Abstract
The North European species of the Hygrophorus agathosmus group in subsection Tephroleuci were studied. Three new species are identified based on morphology, ecology and sequence data. Two species are associated with Pinus spp. One of these is described here as H. suaveolens, while the other one is only known from one locality in the Nordic countries and seems to have a more South European distribution range. A closely related sister species to H. agathosmus is identified based on ITS sequence data, H. cf. agathosmus. It is confirmed to have an intercontinental distribution range and to be associated with Picea spp. probably on more acidic to neutral soil, whereas H. agathosmus s.s. has a more limited North-East European distribution range and occurs in older and rich Picea abies forests. A neotype for H. agathosmus is here selected from South Sweden. Hygrophorus agathosmus f. albus and H. agathosmus f. aureofloccosus are confirmed as forms. No genetic differences in the ITS region between specimens with grey cap colour and the two forms were observed. A key to the species in Northern Europe is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Hygrophorus Fr.: Fr includes species that form ectomycorrhizal associations with roots of trees and are mainly distributed in the temperate zones of the Northern Hemisphere (Tedersoo et al. 2010). Many species show strong host preferences, a character that has proved to be useful for separation of morphologically similar species (Larsson and Jacobsson 2004; Jacobsson and Larsson 2007). The genus is considered rather well-known with about 50 accepted species in Europe (e.g. Candusso 1997) and about 40 species in the Nordic countries (Kovalenko 2012; Larsson et al. 2011).
Lodge et al. (2014) revised the family Hygrophoraceae Lotsy and proposed a DNA-based classification of Hygrophorus. In this new classification, the subgenus Colorati (Bataille) E. Larss. was divided into three sections: Olivaceoumbrini (Bataille) Konrad & Maubl., Pudorini (Bataille) Konrad & Maubl. and Aurei (Bataille) E. Larss. Section Olivaceoumbrini is further divided into subsections Olivaceoumbrini (Bataille) Singer and Tephroleuci (Bataille) Singer.
The species in the H. agathosmus group belong to subsection Tephroleuci and are all united by having a sweetish smell reminiscent of bitter almonds, hyacinths, or bunch-flowered daffodil (Narcissus tazetta). The smell can be more or less pronounced depending on species. Traditionally, two species with a sweetish smell have been recognised in the Nordic countries: H. agathosmus (Fr.) Fr. which is common in moist, nutrient-rich and old forest stands of Picea abies and H. hyacinthinus Quél. (Fig. 1a). which is associated with Picea abies in herb-rich forests on calcareous soils (Kovalenko 2012). Hygrophorus agathosmus is wide-spread and often produces large amounts of basidiomata, even fairy rings in suitable habitats, while H. hyacinthinus is rare and in most Nordic countries a redlisted species, classified as endangered (EN) in Norway (Henriksen and Hilmo 2015) and Sweden (Gärdenfors 2010), and as vulnerable (VU) in Finland (Rassi et al. 2010).
A rather recently described species in subsection Tephroleuci, H. exiguus E. Larss., E. Campo & M. Carbone (Fig. 1b, Larsson et al. 2014), occurs in old growth Picea abies forests often in close association with Tricholoma inamoenum (Fr.) Gillet. The species is small, with a pileus rarely exceeding 3 cm in diameter and with a long stipe often deeply buried in the moss. In Northern Europe, it was probably overlooked because of the small basidiomata, growing in the same type of forests and having a similar smell of bitter almonds as H. agathosmus. The species was encountered and reported from Southern Europe under the name H. odoratus A. H. Sm. & Hesler (Bon 1990; Candusso 1997; Bidaud 2007), but the molecular data clearly shows that the two are separate species (Larsson et al. 2014).
Thorough analyses of sequence data of specimens from a wide, intercontinental geographic distribution Moreau et al. (2018) demonstrated an unexpectedly high genetic diversity within the yellow waxcap H. hypothejus Fr.: Fr. (section Aurei). The diversity could be correlated to geographic origin, ecology and host preferences. This suggests that the diversity and number of species within Hygrophorus may be higher than current estimations.
In this study, well-documented specimens from the H. agathosmus group from Northern Europe were sequenced with the aim of further exploring the genetic diversity, host preferences and geographic distribution of species. In addition, specimens of the white form H. agathosmus f. albus Candusso and the form with yellow floccules at stipe apex and cap margin H. agathosmus f. aureofloccosus Bres. were sampled and their taxonomic status was evaluated.
Material and methods
Morphological data
The majority of the sequenced specimens in this study were collected by the authors. Additional specimens were received as loan from the herbaria UPS, TU, AMNH and MA. Permission to extract DNA and sequence the ITS region was granted. Abbreviation of herbaria follows Index Herbariorum (http://sweetgum.nybg.org/science/ih/).
Macro-morphological characters and ecology were observed in the field, and fresh basidiomata photographed. Colour codes refer to Kornerup and Wanscher (1962). Micro-morphological characters were measured from dried material mounted in Congo red NH3 solution at × 1000 magnification. Photos of micro-morpholgical characters were made using Axioskop 2 (Zeiss, Oberkochen, Germany) light microscope and the AxioVision software (http://www.zeiss.com/microscopy/int/home.html). For each specimen, the length and width of a minimum of 30 spores were measured and the ratio calculated (Q = length/width). In addition, 10 basidia and sterigmata, as well as hyphae of trama, pilei- and stipetipellis, were measured.
Molecular data
Forty specimens from Northern Europe, Germany, Spain and Canada were targeted for sequencing. In addition, ITS and LSU sequence data of specimens from subsection Tephroleuci were included from previous studies (Larsson et al. 2014; Lodge et al. 2014). ITS sequences type was also blasted in GenBank (Clark et al. 2016) and the UNITE database (Kõljalg et al. 2013) to search for additional data generated from curated specimens or from environmental samples as soil and ectomycorrhizae. In total, 12 sequences were found and added to the data set. Sequence data of H. pustulatus (Pers.) Fr. (Fig. 1f) was selected as out-group for rooting of trees. The species belongs in subsection Tephroleuci characterised by the greyish-brown colour of the cap and the non-viscid stipe but is odourless, lacking the characteristic sweetish smell of the H. agathosmus group (Kovalenko 2012).
Sequences from the complete internal transcribed spacer (ITS) region and about 900 base pairs (bp) of the 5′ end of the large subunit (LSU) of the nuclear ribosomal DNA were generated. Protocols for DNA extraction, PCR and primers follow Larsson et al. (2018). The type specimens were extracted using a modified CTAB method and PCR and sequencing followed protocols described in Larsson and Jacobsson (2004).
Sequences were edited and assembled using Sequencher 5.1 (Gene Codes, Ann Arbor, Michigan). Alignment was performed using the L-INS-i strategy as implemented in MAFFT v. 7.017 (Katoh and Standley 2013). The alignment was adjusted using Aliview 1.17.1 (Larsson 2014). Sequences generated for this study have been deposited in GenBank with accession numbers MH656439–MH656479. Specimens sequenced for this study are indicated with an asterisk (*) in the list of studied material and the GenBank number is specified.
For inferring phylogenetic relationships among species, heuristic searches were performed using the maximum parsimony method in PAUP* (Swofford 2003). All transformations were considered unordered and equally weighted. Variable regions with ambiguous alignment were excluded and gaps were treated as missing data. Heuristic searches with 1000 random-addition sequence replicates and TBR branch swapping were performed, saving at most 25 trees in each replicate. Relative robustness of clades was assessed by the bootstrap method using 1000 heuristic search replicates with 10 random taxon addition sequence replicates and TBR branch swapping, the latter saving at most 100 trees in each replicate.
Bayesian analysis was carried out in MrBayes 3.2.6 (Ronquist and Huelsenbeck 2012), with a best-fit model of nucleotide evolution supplied by MrModeltest 2.2 (Nylander 2004). Eight default-setting Metropolis-Coupled Markov Chain Monte Carlo (MCMCMC) chains were run for 10 million generations with trees sampled every 5000 generations and an initial burn-in of 1000 trees. A 50% majority-rule consensus phylogram was computed from the remaining trees.
Results
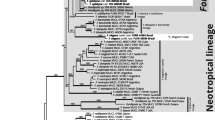
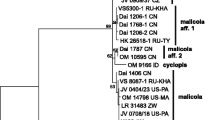
The aligned complete dataset consisted of 65 sequences and 1521 characters. After exclusion of ambiguous regions, mainly from the beginning and the end of the data set 1474 characters remained for the analyses. Of these, 1338 were constant, 14 were variable but parsimony uninformative and 122 (8.3%) were parsimony informative. The maximum parsimony analysis yielded 9610 equally most parsimonious trees (length = 195 steps, CI = 0.8051 and RI = 0.9604). One of the trees is presented as a phylogram (Fig. 2).
Phylogram showing the phylogenetic relationships among the species in Hygrophorus subsection Tephroleuci, based on ITS and LSU sequence data. Bootstrap values and Bayesian Posterior Probabilities are indicated on branches. Clades discussed in the text are indicated with bars and species epithets. Sequences originating from type specimens are marked
The bootstrap analysis recovered seven terminal clades with low to strong bootstrap support and three single branches. The clades correspond to H. agathosmus (74%), H. cf. agathosmus (64%), Hygrophorus sp. (100%), H. hyacinthinus (100%), H. suaveolens (97%), H. exiguus (100%) and H. odoratus (86%). The single branch with sequence data from a mycorrhizal study on Arbutus menziesii originating from North America is recovered close to H. agathosmus. The sequence named H. morrisii Pk. and the sequence data originating from a soil sample of Pinus from Mexico both were recovered close to H. odoratus with strong support (Fig. 2).
As suggested by MrModeltest, the nucleotide evolution model HKY + G was used for the ITS1 spacer, K80 was used for the 5.8S gene and GTR + 1 was used for the ITS2 spacer and LSU region in the Bayesian analysis. The MCMC analysis converged well in advance of the burn-in threshold and chain mixing was found to be satisfactory, as assessed by using Tracer v1.5 (Drummond et al. 2012). In the Bayesian analysis, the same terminal clades were recovered as monophyletic with support as in the bootstrap analysis, except that the clade H. cf. agathosmus collapsed within the major clade of H. agathosmus s. l. The Bayesian tree topology is similar to the MP bootstrap tree. BPP values are indicated on corresponding branches (Fig. 2).
The specimens identified in the field as H. agathosmus f. albus clustered in both the H. agathosmus and H. cf. agathosmus clades and also the specimens of H. agathosmus f. aureofloccosus clustered in the H. agathosmus clade (Fig. 2) suggesting they are just colour forms.
Taxonomy
Hygrophorus agathosmus (Fr.) Fr., Epicr. syst. mycol.: 325 (1838) Figs. 4a–c
≡ Agaricus agathosmus Fr., Observ. mycol. 1: 16 (1815)
≡ Hygrophous agathosmus (Fr.) Fr., Epicr. syst. mycol.: 325 (1838) f. agathosmus
≡ Hygrophous agathosmus (Fr.) Fr., Epicr. syst. mycol.: 325 (1838) var. agathosmus
≡ Limaceum pustulatum var. agathosmum (Fr.) P. Kumm. Führ Pilzk.:119 (1871)
≡ Limaceum agathosmum (Fr.) Wünsche, Die Pilze: 116 (1877)
= Agaricus cerasinus Berk., Engl. Fl. Fungi 5(2): 12 (1836)
= Hygrophorus cerasinus (Berk.) Berk., Outl. Brit. Fung.: 197 (1860)
= Hygrophous agathosmus f. aureofloccosus Bres., Iconogr. Mycol. 7:tab. 320 (1928)
= Hygrophous agathosmus var. aureofloccosus (Bres.) A. Pearson & Dennis, Trans. Br. mycol Soc., 31(3–4): 163 (1948)
= Hygrophous agathosmus f. albus Candusso, Fungi europ. 6:104 (1997)
Original diagnosis 1815:
Agaricus agathosmus, pileo convexiusculo carnoso papilloso suaveolenti, cinereo, lamellis albis decurrentibus, stipite folido albo. In silvis abietinis densis, una cum Ag. inamoeno, sed rarior. Septembr. Octobr.
Descr. Stipes solidus 2 uncialis crassit. pennae anserinae aequalis glaber basi albido pubescens laevis albus rectus, raro curvatus, elasticus, demum cavus. Variat sursum subattenuatus et squamuloso-pubescens. Pileus carnosus convexus demum planiusculus 1–1½ unc., interdum subviscosus, papillis minutissimis dilutioribus obsitus. Lamellae albae 2-natae decurrentes destinctae latiusculae. Odor gratissimus.
Diu pro varietate Agar. pustulati P. habui, a quo charactere aegre distinguitur, sed odore gratissimo, colore et magnitudine satis differre videtur. Ceterum nullo modo Ag. eburneo affinis, ut de fungo hoc attulerunt observatores diligentissimi de Albertini et de Schweiniz. - Noster Ag. fragranti potius affinis et hinc summa differentia apparet!
When Fries described Agaricus agathosmus (Fries 1815), he did not refer to any painting, drawing or authentic material and he regarded A. agathosmus as a rather rare species. Later (Fries 1838, as Hygrophorus), he emended the description to partly include H. pustulatus, but still characterised the species as having a pleasant smell of aniseed. In Fries herbarium (UPS), two collections of H. agathosmus are preserved: one from the Presovsky area (Slovakia) collected by Kalchbrenner but not dated, and the other from Tammela, Finland, collected by Karsten and dated 2 October 1869. Obviously, none of them were at hand when Fries described the species. In the Uppsala, herbarium there is also a painting of H. agathosmus, signed by Fries (No. 737) and dated 21 September 1856 (Strid 1994). This painting is certainly representative for the species (Fig. 3). Since no material suitable for lectotypification is known, we have to select a neotype. For this, we then prefer recently collected material with a photo of fresh basidiomata from which we have been able to generate genetic information.
Emended description:
Neotype (designated here): MykoBank no.: MBT383487
SWEDEN: Öland, Böda, Trollskogen NR, herb-rich, mixed coniferous forest, 5 Oct 2017, E. Larsson 398-17 (GB-0159607!, isoneotype UPS, GenBank Acc. No. MH656445)
Pileus 30–50 (− 80) mm wide, campanulate, obtusely conical with involute margin when young, soon becoming plano-convex, applanate, with a broad umbo or slightly depressed with age, grey to greyish-brown, usually slightly paler towards the margin, margin whitish or yellowish, finely fibrillose, pale or completely white pileus forms common, glabrous, minutely fibrillose at centre, when moist viscid. Lamellae arcuate, subdecurrent to short-decurrent, L = 35–60, distant to subdistant, thick, waxy, with lamellulae, intervenose, white, sometimes with a pale greyish or pinkish tint. Stipe up to 5–8 × 0.5–1.5 cm, cylindrical, slightly clavate, tapering towards the base, whitish with floccules especially in the upper half, with age pale greyish, at apex with white or sometimes yellow floccules. Context whitish, taste mild, smell strong, sweetish like bitter almonds. Spore deposit white.
Spores [n = 159] elliptical, oblong to ovoid, with a distinct obtuse hilar appendage, smooth, hyaline, inamyloid, (8.6–) 9.4–9.6–9.8 (− 11.0) × (4.9–) 5.6–5.7–5.9 (− 6.4) μm, average Q = 1.64–1.69–1.73. Basidia mainly 4-spored, narrowly clavate, 40–55 × 7–9 μm, sterigmata 6–7.5 μm long. Hymenophoral trama bilateral to slightly divergent composed of interwoven cylindrical hyphae 5–8 μm wide and thin-walled inflated hyphae with terminal cells up to 20 μm wide.
Pileipellis an ixotrichoderm up to 400 μm thick; made up of loosely arranged interwoven, branched hyphae 3.5–7 μm wide, in matrix smooth, intracellularly pigmented. Subpellis composed of densely arranged, interwoven hyphae up to 15 μm broad.
Stipitipellis a cutis, up to 60 μm thick, made up of 3–5 μm wide loosely interwoven branched hyphae with scatted free ends, smooth, hyaline. Stipititrama composed of hyaline more or less parallel hyphae 8–14 μm wide. Floccules at apex made up of compact erect branched hyphae, 3.5–6.0 μm wide, loosely scattered free ends cylindrical or slightly enlarged, up to 8 μm wide.
Clamp connections present in all tissues.
Ecology and distribution
Associated with Picea abies usually on nutrient-rich soil in moist herb-rich forests. Confirmed sequence specimens were all collected from old-growth forests, most of them situated on more or less calcareous soils. Other species found on the same localities are H. piceae, H. discoideus, Tricholoma inamoenum, Sarcodon imbricatus and Lactarius scrobiculatus. The sequenced specimens originate from Estonia, Finland, Norway, Russia and Sweden (Fig. 4).
Material studied
Hygrophorus agathosmus ESTONIA: Saare, Kärla, Sauvere, mixed forest on calcareous soil, 3 Sep 2007, leg. V. Liiv, det. E. Larsson (*TU106100, MH656442); Saare, Lümanda, Audaku, Viidumää NR, spruce forest, 4 Sep 2007, leg. V. Liiv, det. V. Liiv (*TU106085, MH656443). FINLAND: Etälä-Hämä, Lammi, rich, mixed coniferous forest with Picea abies and Corylus avellana, 2 Sep 2000, leg. E. Larsson 1-00, det. E. Larsson (GB-0150351). NORWAY: Telemark, Bamble, Røsskleiva, mixed coniferous forest on calcareous soil dominated by Picea abies, Corylus avellana, Pinus sylvestris, 10 Oct 2015, leg. E. Larsson, M. Jeppson, det. E. Larsson (*EL191-15, MH656454). SWEDEN: Bohuslän, Tossene, Anneröd Hogsäm NR, in grass under Picea abies, 19 Oct 2013, leg. E. Larsson, det. E. Larsson (*EL429-13, MH656455); Dalsland, Skållerud, Håverud, Östebo Ö, herb-rich coniferous forest with Picea abies and Corylus avellana, 17 Sep 2011, leg. E. Larsson, A. Stridvall, S. Jacobsson, det. E. Larsson (*EL249-11, MH656452); Gotland, Ardre, Ollajvs NR, moist Picea abies forest on calcareous soil with Quercus robur, Betula pendula and Corylus avellanea, 27 Sep 2010, leg. E. Larsson, A. Stridvall, S. Jacobsson, det. E. Larsson (EL327-10, KJ720195); Ibidem, (*EL345-10, MH656446); Gotland, Dalhem, Malmskogen, moist coniferous forest on calcareous soil, 30 Sep 2011, leg. E. Larsson, det. E. Larsson (*EL331-11, MH656453); Medelpad, Sättna, 2–3 km V. Västmansjö, old-growth forest with Picea abies, 27 Sep 2003, leg. S. Muskos 03-10, det. S. Muskos (GB-0076510, KJ720194); Närke, Axberg, Kvinnerstatorp, Picea abies dominated mixed forest on calcareous ground with Populus tremula and Betula pendula, 10 Sep 2008, leg. E. Larsson, det. E. Larsson (*EL110-08, MH656450); Västergötland, Medelplana, Eriksberg, moist Picea abies forest, 19 Sep 2012, leg. E. Larsson, det. E. Larsson (*EL229-12, MH656456); Västergötland, Skepplanda, Slereboåns dalgång NR, 16 Sep 2012, leg. E. Larsson, det. E. Larsson (*EL194A-12, MH656447); Öland, Böda, Trollskogen NR, herbrich mixed coniferous forest on sand, 5 Oct 2017, leg. E. Larsson, det. E. Larsson (*EL398-17, MH656445).
Hygrophorus agathosmus f. albus FINLAND: Etelä-Häme, Lammi, rich, mixed coniferous forest with Picea abies and Corylus avellana, 2 Sep 2000, leg. E. Larsson, det. E. Larsson (*EL2-00, GB-0150352, MH656440). NORWAY: Akershus, Nesodden, Torvet, Sörby S, rich, mixed coniferous forest with Picea abies and Corylus avellana, 11 Sep 2010, leg. E. Larsson, det. E. Larsson (*EL304–-10, MH656439). SWEDEN: Bohuslän, Tossene, Anneröd Hogsäm NR, in grass under Picea abies, 19 Oct 2013, leg. E. Larsson, det. E. Larsson (*EL430-13, MH656449); Gotland, Viklau, Tjaukle, grazed, mixed Picea abies forest, 30 Sep 2011, leg. G. Gyða Eyjólfsdóttir, det. E. Larsson (*GB-0107410, MH656448); Västergötland, Husaby, coniferous forest on calcareous soil, 13 Sep 1980, leg. L. & A. Stridvall, LAS80-409, det. L. Stridvall (*GB-0063990, MH656441).
Hygrophorus agathosmus f. aureofloccosus NORWAY: Buskerud, Drammen, between Landfallstjern and Myrdammen, spruce forest, 19 Sep 2017, leg. G. Gulden, det. G. Gulden, E. Larsson (*GG6-17, MH656444). SWEDEN: Gotland, Tofta, Smågårde, mixed coniferous forest on sand, under Picea abies, 28 Sep 2010, leg. E. Larsson, A. Stridvall, S. Jacobsson, det. E. Larsson (*EL368-10, MH656451).
Hygrophorus suaveolens Kleine & E. Larss. sp. nov. Figs. 1 e and 5 a–f
MycoBank No.: MB827920
Holotype: Sweden, Uppland, Heliga trefaldighet, Malma Bergsvägen 26, managed meadow area with Picea abies and Pinus sylvestris, 16 Nov 2017, leg. M. Iwarsson & M. Krikorev, det. M. Krikorev & E. Larsson 448B-17 (GB-0159606!, isotype UPS, GenBank Acc. No. MH939180).
Etymology: Refers to the sweetish smell of bitter almonds.
Diagnosis: Small- to medium-sized species fruiting from October to December, in the central to northern parts of Europe. Resembles H. agathosmus but differs by the smaller basidiomata, a darker olive-brown disc zone on the pileus, and by being associated with Pinus spp. on acid to neutral soils.
Pileus 20–35 (− 50) mm broad, campanulate, obtusely conical with involute margin when young, soon becoming applanate, with a broad umbo or slightly depressed, sometimes becoming crenulate at margin, grey-brownish (5:3E, 5:4D) to somewhat olive-brownish (4:4F, 4:5F) in the centre, distinctly paler towards the margin, becoming more uniformly grey when drying, when moist viscid. Lamellae arcuate, subdecurrent to decurrent, L = 18–44, distant to subdistant, thick, waxy, with lamellulae, white, sometimes with a pale incarnate tint in young basidiomata. Stipe up to 5–8 × 0.5–1 cm, cylindrical, slightly clavate or tapering towards the base, fragile, glutinous when young, remaining distinctly viscid when old, first whitish with floccules especially at the top, later pale greyish brown to brownish and less pronounced flocculose. Context whitish, taste mild, smell sweetish reminding of bitter almonds, similar to H. agathosmus. Spore deposit white.
Spores [n = 190] elliptical to ovoid, sometimes with a suprahilar depression, more rarely subamygdaliform, with a distinct obtuse hilar appendage, smooth, hyaline, inamyloid, (8.4–) 9.6–9.9–10.1 (− 11.2) × (4.9–) 5.8–5.9–6.0 (−6.4) μm, average Q = 1.61–1.68–1.73. Basidia mainly 4-spored, narrowly clavate, 45–60 × 8–10 μm, sterigmata 6–8 μm long. Hymenophoral trama bilateral to slightly divergent, composed of interwoven cylindrical hyphae 4–8 μm wide and thin-walled inflated hyphae with terminal end cells up to 20 μm wide.
Pileipellis an ixotrichoderm up to 300 μm thick; made up of loosely arranged interwoven, branched hyphae 1.5–5 μm wide, in matrix smooth, hyaline or intracellular pigmented, upper layer with incrusted pigmented hyphae. In water and carbol fuchsin with visible small droplets on hyphae. Subpellis composed of densely arranged, sub-parallel interwoven hyphae up to 17 μm broad.
Stipitipellis a cutis, up to 50 μm thick, made up of 5–8 μm wide interwoven branched hyphae, with scattered free ends, smooth, hyaline, or with intracellular pigments. Stipititrama of hyaline more or less parallel interwoven hyphae 6–12 μm wide. Floccules at apex made up of compact erect branched hyphae, 3.5–5 μm wide, loosely scattered free ends, cylindrical or slightly enlarged up to 7 μm wide. In water and carbol fuchsin, with small visible droplets on hyphae.
Clamp connections present in all tissues.
Ecology and distribution
Growing solitary or in groups under Pinus sylvestris and Pinus nigra. Fruiting late in the season from October to December. So far confirmed from Germany and Sweden. However, sequence data from environmental soil samples originating from Canada suggest that the species also occurs in North America (Fig. 5).
Hygrophorus suaveolens. a Basidiomata of the holotype (EL448B-17, GB-0159606). b Basidia and spores from the lamellae edge. c Floccules at stipe apex, with spores among the scattered terminal hyphae. d Pilpeipellis hyphae with droplets, stained with carbol fuchsin. e Spores close to hymenium. f Inflated hyphae in the lamella trama
Material studied
GERMANY: Sachsen, Markkleeberg, Neue Harth Süd, reforestation area with Pinus nigra, 20 Nov 2007, leg. J. Kleine 07112001, det. J. Kleine and E. Larsson (*GB-0159604, MH656475); Ibidem, 27 Nov 2014, leg J. Kleine 14112,01, det. J. Kleine and E. Larsson (*GB-0159602, MH656477); Ibidem Markkleeberger See, reforestation area with Pinus nigra and P. sylvestris, 25 Nov 2014, leg. J. Kleine 1411201, det. J. Kleine and E. Larsson (*GB-0159603, MH656476). SWEDEN: Uppland, Heliga trefaldighet, Malma Bergsväg 26, managed meadow garden with Picea abies and Pinus sylvestris, 2 Dec 2014, leg. M. Iwarsson, det. E. Larsson (*GB-0159605, MH656478); Öland, Persnäs, Jordhamn, under Pinus sylvestris on sandy soil, 8 Dec 2017, leg. Jan Olsson, det. E. Larsson (*GB-0159600, MH656473); Ibidem, Algutsrum, S. om Törnbottens stugby, in grass under Pinus sylvestris, 31 Oct 2015, leg. M. Jeppson (MJ10154) and A. Molia, det. E. Larsson (GB-0159601); Västergötland, Göteborg, Högsbotorp, under Pinus sylvestris, 18 Nov 2016, leg. Anders Aronsson, det. E. Larsson 390-16, (*GB-0159609, MH656474).
Additional material studied
Hygophorus aff. agathosmus. CANADA: Quebec, L’Islet Co., St. Aubert, 20 Sep 1963, leg. J.W. Groves, det J.W. Groves (UPS F-528300, MH656464); Newfoundland, mixed coniferous forest, 28 Sep 2012, leg. J. Graham, det J. Graham (*MH656463). NORWAY: Trøndelag, Steinkjer, Skrattåsen, mixed forest with Picea abies, 5 Sep 2009, leg E. Larsson, M. Jeppson, det. E. Larsson, M. Jeppson (*EL179-09, MH656458); Troms, Storfjord, Lulledalen, close to Mullejokka, mixed coniferous forest on calcareous soil, under Picea abies, 30 Aug 2013, leg. E. Larsson, K. Bendiksen, J. Vauras, det E. Larsson (*EL294-13, MH656460). SWEDEN: Lule Lappmark, Jokkmokk, Kassavare, moist mixed coniferous forest with Picea abies, Betula pendula, Pinus sylvestris, 1 Sep 2011, leg E. Larsson, det E. Larsson (*EL175-11, MH656462); Ibidem, Sitoätno, mixed Picea abies dominated forest, 31 Aug 2011, leg. E. Larsson, A. Dahlberg, S. Lund, det. E. Larsson (*EL160-11, MH656459); Lycksele Lappmark, Tärna, Voitatjaure, mixed coniferous forest, 21 Aug 2015, leg. Nico Dam, det. E. Larsson (*EL141-15, MH656467); Öland, Böda, Kesnäsudden, grazed meadow area with Pinus sylvestris and Picea abies, 5 Oct 2017, leg. E. Larsson, det. E. Larsson (*EL384-17, MH656457).
Hygophorus aff. agathosmus f. albus. ICELAND: Vaglaskógur Fnósadal, Arnpórslunur, under Picea and Betula, 13 Sep 2009, leg G.G. Eyjólfsdóttir, det. G.G. Eyjólfsdóttir (*AMNH-185145, MH656461). DENMARK: Lolland, Fuglsang Storskov, under Picea abies, 3 Oct 2007, leg. E. Larsson, det. E. Larsson (*EL134-07, MH656465); Sjælland, Store Dyrehave S of Hillerød, under Picea abies, 10 Nov 1999, leg B.T. Olsen, det. B.T. Olsen (*C-F41536, MH656466).
Hygrophorus sp. SPAIN: Madrid, Zarzalejo, Puerto de la Cruz Verde, en humus de Pinus sylvestris y P. pinaster, leg. F. Prieto, A. González & et al., det. F. Prieto (*MA-F74970, MH656469). SWEDEN: Gotland, Gammelgarn, Danbo, Sjausru, sandy heathland with Pinus sylvestris, 27 Sep 2011, leg. E. Larsson, A. Stridvall, S. Jacobsson, det. E. Larsson (*EL289-11, MH656468).
Hygrophorus hyacinthinus. SLOVAKIA: Banskobystricky Kraj, Muránska Planina NP, Murán, Velka Lúka, Sedo Javorinko, rich mixed Picea forest, 10 Oct 2008, leg. E. Larsson, I. Kautmanova, M. Jeppson, det E. Larsson (EL259-08, KJ720191). SWEDEN: Gotland, Ardre, Ollajvs NR, moist Picea abies forest on calcareous soil with scattered Quercus robur, Betula pendula and Corylus avellanea, 27 Sep 2010, leg. E. Larsson, A. Stridvall, S. Jacobsson, det. E. Larsson (*EL326-10, MH656470); Medelpad, Matfors, rich forest with Picea abies on calcareous ground, 30 Aug 1995, leg. S. Muskos, det. S. Muskos (GB-0124675, HM143012).
Hygrophorus exiguus. FINLAND: Koillismaa, Kuusamo, Oulanka NP, Ampumavaara, in moist Picea abies dominated mixed forest, 18 Aug 2009, leg. F. Calledda, E. pini, G. Boerio, M. Carbone, det. E. Campo, M. Carbone, E. Larsson (TUR-A190791!, KJ720198). ITALY: Lombardia, Bergamo, Schilpario, in mossy mixed Picea abies and Abies alba forest, 6 Aug 2011, leg. Mantovani, Calledda (GB-0179914, KJ720200). SWEDEN: Västerbotten, Degerfors, Klubbrännan, in old-growth forest with Picea abies, 26 Aug 2011, leg. N. Dam, det. N. Dam, E. Larsson (GB-0179915, KJ720199); Medelpad, Borgsjö, Gammelbodarna, mixed grazed forest with Picea abies, 25 Aug 2010, leg. E. Larsson, A. Stridvall, S. Jacobsson, leg. E. Larsson (*EL187-10, MH656472); Västergötland, Skepplanda, Slereboåns dalgång NR, moist old-growth forest with Picea abies, 21Sep 2014, leg. E. Larsson, K-H Larsson, det. E. Larsson (*EL189-14, MH656471).
Hygrophorus occidentalis. USA: Michigan, Ann Arbor, Saginaw Forest, Ann, 8 April 1936, leg. Smith, det. Smith (*TENN10197!, MH656479).
Hygrophorus odoratus. USA: Oregon, Paradise Park, Mt. Hood, Clackamas Co., 20 Oct 1947, leg. A.H. Smith 28387 (MICH54605!, KJ720193); Mile Bridge, Mt. Hood Nat. Forest, 26 Oct 1947, leg. A.H. Smith 28300 (MICH28300, KJ720192).
Hygrophorus pustulatus. DENMARK: Lolland, Guldborgsund, Fuglsang, 3 Oct 2007, leg. E. Larsson, det. E. Larsson (EL130-07_KJ720196). SLOVAKIA: Banskobystricky Kraj, Veporské vrchy Mts, Cierny Balog, Dobrocsky prales Nature Reserve, 7 Oct 2008, leg. E. Larsson, det. E. Larsson (EL223-08, KJ720197).
Key to the North European species in subsection Tephroleuci
Cap colour white, grey to greyish-brown. Veil absent or fragmentary. Stipe not viscid or at most slightly viscid, usually mat when dry.
• 1. Smell distinctly sweetish . . . . . . . . . . . . . . . . . . . . . . . . . 2
- Smell indistinct . . . . . . . . . . . . . . . . . . . . . . . . H. pustulatus
• 2. Smell of Hyacinthus or Narcissus tazetta; with Picea on calcareous soils; rare . . . . . . . . . . . . . . H. hyacinthinus
- Smell of bitter almonds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
• 3. Pileus rarely exceeding 2.5–3 cm in diameter; with Picea; usually deep in moss/needle mat; often close to Tricholoma inamoenum . . . . . . . . . . . . . . . . . . H. exiguus
- Pileus on average larger to much larger than 2.5–3 cm in diameter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
• 4. Pileus white . . . . . . . . . . . . . . . . .H. agathosmus f. albus or H. cf. agathosmus (white form)
- Pileus grey to greyish brown . . . . . . . . . . . . . . . . . . . . 5
• 5. With Pinus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
- With Picea . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
• 6. On calcareous soil; pileus colour uniformly greyish brown . . . . . . . . . . . . . . . . . . . . . . . . . . . Hygrophorus sp.
- On more acid to neutral soil; pileus with distinct darker disc . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. suaveolens
• 7. Usually in rich old-growth forest . . . . . . . . . . . . . . . . . . 8
- Usually on more acid to neutral soils . . . . . H. cf. agathosmus
• 8. Floccules at stipe apex white . . . . . . . . . H. agathosmus
- Floccules at stipe apex yellow . . . . . . . H. agathosmus f. aureofloccosus
Discussion
Despite forming mycorrhiza, Hygrophorus sequences are surprisingly rare in metabarcoding studies of forest soils and therefore to a large extent missing from public database repositories. Compared to many other groups of ectomycorrhizal fungi information on genetic variation, distribution range and occurrence of Hygrophorus species are scanty. With increased sequencing of herbarium collections, new information on species diversity, ecology and distribution will become available (Moreau et al. 2018).
We identified seven clades within subsection Tephroleuci, all representing species having the characteristic sweetish smell. Three other sequences downloaded from public databases seem to represent additional taxa. One sequence identified as H. morrisii and originating from North America and another sequence generated from ectomycorrhiza of Pinus from Mexico are sister taxa to H. odoratus. The third sequence was generated from ectomycorrhizae of Arbutus menziesii originating from North America and is recovered as a sister taxon to H. agathosmus (Fig. 2). This suggests that at least ten species are present within the group. Six clades represent species that occur in Northern Europe, H. agathosmus (Fig. 4a), H. cf. agathosmus (Fig. 1d), Hygrophorus sp. (Fig. 1c), H. hyacinthinus (Fig. 1a), H. suaveolens (Figs. 1e, 5a) and H. exiguus (Fig. 1b). So far, H. odoratus is not confirmed from Europe. The three identified new species, here named H. cf. agathosmus, H. suaveolens and Hygrophorus sp. all have the same sweetish smell of bitter almonds and the name H. agathosmus has probably been applied to all of them.
Sequences of H. agathosmus specimens associated with Picea spp. are in the maximum parsimony tree split into two supported sister clades, while in the Bayesian tree the branch to the cf. agathosmus clade collapses (Fig. 2). This is surprising since the clades differ by one substitution and three 1–4 bp insertion/deletion events in the ITS1 region and one substitution and two 3 bp insertion/deletion events in the ITS2 region. The status of this taxon will be further studied, but in this paper we provisionally treat it at species level. Hygrophorus agathosmus s.s. is here neotypified with a collection from South Sweden, thus defining the name in accordance with the description by Fries. This genotype seems to be restricted to Europe, with its main distribution in the north-eastern parts, and is confirmed from Fennoscandia, Estonia and Russia, always associated with Picea abies in nutrient-rich old-growth forests on moist and often also calcareous ground.
Hygrophorus cf. agathosmus (Fig. 5d) is in morphology very similar to H. agathosmus s.s. but seems to have a much wider distribution range and is present in both Europe and North America. In Europe, it is here confirmed from Denmark, Estonia, Norway and Sweden. In Northern Europe, it seems to have its main distribution in the northern and western parts and was even collected from a Picea abies plantation on Iceland. The sequenced Canadian specimens misidentified as H. occidentalis A.H. Sm. & Hesler are nested within this clade. We studied the isotype of H. occidentalis (TENN) and generated an ITS2 sequence. Although the species has a greyish cap like H. cf. agathosmus, it differs in micromorphology e.g. by smaller spores (7.2–) 8.0 (− 8.8) × (3.8–) 4.5 (− 5.0) μm, average Q = 1.78. Smith and Hesler (1939) placed the species in subsection Fuliginei and said it was associated with oak-pine woods and lacking a specific smell. The sequence data suggest an affinity to H. eburneus (Bull.) Fr.. An older name to consider for this clade is H. cerasinus (Berk.) Berk. which has been synonymized with H. agathosmus (e.g. Hesler and Smith 1963). The species is described as having an odour exactly like the leaves of Prunus laurocerasus, a pleasant smell of bitter almonds. However, H. cerasinus was collected in a fir plantation, suggesting it may also be a name to consider for Hygrophorus sp.
Two specimens from the limestone islands in the Baltic Sea (Gotland, Sweden and Saarema, Estonia) generated sequences that cluster with a sequence from a Spanish specimen determined to H. agathosmus. All three specimens were associated with Pinus spp., and the clade is here named Hygrophorus sp. (Fig. 2). The species is clearly distinct in both sequence data and morphology, with a darker and more even greyish brown pileus (Fig. 5c). This so far unnamed species may have a predominantly South European distribution and will be dealt with in a separate paper.
Hygrophorus suaveolens was first identified from a reforestation area of a former strip-mining pit in North Western Saxony, Germany, replanted with Pinus nigra. It is characterised by the rather small basidiomata, with a pileus that is greyish brown with a darker central disc and often becomes crenulate at margin (Fig. 5a). Moreover, parts of the pileipellis and stipetipellis hyphae are densely covered with conspicuous droplets, which are easily observed in water and can be stained with carbol fuchsin (Fig. 5d), but completely disappear in Congo red NH3 KOH or SDS solutions. Nevertheless, similar droplets, but considerably smaller and less conspicuous, can also be observed in H. agathosmus. More recently, H. suaveolens was also observed in several localities in Sweden, always under Pinus sylvestris, very late in the season and often affected by frost. It is seemingly rare but might have been overlooked because of the late-season fruiting and because it is reminiscent of H. agathosmus. Three sequences from a soil environmental study of Picea mariana in Alaska were retrieved from GenBank. These sequences are nested within the H. suaveolens clade with strong support (Fig. 2), suggesting that H. suaveolense or a closely related species occurs in North America. The two genotypes differ by four to five insertion/deletion events throughout the ITS region. No collections were available for morphological comparisons.
Different forms and varieties of H. agathosmus have been described and here we can confirm that white forms of both H. agathosmus (Fig. 4b) and H. cf. agathosmus are rather common (Fig. 2), and that no sequence variation within the ITS region has been observed in comparison with grey forms. White and grey forms often fruit in the same forest, but probably develop from different mycelia. Intermediate forms, having basidiomata with pale grey pileus, are also frequently observed. The white form of H. agathosmus can easily be mistaken for H. piceae Kühner, and probably many such collections are deposited under the wrong name in our herbaria. By checking the presence of the sweetish bitter almond smell in fresh basidiomata such mistakes can easily be avoided.
The occurrence of bright yellow floccules on pileus margin and at stipe apex (Fig. 4c) is confirmed in H. agathosmus (Fig. 2). However, no specimen of H. cf. agathosmus with yellow floccules was included in this study and, thus, the occurrence of such colour form in the latter taxon could not be confirmed. One specimen included in this study was completely white and with yellow scales (GG6-17) and was thus macro-morphologically reminiscent of H. chrysodon (Batsch) Fr. However, the sweetish smell of bitter almonds is always present, making it rather easy to separate it from H. chrysodon.
References
Bidaud A (2007) Journée des espèces rares ou intéressantes 2005 – 3 parte. Bull Mycol Bot Dauphiné-Savoie 184:33–34
Bon M (1990) Flore Mycologique d’Europe. 1. Les Hygrophores. Hygrophoraceae Lotsy. Doc Mycol mém hors série 1:1–99
Candusso M (1997) Hygrophorus s. l. Fungi Europei, vol 6. Libreria Basso, Alassio
Clark K, Karsch-Mizrachi LDJ, Ostell J, Sayers E (2016) Genbank. Nuc Acid Res 44(Database issue):D67–D72. https://doi.org/10.1093/nar/gkv1276
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973 https://doi-org.ezproxy.ub.gu.se/10.1093/molbev/mss075
Gärdenfors U (ed) (2010) The 2010 red list of species. Artdatabanken, SLU, Uppsala https://www.artdatabanken.se/publikationer/bestall-publikationer/rodlistan2015/
Henriksen S, Hilmo O (eds) (2015) Norsk rødliste for arter 2015. Artsdatabanken, Norge
Hesler LR, Smith AH (1963) North American species of Hygrophorus. Kingsport Press, Knoxville
Jacobsson S, Larsson E (2007) Hygrophorus penarioides, a new species identified using morphology and ITS sequence data. Mycotaxon 99:337–343
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates SB, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Griffith TGW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín RMP, Matheny PB, Nguen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüssler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria T, Weiss M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Kornerup A, Wanscher JH (1962) Farver i farver. Politikens Forlag, København
Kovalenko A (2012) Hygrophorus Fr. In: Knudsen H, Vesterholt J (eds) Funga Nordica. Agaricoid, boletoid, cyphelloid and gasteroid genera. Nordsvamp, Copenhagen, pp 282–293
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 22:3276–3278. https://doi.org/10.1093/bioinformatics/btu531
Larsson E, Jacobsson S (2004) Controversy over Hygrophorus cossus settled using ITS sequence data from 200 year-old type material. Mycol Res 113:1154–1171
Larsson E, Jacobsson S, Stridvall A (2011) Släktet Hygrophorus, Skogsvaxskivlingar i Sverige En fältguide till SMF:s svampväkteri – Mykologiska Publikationer. 3:1–56, ISSN 1654-546x
Larsson E, Campo E, Carbone M (2014) Hygrophorus exiguus, a new species in subgenus Colorati section Olivaceoumbrini, subsection Tephroleuci. Karstenia 54:41–48
Larsson E, Vauras J, Cripps CL (2018) Inocybe praetervisa group - a clade of four closely related species with partly different geographical distribution ranges in Europe. Mycoscience 59:277–287. https://doi.org/10.1016/j.myc.2017.11.002
Lodge DJ, Padamsee M, Matheny PB, Aime MC, Cantrell SA, Boertmann D, Kovalenko A, Vizzini A, Dentinger BTM, Kirk PM, Ainsworth AM, Moncalvo J-M, Vilgalys R, Larsson E, Lücking R, Griffith GW, Smith ME, Norvell LL, Desjardin DE, Redhead SA, Ovrebo CL, Lickey EB, Ercole E, Hughes KW, Courtecuisse R, Young A, Binder M, Minnis AM, Lindner DL, Ortiz-Santana B, Haight J, Læssøe T, Baroni TJ, Geml J, Hattori T (2014) Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Divers 64:1–99. https://doi.org/10.1007/s13225-013-0259-0
Moreau P-A, Bellanger J-M, Lebeuf R, Athanassiou Z, Athanasiades A, Lambert H, Schwartz C, Larsson E, Loizides M (2018) Hidden diversity uncovered in Hygrophorus sect. Aurei (Hygrophoraceae), including the Mediterranean H. meridionalis and the North American H. boyeri spp. nov. Fungal Biol. in Press. https://doi.org/10.1016/j.funbio.2018.04.009
Nylander JAA (2004) MrModeltest v2. – Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala
Rassi P, Hyvärinen E, Juslén A, Mannerkoski I (eds) (2010) The 2010 red list of Finnish species. Helsinki, Ympäristöministeriö & Suomen ympäristökeskus, 685 p
Ronquist F, Huelsenbeck JP (2012) MrBayes 3.2, efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Smith AH, Hesler LR (1939) Studies in North American species of Hygrophorus – I: the subgenus Limacium. Lloydia 2:1–62
Strid Å (1994) A catalogue of fungus plates painted under the supervision of Elias Fries. Swedish Museum of Natural History, Stockholm
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (* and other methods). Version 4. Sinauer Associates, Sunderland
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal life style in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Acknowledgments
Curators of herbaria C, UPS, TU, AMNH and MA are gratefully acknowledged for arranging loans. Gro Gulden, Mattias Iwarsson, Jan Olsson, Anders Aronsson, G. Gyda Eyjólfsdóttir, Jan Nilsson and Mikael Jeppson for sharing interesting collections and photos. Svengunnar Ryman is acknowledged for the support on nomenclatural issues and Stefan Ekman at Herbarium UPS for the permission to publish Fries painting.
Funding
Financial support was received from The Swedish Taxonomy Initiative, ArtDatabanken SLU Uppsala and Carl Tryggers Stiftelse för Vetenskaplig Forskning.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Zhu-Liang Yang
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Larsson, E., Kleine, J., Jacobsson, S. et al. Diversity within the Hygrophorus agathosmus group (Basidiomycota, Agaricales) in Northern Europe. Mycol Progress 17, 1293–1304 (2018). https://doi.org/10.1007/s11557-018-1445-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-018-1445-y