus (i.e. taxon 2) form a well-supported lineage, distinct from all the other known species of Ceratocystiopsis in the ITS, LSU, TUB2, CAL and TEF1 analyses (Figs. 2, 3, 4, 5 and 6). In the ITS tree, five isolates of taxon 2 clustered into a distinct clade next to Ceratocystiopsis strains CBS 116963 and UM 1534 from Poland that belonged to the ‘C. minuta’ clade C and E, respectively (Fig. 2). Six isolates representing taxon 3 and taxon 4 also form well-supported lineages distinct from other known species of Ceratocystiopsis (Figs. 2, 3, 4, 5 and 6). Taxon 3 appears to represent a phylogenetically distinct lineage, potentially sharing ancestry with C. neglecta (Figs. 2, 3 and 4). Members of taxon 4 form a lineage adjacent to C. minima within the LSU based phylogenetic tree (Fig. 3). However, the other datasets do not support monophyly for these two species (Figs. 2, 4 and 7).
The analyses of the combined ITS+LSU+TUB2 datasets clearly separated taxa 1 to 4 from the other known species of Ceratocystiopsis and also from each other (Fig. 7). Nineteen isolates of taxon 5 grouped with the ex-type isolate of C. rollhanseniana (UM113) and other isolates of this species based on LSU (Fig. 3). The TUB2, CAL and TEF1 data also confirmed that those isolates represented C. rollhanseniana (Figs. 4, 5 and 6). The ITS, LSU, TUB2, CAL and TEF1 datasets confirmed that four isolates of taxon 6 grouped with ex-type isolate of C. neglecta (Figs. 2, 3, 4, 5 and 6).
Taxonomy
The morphological characterization and phylogenetic comparisons based on five genetic loci, showed that four herein described morphotypes (taxon 1, 2, 3 and 4) are distinct from each other and from other known Ceratocystiopsis taxa. Therefore, they are described here as new species:
Ceratocystiopsis chalcographii Jankowiak & H. Solheim, sp. nov., Fig. 8
Morphological characteristics of Ceratocystiopsis chalcographii (CBS 147954, Taxon 1). a–b Perithecium; c Ostiolar hyphae; d Ascospores; e–f Micronematous conidiophores; g slightly branched micronematous conidiophores; h Conidia; i 14-day-old culture on 2% MEA. Scale bars: a = 25 μm; b = 50 μm; c = 25 μm; d = 10 μm; e = 25 μm; f–g = 10 μm
MycoBank: 841844
Etymology: The epithet chalcographii (Latin) refers to the species name of the bark beetle vector of this fungus, Pityogenes chalcographus.
Diagnosis: This species is closely related to C. minuta and C. norroenii. Ceratocystiopsis chalcographii has longer perithecial necks (36–124 μm vs. 20–45 μm), ostiolar hyphae (7.7–16.4 vs. to 12 μm) and ascospores (9.9–15 vs. 10–13 μm) compared to C. minuta. Ceratocystiopsis chalcographii has obovoid conidia, whereas C. minuta has oblong conidia (Reid and Hausner 2010). Ceratocystiopsis chalcographii has longer conidia (2.9–6 μm) compared to C. minuta (2–4 μm) (Reid and Hausner 2010). In addition, conidiophores of C. chalcographii are simple or slightly branched while C. minuta produces simple or verticillate to irregular branched conidiophores (Reid and Hausner 2010). Ceratocystiopsis norroenii also produces branched conidiophores; however, this taxon could be distinguished from C. minuta by oblong and larger conidia (2.7–5 × 1.1–2.3 vs. 2–4 × 1.2 μm). In addition, C. norroenii does not produce a sexual morph.
Type: Norway, Nannestad, from Pityogenes chalcographus infesting Picea abies, 15 Oct. 2014, H. Solheim and M.E. Waalberg (O-F-259424 – holotype; CBS 147954 = CMW 57280 – ex-type culture).
Description: Perithecia abundantly produced on media and sterilised spruce twigs, formed by single isolates, variable in shape, with a globose or sub-prolate black base, (46–)55–101(–125) μm diam, ending in black, straight or slightly curved necks, (36–)55–88(–124) μm long, (20.3–)23.9–32.3(39.7) μm diam at the base and (5.9–)8.2–13(–17) μm diam at the apex, ending in a tuft of 5–8 ostiolar hyphae, hyaline, aseptate, straight, tapering toward the apex, (7.7–)9.2–13(–16.4) μm long. Asci not observed. Ascospores one-celled, hyaline, including the sheath falcate in side view (9.9–)11.6–14(–15) × (1–)1.2–1.6(–1.8) μm, accumulating in white-coloured mucoid masses at tip of neck.
Asexual morph hyalorhinocladiella-like. Conidiophores mononematous, micronematous or semi-macronematous, abundantly formed, arising from vegetative hyphae, simple or seldom slightly branched, upright, straight or undulate, producing conidiogenous cells at their apices. Conidiogenous cells integrated, hyaline, proliferating percurrently, not denticulate, (1.7–)6.9–23.4(–40.4) × (0.7–)1–1.8(–2.2) μm. Conidia hyaline, aseptate, obovoid, the upper part swollen, apex rounded, tapering toward base, sometimes pyriform, (2.9–)3.6–5(–6) × (0.9–)0.9–1.2(–1.9) μm, without sheath.
Colonies with optimal growth at 30 °C on 2% MEA, with radial growth rate of 1.11 (± 0.05) mm/day. Colonies white, margin smooth, flocculose, due to abundant aerial mycelium with white lumps of slimy conidia; reverse pale orange (5A3). Hyphae hyaline to pale grey in colour, smooth, submerged in medium, not constricted at septa, 1–2.6 (mean 1.7 ± 0.4) μm diam.
Ecology: Isolated from Pityogenes chalcographus infesting Picea abies and Pinus sylvestris.
Habitat: Picea abies and Pinus sylvestris forests.
Distribution: Currently only known from Norway.
Notes: Ceratocystiopsis chalcographii and C. norroenii formed two distinct, well-supported clades within the European and Japanese putative strains of C. minuta (clades C and E according to Plattner et al. 2009) (Figs. 2, 3 and 4). They can be both differentiated from C. minuta by the morphology of their sexual and asexual morphs. Ceratocystiopsis norroenii is distinct from C. minuta by producing oblong and larger conidia and C. chalcographii produces avoid and longer conidia on simple conidiophores. Ceratocystiopsis minuta produces oblong smaller conidia on highly branched conidiophores (Reid and Hausner 2010) and the ascocarps in C. chalcographii have longer necks, longer ostiolar hyphae and the ascospores are larger compared to those produced by C. minuta (see diagnosis and description above, Reid and Hausner 2010).
Ceratocystiopsis chalcographii was found only in association with P. chalcographus on Scots pine in Rendalen and on Norway spruce in Nannestad and was isolated from bark beetles infesting P. abies and P. sylvestris with a frequency of 11% (Table 3). This species is one of two Ceratocystiopsis species isolated from P. chalcographus (i.e. Ceratocystiopsis chalcographii and Ceratocystiopsis debeeria).
Additional specimen examined: Norway: Rendalen, from Pityogenes chalcographus infesting Pinus sylvestris, 29 Oct. 2014, H. Solheim, (O-F-259425, culture CBS 147955 = CMW 57282).
Ceratocystiopsis norroenii Jankowiak & H. Solheim, sp. nov., Fig. 9
Morphological characteristics of Ceratocystiopsis norroenii (CBS 147956, Taxon 2). a–c Simple (a, b) and slightly (c) branched micronematous conidiophores; d–e Conidia arising directly from hyphae; f–g branching macronematous conidiophores; h conidia; i 14-day-old culture on 2% MEA. Scale bars: a–d = 10 μm, e = 25 μm, f–h = 10 μm
MycoBank: 841845
Etymology: The epithet norroenii (Latin) refers to the word Norrøn, used for the North in norse mythology.
Diagnosis: See comparisons between C. norroenii and C. minuta under C. chalcographii.
Type: Norway, Karasjok, from Ips acuminatus infesting Pinus sylvestris, 21 Jun. 2015, H. Solheim (O-F-259426 – holotype; CBS 147956 = CMW 57283–ex-type culture).
Description: Sexual morph not observed.
Asexual morph hyalorhinocladiella-like. Conidiophores micronematous or macronematous, abundantly formed. Micronematous conidiophores arising from vegetative hyphae, simple or branched (1 branches), hyaline, upright, straight or undulate, producing apical conidiogenous cells; forming often a single conidium only, formed either laterally on vegetative hyphae or on short lateral branches; conidiogenous cells integrated, hyaline, proliferating percurrently, sometimes denticulate (1.4–)12.1–28.1(–33.3) × (0.9–)1.2–1.7(–2.1) μm; macronematous conidiophores arising from vegetative hyphae, hyaline, (22.2–)29.6–48.2(–64.9) long, and (8.5–)11.2–19.4(–15.8) μm wide at the apex, arising from a basal cell, (4.3–)6.2–11.7(–16) × (1.4–)1.7–2.6 (–3.1) μm, irregularly branched or penicillate, with 1or -2 branches producing 3–4 apical conidiogenous cells per branch; conidiogenous cells integrated, hyaline, proliferating percurrently, sometimes denticulate (13.1–)15.9–27.2(–35.8) × (1–)1.2–1.5(–1.8) μm; conidia hyaline, smooth, unicellular, oblong, sometimes slightly obovoid, (2.7–)3.2–4.1(–5.1) × (1.1–)1.2–1.6(–2.3) μm, without mucilaginous sheath.
Colonies with optimal growth at 25 °C on 2% MEA, with radial growth rate of 1.11 mm (± 0.15 mm) per day. Colonies orange white (5A2), reverse pale orange (5A3), margin smooth, with suede-like surface, but occasionally funiculose; hyphae hyaline, smooth, submerged in medium and aerial mycelium rarely present, 0.5–1.8 (mean 1 ± 0.3) μm diam.
Ecology: Isolated from Ips acuminatus and Pityogenes quadridens infesting Pinus sylvestris.
Habitat: Pinus sylvestris forests.
Distribution: Currently only known from Norway.
Notes: See comparisons between C. norroenii and C. minuta under C. chalcographii. Ceratocystiopsis norroenii was frequently isolated from Ips acuminatus and Pityogenes quadridens infesting Scots pine growing in the north part of Norway.
Additional specimen examined: Norway: Målselv, from Ips acuminatus infesting Pinus sylvestris, 21 Jun. 2015, H. Solheim (O-F-259427, culture CBS 147957 = CMW 57284).
Ceratocystiopsis debeeria Jankowiak & H. Solheim, sp. nov., Fig. 10
MycoBank: 841846
Etymology: Named for Prof. Wilhelm de Beer who has contributed greatly to the taxonomy of ophiostomatoid fungi.
Diagnosis: Ceratocystiopsis debeeria is the closest phylogenetic relative of C. neglecta, from which it is differentiated by the absence of perithecia in in vitro culture, simple or slightly irregularly branched conidiophores, and by conidial size, which is 2.7–5.2 × 0.9–1.7 μm in the former and 3–4.5 × 1–1.5 μm in the latter.
Type: Norway, Flesberg, from Pityogenes chalcographus infesting Picea abies, 31. Oct. 2014, M.E. Waalberg and R.A. Lindseth (O-F-259428 – holotype; CBS 147958 = CMW 57288–ex-type culture).
Description: Sexual morph not observed.
Asexual morph hyalorhinocladiella-like. Conidiophores mononematous, abundantly formed, micronematous, arising from vegetative hyphae, simple or seldom slightly and irregularly branched, upright, straight or undulate, producing apical conidiogenous cells; conidiogenous cells integrated, hyaline, proliferating percurrently, rarely denticulate (7.8–)10.6–25.8(–37.9) × (0.8–)1–1.4(–1.8) μm; conidia hyaline, aseptate, obovoid with the upper part swollen, apex round, tapering toward base, sometimes oblong, without mucilaginous sheath, (2.7–)3.1–4.2(–5.2) × (0.9–)1.1–1.4(–1.7) μm.
Colonies with optimal growth at 25 °C on 2% MEA, with radial growth rate of 0.82 (± 0.18) mm/day; no growth at 35 °C. Colonies yellowish white (2A2), reverse pale orange (5A3), margin smooth, with suede-like surface; hyphae hyaline to pale grey (B1) in colour, smooth, submerged in the medium and aerial mycelium rarely observed, 0.8–2.9 (mean 1.6 ± 0.4) μm diam.
Ecology: Isolated from Pityogenes chalcographus infesting Picea abies and Pinus sylvestris.
Habitat: Picea abies and Pinus sylvestris forests.
Distribution: Currently only known from Norway.
Notes: In a phylogenetic perspective, C. debeeria is closely related to C. neglecta (Figs. 2, 3 and 4). Ceratocystiopsis debeeria is characterised by the absence of perithecia in in vitro culture, which are present in C. neglecta (Kirschner and Oberwinkler 1999). In addition, C. debeeria and C. neglecta can be distinguished by their conidial size.
Ceratocystiopsis debeeria appears to be rare and it was only isolated on a few occasions from Pityogenes chalcographus infesting Picea abies in Rendalen and Pinus sylvestris in Flesberg.
Additional specimen examined: Norway, Rendalen, from Pityogenes chalcographus infesting Pinus sylvestris, 29 Oct. 2014, H. Solheim (O-F-259429, culture CBS 147959 = CMW57290).
Ceratocystiopsis troendelagii Jankowiak & H. Solheim, sp. nov., Fig. 11
Morphological characteristics of Ceratocystiopsis troendelagii (CBS 147962, Taxon 4). a, b. Perithecium; c Ostiolar hyphae; d Ascospores; e Micronematous conidiophores; f–g branch macronematous conidiophores; h Conidia; i Fourteen-day-old culture on MEA. Scale bars: a–b = 25 μm; c–f = 10 μm; g = 25 μm; h = 10 μm
MycoBank: 841847
Etymology: The epithet troendelagii (Latin) refers to the area in Mid-Norway, Trøndelag, from which this fungus was collected.
Type: Norway, Lierne, from Polygraphus subopacus infesting Picea abies, 8 Aug. 2015, T. Kvamme and Å. Lindelöv (O-F-259430 – holotype; CBS 147962 = CMW57294 ex-type culture).
Diagnosis: The species is characterised by having a sexual and an asexual, hyalorhinocladiella-like morph, differentiated from the closely related species C. minima by smaller perithecial base diameter 25–54 μm vs. 40–85 μm, mainly obovoid vs clavate or oblong conidia, which are also shorter (2.7–5.2 μm vs. 2.5–8.0) μm.
Description: Perithecia abundantly produced on media and sterilised spruce twigs, formed by single isolates, with a globose or sub-globose black base, (25–)33–45(–54) μm diam, ending in black, straight or slightly curved necks, (9–)11–29(–72) μm long, (12.5–)15–20(24.6) μm at the base and (4–)4.7–7.7(–10.7) μm diam at the apex, with 3–6 ostiolar hyphae hyaline, aseptate, straight, (3.9–)7.2–10.5(–11.8) μm long, tapering extensively toward the apex. Asci not observed. Ascospores one-celled, hyaline, including the sheath falcate in side view (7–)8.8–11.8(–13.5) × (0.7–)0.8–1.1(–1.3) μm, accumulating in a white-coloured drop at tip of necks.
Asexual morph hyalorhinocladiella-like. Conidiophores micronematous or macronematous, abundantly formed. Micronematous conidiophores arising from vegetative hyphae, simple, hyaline, upright, straight or undulate, producing apical conidiogenous cells; conidiogenous cells integrated, hyaline, proliferating percurrently, sometimes denticulate, (5.9–)8.7–20.4(–30.4) × (0.8–)1.3–2.2(–2.7) μm; macronematous conidiophores arising from vegetative hyphae, hyaline, (17.8–)21.9–34.8(–41.2) long, and (4.4–)11.7–28.8(–37.7) μm wide at the apex, arising from a basal cell, (3.5–)5.1–10.9(–16.5) × (1.7–)2.2–3.3(–4.1) μm, irregularly branched with 2-3 branches, producing 2-4 apical conidiogenous cells per branch; conidiogenous cells integrated, hyaline, proliferating percurrently, sometimes denticulate, (10.5–)12.1–17.9(–23.6) × (1.4–)1.6–2.2(–2.7) μm; conidia hyaline, smooth, unicellular, oblong or obovate (2–)2.6–3.5(–4.1) × (0.8–)1.1–1.8(–2.2) μm, without mucilaginous sheath.
Colonies with optimal growth at 20 °C on 2% MEA with radial growth rate 0.72 (± 0.05) mm/day; no growth at 35 °C. Colonies yellowish white (2A2), reverse pale orange(5A3), margin smooth, with suede-like surface, occasionally funiculose; hyphae hyaline, smooth, submerged in medium and aerial mycelium rarely observed, 0.7–2.2 (mean 1.2 ± 0.4) μm diam.
Ecology: Isolated from Pityogenes saalasi and Polygraphus subopacus infesting Picea abies and Pinus sylvestris
Habitat: Picea abies and Pinus sylvestris forests
Distribution: Currently only known from Norway.
Notes: Ceratocystiopsis troendelagii, based on LSU data is phylogenetically related to C. minima (Fig. 3) but formed a distinct clade in both the ITS and the TUB2 based tree (Figs. 2 and 4). It can be differentiated from C. minima by dimension of the perithecial base and conidial morphology.
Ceratocystiopsis troendelagii was the rarest Ceratocystiopsis species isolated in this study. It was isolated twice in the same plot, once from Pityogenes saalasi and once from Polygraphus subopacus. Both bark beetles have a northern distribution in Fennoscandia.
Additional specimen examined: Norway, Lierne, from Pityogenes saalasi infesting Picea abies, 8 Aug. 2015, T. Kvamme and Å. Lindelöv (O-F-259431, culture: CBS 147963 = CMW57295).
Discussion
We collected 22 species of bark beetles from Picea abies and Pinus sylvestris located in Norway. From these beetles, we recovered 126 isolates that based on morphology can be assigned to Ceratocystiopsis, representing six species. Both molecular and morphological data revealed four undescribed species, herein described as C. chalcographii, C. debeeria, C. norroenii and C. troendelagii, and two known species, C. neglecta and C. rollhanseniana.
Description of these new species brings the total number of recognized species in this genus to 25, of which seven occur in Norway. These include the six species recovered in this study as well as C. minuta, which was previously found in association with I. typographus (Solheim 1986, 1992, 1993). Ceratocystiopsis rollhanseniana was described by Hausner et al. (2003) based on the Norwegian isolates collected by James Reid from P. sylvestris.
Ceratocystiopsis norroenii and C. rollhanseniana were the most frequently isolated Ceratocystiopsis species. However, these species appear to have different distribution and autecology including insect associations. Ceratocystiopsis norroenii appears to be a consistent associate of I. acuminatus on P. sylvestris while C. rollhanseniana has broader host/vector associations including different bark beetle species found on P. abies and P. sylvestris. Our phylogenetic analysis of the LSU sequences (Fig. 3) showed that some Polish isolates (CBS 116963, CBS 116796) previously identified as C. minuta and collected from P. abies infested by I. typographus actually represented C. rollhanseniana, showing a wider distribution in Europe.
Ceratocystiopsis norroenii belongs to the C. minuta sensu lato lineage that includes several sublineages in Europe and Japan (Plattner et al. 2009), representing a different, morphologically well-defined phylogenetic species. To stabilize the concept of C. minuta, an epitype was designated by Reid and Hausner (2010), RJ705 = UAMH 11218 = WIN(M) 1532. The C. norroenii lineage was not represented in Plattner et al. (2009), suggesting that the C. minuta species complex could accommodate numerous morphologically similar species. For example, C. weihaiensis, a species from China, was also shown to be phylogenetically close to C. minuta (Chang et al. 2021).
Ceratocystiopsis chalcographii belongs also to the C. minuta species complex. These isolates were recovered from P. chalcographus on P. abies. Several isolates from Scotland, Austria and Japan, previously identified as C. minuta and placed in clade D (Plattner et al. 2009), were shown to have ITS, LSU and TUB2 sequences identical or very similar to those of C. chalcographii (Figs. 2, 3 and 4). Therefore, C. chalcographii may have a wider geographical distribution in Europe and possibly Asia. This species also shares an identical LSU sequence with an isolate from the USA (CBS 145.59). More studies are needed on the taxonomic/genetic diversity of the C. minuta species complex to resolve the various cryptic species that might be part of this group. In this study, P. chalcographus is also the only vector of C. debeeria, suggesting in this case species-specific ectosymbiont relationships between them.
Ceratocystiopsis troendelagii and C. neglecta were found only on a few occasions in our study. Ceratocystiopsis troendelagii seems to be preferably associated with P. saalasi and P. subopacus, while C. neglecta was found associated with H. palliatus and P. subopacus. This is the first report of C. neglecta in association with P. subopacus. Previously, C. neglecta was only known from different bark- and ambrosia beetles including C. cinereus, C. pusillus, D. autographus, H. palliatus, I. typgraphus, P. chalcographus and T. lineatum infesting P. abies and P. sylvestris in Germany (Kirschner 2001; Kirschner and Oberwinkler 1999).
Most of the formally described species of Ceratocystiopsis are known only from conifers, although C. lunata and C. synnemata were recently collected from hardwood-infesting beetles (Li et al. 2018; Strzałka et al. 2020; Nel et al. 2021). The results of the present study confirm that Ceratocystiopsis species have a close affinity to conifers.
Based on morphological characters and DNA sequence data, four new species of ophiostomatalean fungi were described and one is reported from Norway for the first time. The relationships between fungi and bark beetles are continuously studied in Europe and worldwide (Kirisits 2004; Linnakoski et al. 2012; Jankowiak et al. 2020; Marincowitz et al. 2020; Chang et al. 2021; Nel et al. 2021; Trollip et al. 2021). Our survey led to the discovery and description of four new species, suggesting that there is still a lot of unknown taxonomic and ecological diversity to be uncovered for the ophiostomatalean fungi in Europe.
Data availability
All data generated or analysed during this study are included in this published article and/or is available from the corresponding author upon request.
References
Aas T, Solheim H, Jankowiak R, Bilański P, Hausner G (2018) Four new Ophiostoma species associated with hardwood-infesting bark beetles in Norway and Poland. Fungal Biol 122:1142–1158. https://doi.org/10.1016/j.funbio.2018.08.001
Bridges JR, Perry TJ (1987) Ceratocystiopsis ranaculosus sp. nov. associated with the Southern Pine Beetle. Mycologia 79:630–633
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies filamentous ascomycetes. Mycologia 91:553–556. https://doi.org/10.2307/3761358
Chang R, Zhang X, Si H, Zhao G, Yuan X, Liu T, Bose T, Dai M (2021) Ophiostomatoid species associated with pine trees (Pinus spp.) infested by Cryphalus piceae from eastern China, including five new species. MycoKeys 83:181–208. https://doi.org/10.3897/mycokeys.83.70925
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
De Beer ZW, Duong TA, Wingfield MJ (2016) The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 83:165–191. https://doi.org/10.1016/j.simyco.2016.07.001
De Beer ZW, Seifert KA, Wingfeld MJ (2013a) The ophiostomatoid fungi: their dual position in the Sordariomycetes. In: Seifert KA, De Beer ZW, Wingfeld MJ (eds) The ophiostomatoid fungi: expanding frontiers, CBS Biodiversity Series, vol 12. CBS-KNAW Biodiversity Centre, Utrecht, The Netherlands, pp 1–19
De Beer ZW, Seifert KA, Wingfield MJ (2013b) A nomenclator for ophiostomatoid genera and species in the Ophiostomatales. In: Seifert KA, De Beer ZW, Wingfeld MJ (eds) The ophiostomatoid fungi: expanding frontiers, CBS Biodiversity Series, vol 12. CBS-KNAW Biodiversity Centre, Utrecht, The Netherlands, pp 245–322
De Beer ZW, Wingfeld MJ (2013) Emerging lineages in the Ophiostomatales. In: Seifert KA, De Beer ZW, Wingfeld MJ (eds) The ophiostomatoid fungi: expanding frontiers, CBS biodiversity series, vol 12. CBS-KNAW Biodiversity Centre, Utrecht, The Netherlands, pp 21–46
Ehnström B, Axelsson R (2002) Insektsgnag i bark och ved. ArtDatabanken, SLU, Uppsala
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhiza and rusts. Mol Ecol 2:113–118
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microb 61:1323–1330
Griffin HD (1968) The genus Ceratocystis in Ontario. Can J Bot 46:689–718
Grobbelaar J, De Beer ZW, Bloomer P, Wingfield MJ, Wingfield BD (2010) Ophiostoma tsotsi sp. nov., a wound- infesting fungus of hardwood trees in Africa. Mycopathologia 169:413–423. https://doi.org/10.1007/s11046-009-9267-8
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704. https://doi.org/10.1090/1063515150390235520
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hausner G, Eyjolfsdottir GG, Reid J (2003) Three new species of Ophiostoma and notes on Cornuvesica falcata. Can J Bot 81:40–48. https://doi.org/10.1139/b03-009
Hausner G, Reid J, Klassen GR (1993) The genus Ceratocystiopsis: a reappraisal based on molecular criteria. Mycol Res 97:625–633
Hsiau PT-W, Harrington TC (1997) Ceratocystiopsis brevicomi sp. nov., a mycangial fungus from Dendroctonus brevicomis (Coleoptera: Scolytidae). Mycologia 89:661–669
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat DJ, Jones EBG, Bundhun D, Chen YJ, Bao DF, Boonmee S, Calabon MS, Chaiwan N, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J, Zeng XY, Zhang SN, Xiang MM (2020) Refined families of Sordariomycetes. Mycosphere 11(1):305–1059
Jankowiak R (2005) Fungi associated with Ips typographus on Picea abies in Southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. Forest Pathol 35:37–55
Jankowiak R, Solheim H, Bilański P, Marincowitz S, Wingfield MJ (2020) Seven new species of Graphilbum from conifers in Norway, Poland, and Russia. Mycologia 112(6):1240–1262. https://doi.org/10.1080/00275514.2020.1778375
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7, improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kirisits T (2004) Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi. In: Lieutier F, Day KR, Battisti A, Gregoire JC, Evans H (eds) Bark and Wood Boring insects in living trees in Europe, a synthesis. Kluwer, Dordrecht, pp 185–223
Kirschner R (2001) Diversity of filamentous fungi in bark beetle galleries in central Europe. In: Misra JK, Horn BW (eds.) Trichomycetes and Other Fungal Groups. Robert W. Lichtwardt Commemoration Volume, pp 175–196
Kirschner R, Oberwinkler F (1999) A new Ophiostoma species associated with bark beetles infesting Norway spruce. Can J Bot 77:247–252
Kornerup A, Wanscher JH (1978) Methuen Handbook of Colour. Third Edition. Eyre Methuen, London, pp 1–252
Lekander B, Bejer-Petersson B, Kangas E, Bakke A (1977) The distribution of bark beetles in the Nordic countries. Acta Entomol Fenn 32:1–32 + 78 Maps
Li Y, Huang Y-T, Kasson MT, Macias AM, Skelton J, Carlson PS, Yin M, Hulcr J (2018) Specific and promiscuous ophiostomatalean fungi associated with Platypodinae ambrosia beetles in the southeastern United States. Fungal Ecol 35:42–50
Linnakoski R, de Beer ZW, Niemelä P, Wingfield MJ (2012) Associations of conifer-infesting bark beetles and fungi in Fennoscandia. Insects 3:200–227
Marincowitz S, Duong TA, Stephen SJ, De Beer ZW, Wingfield MJ (2020) Fungal associates of an invasive pine-infesting bark beetle, Dendroctonus valens including seven new ophiostomatalean fungi. Persoonia 45:177–195. https://doi.org/10.3767/persoonia.2020.45.07
Marmolejo JG, Butin H (1990) New conifer-inhabiting species of Ophiostoma and Ceratocystiopsis (Ascomycetes, Microascales) from Mexico. Sydowia 42:193–199
Nel WJ, Wingfield MJ, de Beer ZW, Duong TA (2021) Ophiostomatalean fungi associated with wood boring beetles in South Africa including two new species. Anton Leeuw 114(6):667–686. https://doi.org/10.1007/s10482-021-01548-0
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7:103–116. https://doi.org/10.1006/mpev.1996.0376
O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. P Natl Acad Sci USA 95:2044–2049. https://doi.org/10.1073/pnas.95.5.2044
O'Donnell K, Nirenberg H, Aoki T, Cigelnik E (2000) A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41:61–78. https://doi.org/10.1007/BF02464387
Olchowecki A, Reid J (1974) Taxonomy of the genus Ceratocystis in Manitoba. Can J Bot 52:1675–1711
Plattner A, Kim J-J, Reid J, Hausner G, Lim YW, Yamaoka Y, Breuil C (2009) Resolving taxonomic and phylogenetic incongruence within species Ceratocystiopsis minuta. Mycologia 101:878–887. https://doi.org/10.3852/08-132
Rambaut A, Drummond AJ (2007) Tracer v1.4, Available from http://beast.bio.ed.ac.uk/Tracer
Reid J, Hausner G (2010) The epitypification of Ophiostoma minutum, now Ceratocystiopsis minuta. Mycotaxon 113:463–474. https://doi.org/10.5248/113.463
Robert V, Vu D, Amor AB, Van de Wiele N, Brouwer C et al (2013) MycoBank gearing up for new horizons. IMA Fungus 4:371–379. https://doi.org/10.5598/imafungus.2013.04.02.16
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/sysbio/sys029
Siemaszko W (1939) Zespoły grzybów towarzyszących kornikom polskim. Planta Pol 7:1–54
Solheim H (1986) Species of Ophiostomataceae isolated from Picea abies infested by the bark beetle Ips typographus. Nord J Bot 6:199–207
Solheim H (1992) Fungal succession in sapwood of Norway spruce infested by the bark beetle Ips typographus. Eur J For Pathol 22:136–148
Solheim H (1993) Fungi associated with the spruce bark beetle Ips typographus in an endemic area in Norway. Scand J For Res 8:118–122
Strzałka B, Jankowiak R, Bilański P, Patel N, Hausner G, Linnakoski R, Solheim H (2020) Two new species of Ophiostomatales (Sordariomycetes) associated with the bark beetle Dryocoetes alni from Poland. MycoKeys 68:23–48
Swofford DL (2003) PAUP* 4.0: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts
Trollip C, Carnegie AJ, Dinh Q et al (2021) Ophiostomatoid fungi associated with pine bark beetles and infested pines in south-eastern Australia, including Graphilbum ipis-grandicollis sp. nov. IMA Fungus 12: 24. https://doi.org/10.1186/s43008-021-00076-w
Upadhyay HP (1981) A monograph of Ceratocystis and Ceratocystiopsis. the University of Georgia Press, Athens
Upadhyay HP, Kendrick WB (1975) Prodromous for a revision of Ceratocystis (Microascales, Ascomycetes) and its conidial states. Mycologia 67:798–805
Vilgalys R Conserved primer sequences for PCR amplification of fungal rDNA Mycology Lab – Duke University. https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/. Accessed 11 June 2021.
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172:4238–4246. https://doi.org/10.1128/JB.172.8.4238-4246.1990
Viljoen CD, Wingfield MJ, Jacobs K, Wingfield BD (2000) Cornuvesica, a new genus to accommodate Ceratocystiopsis falcate. Mycol Res 104(3):365–367
Wang Z, Liu Y, Liu C, Liu Z, Liang L, Lu Q (2021) Morphological and phylogenetic analyses reveal a new species of Ceratocystiopsis (Ophiostomataceae, Ophiostomatales) Associated with Ips subelongatus in Inner Mongolia (China) with Weak Host Pathogenicity. Forests 12:1795. https://doi.org/10.3390/f12121795
Wang Z, Liu Y, Wang H, Meng X, Liu X, Decock C, Zhang X, Lu Q (2020) Ophiostomatoid fungi associated with Ips subelongatus, including eight new species from northeastern China. IMA Fungus 11:3. https://doi.org/10.1186/s43008-019-0025-3
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Snisky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Yamaoka Y, Wingfield MJ, Takahashi I, Solheim H (1997) Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Japan. Mycol Res 101:1215–1227. https://doi.org/10.1017/S0953756297003924
Zipfel RD, de Beer ZW, Jacobs K, Wingfield BD, Wingfield MJ (2006) Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud Mycol 55:75–97. https://doi.org/10.3114/sim.55.1.75
Acknowledgements
We would like to thank and acknowledge the following for their assistance across various aspects of this study. Terje B. Dahl, Torstein Kvamme, Geir Kvammen, Åke Lindelöv Ruben A. Lindseth, Tor H. Sundt and Max E. Waalberg, for their assistance in field sampling. Anne E. Nielsen, Max E. Waalberg and Gro Wollebæk participated in laboratory work. T.K. helped with the identification of bark beetles.
Abbreviations
BI: Bayesian Inference; TUB2: beta-tubulin; CAL: Calmodulin; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; ITS: The internal transcribed spacer; LSU: The large ribosomal subunit (28S); MEA: Malt extract agar; ML: Maximum likelihood; MP: Maximum Parsimony; NCBI: National Center for Biotechnology Information; NIBIO: Culture collection of Norwegian Institute of Bioeconomy, Norway; O: the Mycological Herbarium, Natural History Museum, University of Oslo, Norway; s.l.: sensu lato; s.str.: sensu stricto; TEF1: Translation elongation factor 1-α; UAMH: The UAMH Centre for Global Microfungal Biodiversity, University of Toronto, Canada; WIN(M or UM): Culture Collection of the University of Manitoba (Winnipeg); Canada.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research. This work was funded by the Norwegian biodiversity information centre on the project ‘Ophiostomatoid fungi in Norway’ (No. 51-14) and Norwegian Institute of Bioeconomy Research, (No. 132001). This work was supported by the Ministry of Science and Higher Education of the Republic of Poland (SUB/040013/D019).
Author information
Authors and Affiliations
Contributions
HS conceived the idea of this study, performed isolations and DNA sequencing and edited the final version of the manuscript. RJ performed DNA sequencing, performed microscopy for the species descriptions and edited the description text and wrote the manuscript. PB performed phylogenetic analysis. JM performed DNA sequencing, GH performed DNA sequencing and edited the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Adherence to national and international regulations
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section editor: Hans-Josef Schroers
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jankowiak, R., Solheim, H., Bilański, P. et al. Ceratocystiopsis spp. associated with pine- and spruce-infesting bark beetles in Norway. Mycol Progress 21, 61 (2022). https://doi.org/10.1007/s11557-022-01808-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01808-x