Abstract
Purpose
The aim of this study is to determine if radiomics features extracted from staging magnetic resonance (MR) images could predict 2-year long-term clinical outcome in patients with locally advanced cervical cancer (LACC) after neoadjuvant chemoradiotherapy (NACRT).
Materials and methods
We retrospectively enrolled patients with LACC diagnosis who underwent NACRT followed by radical surgery in two different institutions.
Radiomics features were extracted from pre-treatment 1.5 T T2w MR images.
The predictive performance of each feature was quantified in terms of Wilcoxon–Mann–Whitney test. Among the significant features, Pearson correlation coefficient (PCC) was calculated to quantify the correlation among the different predictors. A logistic regression model was calculated considering the two most significant features at the univariate analysis showing the lowest PCC value.
The predictive performance of the model created was quantified out using the area under the receiver operating characteristic curve (AUC).
Results
A total of 175 patients were retrospectively enrolled (142 for the training cohort and 33 for the validation one).
1896 radiomic feature were extracted, 91 of which showed significance (p < 0.05) at the univariate analysis. The radiomic model showing the highest predictive value combined the features calculated starting from the gray level co-occurrence-based features. This model achieved an AUC of 0.73 in the training set and 0.91 in the validation set.
Conclusions
The proposed radiomic model showed promising performances in predicting 2-year overall survival before NACRT. Nevertheless, the observed results should be tested in larger studies with consistent external validation cohorts, to confirm their potential clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer (CC) is the fourth most frequent cancer and the fourth leading cause of cancer death in women worldwide [1].
The recommended standard treatment of patients with locally advanced cervical cancer (LACC) is currently represented by concomitant chemoradiotherapy (CRT), administered with weekly cisplatin followed by intrauterine brachytherapy [2, 3].
Despite the recent improvements related to these therapeutic strategies, the long-term overall survival rate still ranges about 57–67%, urging novel therapeutic strategies [4].
Local recurrence and distant metastasis remain the main causes of therapeutic failure in CC, proving that the microscopic residual disease, often undetectable with current diagnostic restaging strategies, represents the major risk factor for treatment failure observed after CRT.
In patients with limited response to CRT, neoadjuvant chemoradiotherapy (NACRT) followed by radical hysterectomy seems to be a valuable option [5,6,7].
Several experiences demonstrated that surgery has the potential to remove radio- and chemo-resistant neoplastic foci, improving local control and possibly overall survival. [8,9,10].
In addition, surgery represents a valid alternative to utero-vaginal brachytherapy, where brachytherapy equipment and specialists are or limited.
Magnetic resonance imaging (MRI) is currently considered the standard technique for CC local staging and prognosis evaluation before treatment, where T2-W and diffusion-weighted imaging (DWI) represent the mainstay of diagnostic sequences [11, 12].
A recent pilot study underling the efficacy of MRI in the assessment of treatment response after neoadjuvant chemotherapy plus cold knife conization, in patient affected by early CC (FIGO stage IB2- IIA1) [13].
Radiomics is a translational field of research consisting of mathematical-statistical procedures for extracting data from standard radiological images, resulting in quantitative features that describe tumor heterogeneity and other intrinsic characteristics related to its biological behavior [14,15,16,17].
It has been observed in several experiences that radiomic features extracted from MR images have the potential to predict staging, histology, node status, relapse and survival [18,19,20].
Great interest has been recently paid to radiomics applications focused on CC, with studies predicting treatment response, or long-term outcomes [21,22,23,24].
A recent study investigated the potential role of radiomics in predicting pathological complete response after NACRT in LACC patients, reporting promising performances in terms of receiver operating characteristic curve [25].
Prediction of outcome besides treatment response can be useful to tailoring therapeutic treatment scheduling, potentially reducing treatment toxicity in gynecology cancer [26].
Aim of this study is to determine if radiomics features extracted from T2-weighted 1.5 T MRI could predict 2-year local control (2yLC), distant metastasis-free survival (2yDMFS) and overall survival (2yOS) in patients affected by LACC and treated with NACRT followed by radical hysterectomy.
Materials and methods
Patient enrollment and treatment
We retrospectively enrolled patients affected by LACC, staged IB2 to IVA from International Federation of Gynecology and Obstetrics (FIGO) 2018, treated with NACRT followed by radical hysterectomy plus pelvic lymphadenectomy after 6–8 weeks.
Patients were recruited from two different institutions: Institution A, an academic tertiary hospital and Institution B, a non-academic tertiary center.
This study was approved by the ethics committee of Institution A.
Inclusion criteria were as follows: histological confirmed invasive carcinoma of the cervix, FIGO stage from IB2 to IVA and absence of distant metastasis.
Patients with incomplete documentation, younger than 18 years, treated with palliative intent and did not undergo surgery were excluded.
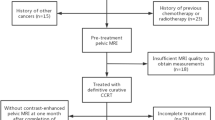
Final cohort of institution A (training set) consisted of 142 patients (from a total of 157, 2 patients have been excluded for progression during NACRT, 13 patients were lost to follow-up); final cohort of institution B (validation set) included 33 patients (from a total of 37 patients, 4 were lost to follow-up) (Fig. 1).
Before treatment, all patients underwent pelvic MRI and total body contrasted enhanced CT scan as staging imaging. 18F-FDG PET-CT was considered and performed only in few selected cases.
All patients included in the analysis underwent NACRT with concurrent weekly cisplatin (Cisplatin dose: 40 mg/sm) alone or plus 5-fluorouracil (Cisplatin dose: 20 mg/sm+5-Fluorouracil dose: 1000 mg) during the first and the last week of treatment.
Radiotherapy volumes were delineated according to consensus guidelines for the clinical target volume (CTV), and organs-at-risk.
CTV was defined as primary tumor (CTV1) plus whole pelvis (CTV2).
In the case of common iliac or lumbo-aortic lymph nodes involvement (cN+) at the staging imaging, lumbo-aortic lymph nodes were also added to the CTV2.
Planning target volumes (PTV) were considered as isotropic expansion of 5 mm from the relative CTV.
Radiotherapy treatment was administered using a simultaneous integrated boost technique delivered in 22 fractions and prescribing 50.6 Gy to PTV1 and 39.6 Gy to PTV2.
All patients were re-evaluated with a pelvic MRI and 18F-FDG PET-CT, according to clinical FIGO stage, for local restaging and subsequently referred to surgery, which was carried out with a laparotomy approach between 7 and 8 weeks from term of radiotherapy.
All patients underwent a Radical hysterectomy ± bilateral annessiectomy performed according to Querleu Morrow’s criteria in relation to the response to radiation treatment, verified by radiological imaging [27].
Systematic pelvic lymphadenectomy was always performed; para-aortic lymph node dissection was added if pelvic or para-aortic lymph node were positive at the pre-treatment imaging or at the intra-operatory frozen section of the pelvic lymph nodes.
Pathological response to treatment was evaluated on surgical specimens.
Pathological complete response was defined as absence of any residual tumor after treatment at any site; microscopic response (pR1) as persistent tumor foci of maximum dimension inferior to 3 mm; macroscopic response (pR2) as persistent tumor foci with maximum dimension exceeding 3 mm [28].
Collected data included age, histology, FIGO stage, presence of positive lymph nodes on PET/CT and pelvic MRI and clinical status at 2 years from surgery.
Treatment outcome evaluation
Post-treatment surveillance consisted of follow-up visits every 3 months for the first 2 years and every 6 months thereafter.
All the patients with an observation period equal to or longer than 2 years with information relative to diagnostic imaging, surgery and pathological staging were included in the analysis.
Pelvic examination, serum tumor markers, abdominopelvic CT or pelvic MRI or PET-CT scans were performed at each follow-up visit, according to clinical stage.
2yLC, 2yDMFS and 2yOS were considered as treatment outcome, using the end of radiotherapy as reference time point.
Local control outcome (LC) was defined as the interval between the date of treatment completion and the date of tumor local recurrence. Distant metastasis-free survival was defined as the interval between the date of treatment completion and the date of onset of distance recurrence.
Overall survival (OS) was defined as the interval between the date of treatment and the date of cancer related death.
Image acquisition protocol, image analysis and features extraction
Radiomic analysis was focused on pre-treatment axial T2-weighted MR images, acquired for tumor staging according to institutional staging protocols. The acquisition parameters adopted are reported in Table 1. A total of 564 radiomic features were extracted for each considered clinical outcome.
Gross tumor volume was considered as region of interest; it was manually contoured in consensus by one radiation oncologist and one radiologist, experts in gynecological imaging, using a radiotherapy treatment planning system (Eclipse, Varian Medical Systems, Palo Alto, CA, USA). (
MR images were resampled to a planar resolution of 0.548 × 0.548 mm2 and preprocessed using the Laplacian of Gaussian filter, considering the filter widths (σ) ranging from 0 to 4.2 mm with steps of 0.35 mm 4–5. Radiomic analysis was performed using Moddicom, a radiomic software included in the IBSI initiative [19, 29, 30].
Three radiomic features families were extracted for the analysis: morphological features were extracted from raw images, while statistical and textural features were extracted from the filtered one [31, 32].
With regards to the textural features, three gray level matrices were considered: run length (rlm), co-occurrence (cm) and size zone (szm) matrices. The complete list of the extracted radiomic features is reported in the supplementary materials and in similar experiences of our group about this topic [32, 33].
Statistical analysis
The heterogeneity between training and validation cohorts was evaluated using χ2 test for categorical features and t test for continuous ones [31].
A comprehensive database including radiomic features, clinical data and outcomes was created.
For each radiomic feature object of the analysis, the normality of data distribution was evaluated using the Shapiro–Wilk test. The ability of each single feature in predicting at the univariate analysis the dichotomic outcomes object of this study (presence or absence of 2yLC, 2yDFS, 2yOS) was evaluated using the Wilcoxon–Mann–Whitney (WMW) test for features showing data characterized by non-Gaussian distribution and t test for data with Gaussian distribution [34, 35].
Feature showing the lowest p value was considered as the most significant feature and selected as first feature for model creation: the use of WMW test was recently considered the most accurate and robust method for feature selection in Radiomics [36]
For each clinical outcome object of this study, different linear logistic regression models with two variables were elaborated, combining the most significant feature at the univariate analysis (lowest p value) with all the others radiomic features object of this study [34].
The predictive performance of the different models created was quantified using the area under the receiver operating characteristic (ROC) curve (AUC) [37].
The logistic model showing the highest AUC value was considered as the best predictive model and evaluated on the validation set. The 95% confidence intervals of the AUC value were calculated using the bootstrap method with 2000 iterations.
The best cut-off threshold was identified maximizing the Youden Index (J), and values of sensitivity and specificity at the best threshold were calculated [33, 38].
The statistical analysis was performed using R software (version 3.6.1, Wien Austria) and dedicated packages [39].
Results
Clinical characteristics
A total of 175 patients with LACC were analyzed, 142 in the training cohort and 33 in the validation one).
Table 2 summarizes the clinical characteristics of both cohorts.
At the end of the follow-up period, 2yLC was observed in 82% of patients in the training cohort and in 78% of patients in the validation cohort; 2yDMFS was observed in 73% of patients in the training cohort and in 82% of patients in the validation cohort; and 2yOS was observed in 86% of patients in the training cohort and in 88% of patients in the validation one.
Radiomic models
A total of 10 features showed significance (p < 0.05) at the univariate analysis for the prediction of 2yOS, 19 features for the 2yLC and 62 for the 2yDMFS. The list of the significant features is reported in the supplementary materials for each outcome.
The two-variables predictive model for 2yDMFS showed moderate performance in training set (0.68) and limited performance in the validation set (0.55).
The predictive model for 2yLC showed moderate performance in both dataset (0.71 in training and validation). The only model showing good performance both in training and validation set was the one predicting 2yOS, with an AUC of 0.77 (95% Confidence interval of 0.70–0.91) in the training set and 0.91 (0.70–1) in the validation set.
The 2yOS model combined two textural features: the correlation based on gray level co-occurrence matrix, calculated after the application of the LoG filter at 0.7 mm and the variance calculated on the size zone matrix after the LOG application at 0.5 mm.
The ROC curves of the three models elaborated are reported in Fig. 2, while the predictive performance is summarized in Table 3. The parameters and the coefficient of all the three models are reported in supplementary materials.
Discussion
Several studies aimed to predict and monitor treatment response and clinical outcome analyzing functional imaging, such as MRI or 18-FDG PET-TC in patients affected by cervical cancer [24, 40,41,42].
In particular, some experiences analyzed temporal variations in tumor heterogeneity patterns on functional imaging such as dynamic contrast enhanced MRI, DWI [24] and FDG PET-CT [42] performed before, during and after CRT course to correlate imaging biomarkers with treatment response outcomes, so defining novel promising prognostic factors.
Our previous experience demonstrated the possibility of using radiomics based on staging MRI to predict pathological complete response after NACRT in cervical cancer. [25]
Despite the important efforts made in terms of treatment response prediction, only few experiences explored the correlation between radiomic predictors and long-terms outcomes, such as distant recurrence or overall survival in cervical cancer [22, 43, 44].
In a study proposed by Ho et al., the mean ADC value extracted from the primary tumor in pre-treatment DWI MR was indicated as a predictor of disease-free survival for CC patients treated with CRT. The authors found that a higher mean ADC value may result in a higher probability of disease-free survival and OS.
The mean ADC value of the primary tumor on pre-treatment MRI was the only imaging feature which resulted to be an independent predictor of disease-free survival [22]
Similar experiences were reported in rectal cancer, where delta radiomic models analyzed the variation of radiomics features extracted from staging and post-treatment MRI to predict tumor behavior and long-term outcomes, such as distant metastasis-free survival and OS [45].
In this study, we aimed to identify radiomic features from staging T2-w MR images which are able to predict 2-year clinical dichotomic outcomes, obtaining promising results in case of OS prediction, but only limited results for LC and distant metastasis--free survival.
In particular, it emerged that the features with the highest performance in predicting the presence of OS after 2 years from the end of treatment are based on the textural analysis.
Being able to predict a long-term outcome from pre-treatment MR images could allow patients to be stratified in different risk groups besides the ones generally used in clinical practice to define the most appropriate treatment schedule (e.g., disease stage).
Furthermore, identifying patients with higher risk of local or distant recurrences could guide clinicians to personalize follow-up schedules or early intercept local or distant recurrence that could represent therapeutic failure. On the other side, less invasive tailored therapeutic strategies may be pursued in patients predicted with low OS probability.
Different limitations burden this work. First of all, the limited replicability of patients setting, as neoadjuvant setting, does not represent the standard of treatment in LACC patient and is not adopted by the largest majority of the centers.
Despite the sound methodology applied (TRIPOD type 3) [46, 47], promising specificity and sensitivity values were reached only in the prediction of 2yOS, this being partly related to the relatively small sample size.
Lastly, the lack of a biological interpretation of the significant features limits the translational value of this experience, even if this issue is not considered mandatory for radiomics study [48].
Future studies are being planned to integrate such evidence with clinical, histopathological and molecular data, which would allow to build multi-omics predictive models to be tested in larger cohorts of patients.
Conclusion
In this study, a radiomic model was proposed able to predict 2yOS in LACC patients before undergoing NACRT.
An accurate outcome prediction before or during oncological treatments could be an added clinical value to provide a guidance for clinicians in their decision-making process to adapt and tailoring treatment.
To confirm the reliability of these results and translate the use of this model into clinical practice, larger studies with external validation are required.
Data availability
Data will be made available on reasonable request due to restrictions, e.g., privacy or ethical.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CC:
-
Cervical cancer
- CRT:
-
Chemoradiotherapy
- DWI:
-
Diffusion-weighted imaging
- FIGO:
-
International federation of gynecology and obstetrics
- LACC:
-
Locally advanced cervical cancer
- MRI:
-
Magnetic resonance imaging
- MR:
-
Magnetic resonance
- NACRT:
-
Neoadjuvant chemoradiotherapy
- OS:
-
Overall survival
- PTV:
-
Planned target volume
- 2yDMFS:
-
Two-year distant metastasis-free survival
- 2yLC:
-
Two-year local control
- 2yOS:
-
Two-year overall survival
References
H Sung J Ferlay RL Siegel M Laversanne I Soerjomataram A Jemal 2020 Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Canc J Clini https://doi.org/10.3322/CAAC.21660
R Pötter K Tanderup C Kirisits A Leeuw de K Kirchheiner R Nout 2018 The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies Clin Transl Radiat Oncol 9 48 60 https://doi.org/10.1016/j.ctro.2018.01.001
NR Abu-Rustum CM Yashar S Bean K Bradley SM Campos HS Chon 2020 NCCN guidelines insights: cervical cancer, version 1 J Nat Compreh Cancer Network JNCCN https://doi.org/10.6004/JNCCN.2020.0027
C Marth F Landoni S Mahner M McCormack A Gonzalez-Martin N Colombo 2017 Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Annal Oncol https://doi.org/10.1093/annonc/mdx220
M Resbeut D Cowen P Viens M Noirclerc T Perez J Gouvernet 1994 Concomitant chemoradiation prior to surgery in the treatment of advanced cervical carcinoma Gynecol Oncol 54 68 75 https://doi.org/10.1006/GYNO.1994.1168
PE Colombo MM Bertrand M Gutowski A Mourregot M Fabbro B Saint-Aubert 2009 Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: Surgical morbidity and oncological results Gynecol Oncol 114 404 409 https://doi.org/10.1016/J.YGYNO.2009.05.043
G Ferrandina F Legge A Fagotti F Fanfani M Distefano A Morganti 2007 Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: Safety, outcome, and prognostic measures Gynecol Oncol 107 S127 S132 https://doi.org/10.1016/j.ygyno.2007.07.006
F Fanfani E Vizza F Landoni P Iaco de G Ferrandina G Corrado 2016 Radical hysterectomy after chemoradiation in FIGO stage III cervical cancer patients versus chemoradiation and brachytherapy: complications and 3-years survival Eur J Surg Oncol 42 1519 1525 https://doi.org/10.1016/J.EJSO.2016.05.011
D Mariagrazia F Anna F Gabriella F Francesco S Daniela D Giuseppe 2005 Preoperative chemoradiotherapy in locally advanced cervical cancer: Long-term outcome and complications Gynecol Oncol 99 S166 S170 https://doi.org/10.1016/j.ygyno.2005.07.074
G Ferrandina A Gambacorta V Gallotta D Smaniotto A Fagotti L Tagliaferri 2014 Chemoradiation With concomitant boosts followed by radical surgery in locally advanced cervical cancer: long-term results of the ROMA-2 prospective phase 2 study Int J Radiat Oncol Biol Phys 90 778 785 https://doi.org/10.1016/J.IJROBP.2014.07.033
E Sala AG Rockall SJ Freeman DG Mitchell C Reinhold 2013 The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know Radiology 266 717 740 https://doi.org/10.1148/RADIOL.12120315
P Balcacer A Shergill B Litkouhi 2019 MRI of cervical cancer with a surgical perspective: staging, prognostic implications and pitfalls Abdom Radiol (New York) https://doi.org/10.1007/S00261-019-01984-7
L Russo B Gui M Miccò C Panico R Vincenzo De F Fanfani 2021 Diagnostic imaging in oncology The role of MRI in cervical cancer > 2 cm (FIGO stage IB2-IIA1) conservatively treated with neoadjuvant chemotherapy followed by conization: a pilot study Radiol med https://doi.org/10.1007/s11547-021-01377-1
P Lambin E Rios-Velazquez R Leijenaar S Carvalho RGPM Stiphout Van P Granton 2012 Radiomics: extracting more information from medical images using advanced feature analysis Eur J Cancer 48 441 446 https://doi.org/10.1016/j.ejca.2011.11.036
RJ Gillies PE Kinahan H Hricak 2016 Radiomics: images are more than pictures, they are data Radiology 278 563 577 https://doi.org/10.1148/radiol.2015151169
P Lambin RTH Leijenaar TM Deist J Peerlings EEC Jong de J Timmeren van 2017 Radiomics: the bridge between medical imaging and personalized medicine Nat Rev Clin Oncol 14 749 762 https://doi.org/10.1038/nrclinonc.2017.141
Z Liu S Wang D Dong J Wei C Fang X Zhou 2019 The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges Theranostics 9 1303 1322 https://doi.org/10.7150/thno.30309
V Nardone L Boldrini R Grassi D Franceschini I Morelli C Becherini 2021 Radiomics in the setting of neoadjuvant radiotherapy: a new approach for tailored treatment Cancers 13 3590 https://doi.org/10.3390/cancers13143590
D Cusumano N Dinapoli L Boldrini G Chiloiro R Gatta C Masciocchi 2018 Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer Radiologia Medica 123 286 295 https://doi.org/10.1007/s11547-017-0838-3
L Boldrini D Cusumano G Chiloiro C Casà C Masciocchi J Lenkowicz 2019 Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach La Radiologia Medica https://doi.org/10.1007/s11547-018-0951-y
M Fang Y Kan D Dong T Yu N Zhao W Jiang 2020 Multi-habitat based radiomics for the prediction of treatment response to concurrent chemotherapy and radiation therapy in locally advanced cervical cancer Front Oncol 10 563 https://doi.org/10.3389/fonc.2020.00563
JC Ho PK Allen PR Bhosale GM Rauch CD Fuller ASR Mohamed 2017 Diffusion-weighted MRI as a predictor of outcome in cervical cancer following chemoradiation Int J Radiat Oncol Biol Phys 97 546 https://doi.org/10.1016/J.IJROBP.2016.11.015
AM Perrone G Dondi M Coe M Ferioli S Telo A Galuppi 2020 Predictive role of MRI and 18F FDG PET response to concurrent chemoradiation in T2b cervical cancer on clinical outcome: a retrospective single center study Cancers 12 659 https://doi.org/10.3390/cancers12030659
SR Bowen WTC Yuh DS Hippe W Wu SC Partridge S Elias 2018 Tumor radiomic heterogeneity: multiparametric functional imaging to characterize variability and predict response following cervical cancer radiation therapy J Magn Reson Imaging 47 1388 1396 https://doi.org/10.1002/JMRI.25874
B Gui R Autorino M Miccò A Nardangeli A Pesce J Lenkowicz 2021 Pretreatment MRI radiomics based response prediction model in locally advanced cervical cancer Diagnostics 11 631 https://doi.org/10.3390/diagnostics11040631
D Albano M Benenati A Bruno F Bruno M Calandri D Caruso 2021 Imaging side effects and complications of chemotherapy and radiation therapy: a pictorial review from head to toe Insight Imag https://doi.org/10.1186/s13244-021-01017-2
D Querleu CP Morrow 2008 Classification of radical hysterectomy Lancet Oncol 9 297 303 https://doi.org/10.1016/S1470-2045(08)70074-3
GF Zannoni VG Vellone A Carbone 2008 Morphological effects of radiochemotherapy on cervical carcinoma: a morphological study of 50 cases of hysterectomy specimens after neoadjuvant treatment Int J Gynecol Pathol: Offi J Int Soci Gynecol Pathol 27 274 281 https://doi.org/10.1097/PGP.0B013E31815B1263
R Gatta M Vallati N Dinapoli C Masciocchi J Lenkowicz D Cusumano 2019 Towards a modular decision support system for radiomics: a case study on rectal cancer Artif Intell Med 96 145 153 https://doi.org/10.1016/J.ARTMED.2018.09.003
D Cusumano G Meijer J Lenkowicz G Chiloiro L Boldrini C Masciocchi 2021 A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer Radiol Medica 126 421 429 https://doi.org/10.1007/S11547-020-01266-Z/FIGURES/2
A Zwanenburg M Vallières MA Abdalah HJWL Aerts V Andrearczyk A Apte 2020 The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping Radiology 295 328 338 https://doi.org/10.1148/RADIOL.2020191145
D Cusumano L Boldrini P Yadav G Yu B Musurunu G Chiloiro 2021 Delta radiomics for rectal cancer response prediction using low field magnetic resonance guided radiotherapy: an external validation Physica Med 84 186 191 https://doi.org/10.1016/j.ejmp.2021.03.038
D Cusumano L Boldrini P Yadav G Yu B Musurunu G Chiloiro 2020 External validation of early regression index (ERITCP) as predictor of pathologic complete response in rectal cancer using magnetic resonance-guided radiation therapy Int J Radiat Oncol Biol Phys 108 1347 1356 https://doi.org/10.1016/j.ijrobp.2020.07.2323
J Taylor 1997 Introduction to error analysis, the study of uncertainties in physical measurements 2 University Science Books NY
D Cusumano L Boldrini P Yadav C Casà SL Lee A Romano 2021 Delta radiomics analysis for local control prediction in pancreatic cancer patients treated using magnetic resonance guided radiotherapy Diagnostics 11 72 https://doi.org/10.3390/diagnostics11010072
C Parmar P Grossmann J Bussink P Lambin HJWL Aerts 2015 Machine learning methods for quantitative radiomic biomarkers Sci Rep 5 13087 https://doi.org/10.1038/srep13087
ICRU Report 79, Receiver Operating Characteristic (ROC) Analysis in Medical Imaging – ICRU n.d. https://www.icru.org/report/receiver-operating-characteristic-roc-analysis-in-medical-imaging-icru-report-79/ (accessed December 1, 2021)
MD Ruopp NJ Perkins BW Whitcomb EF Schisterman 2008 Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection Biometr J Biometr Zeitsch 50 419 430 https://doi.org/10.1002/BIMJ.200710415
X Robin N Turck A Hainard N Tiberti F Lisacek JC Sanchez 2011 pROC: an open-source package for R and S+ to analyze and compare ROC curves BMC Bioinfor https://doi.org/10.1186/1471-2105-12-77
JK Schwarz BA Siegel F Dehdashti PW Grigsby 2012 Metabolic response on post-therapy FDG-PET predicts patterns of failure after radiotherapy for cervical cancer Int J Radiat Oncol Biol Phys 83 185 190 https://doi.org/10.1016/J.IJROBP.2011.05.053
GM Lima A Matti G Vara G Dondi N Naselli EM Crescenzo De 2018 Prognostic value of posttreatment 18F-FDG PET/CT and predictors of metabolic response to therapy in patients with locally advanced cervical cancer treated with concomitant chemoradiation therapy: an analysis of intensity- and volume-based PET parameters Eur J Nucl Med Mol Imaging 45 2139 2146 https://doi.org/10.1007/S00259-018-4077-1/TABLES/4
F Yang MA Thomas F Dehdashti PW Grigsby 2013 Temporal analysis of intratumoral metabolic heterogeneity characterized by textural features in cervical cancer Eur J Nucl Med Molecul Imag https://doi.org/10.1007/S00259-012-2332-4
F Lucia D Visvikis MC Desseroit O Miranda JP Malhaire P Robin 2018 Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy Eur J Nucl Med Mol Imag 45 768 786 https://doi.org/10.1007/s00259-017-3898-7
A Yao Z Haiyan X Congying J Xiance 2020 Radiomics in cervical cancer: Current applications and future potential Critical Rev OncolHematol https://doi.org/10.1016/J.CRITREVONC.2020.102985
G Chiloiro P Rodriguez-Carnero J Lenkowicz C Casà C Masciocchi L Boldrini 2020 Delta radiomics can predict distant metastasis in locally advanced rectal cancer: the challenge to personalize the cure Front Oncol 10 2680 https://doi.org/10.3389/FONC.2020.595012/BIBTEX
GS Collins JB Reitsma DG Altman KGM Moons 2015 Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement Ann Intern Med 162 55 63 https://doi.org/10.7326/M14-0697
AR Localio CB Stack 2015 TRIPOD: a new reporting baseline for developing and interpreting prediction models Ann Inter Med https://doi.org/10.7326/M14-2423
L Fournier L Costaridou L Bidaut N Michoux FE Lecouvet LF Geus-Oei de 2021 Incorporating radiomics into clinical trials: expert consensus endorsed by the european society of radiology on considerations for data-driven compared to biologically driven quantitative biomarkers Eur Radiol 31 6001 6012 https://doi.org/10.1007/S00330-020-07598-8
Funding
Open access funding provided by Catholic University of the Sacred Heart within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
RA, BG, LB and DC involved in conception and design; GP, LR and SP involved in data collection; DC and LB involved in analysis and interpretation of data; BG, RA, GM, and MC involved in resources; GP and LB involved in manuscript writing—original draft preparation; RA, BG, LR, AN, GM, GF, MAG, VV, and RM involved in review and editing:; MAG, GF, VV, and RM involved in supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is an observational study conducted according to the guidelines of the Declaration of Helsinki and it was reviewed and approved by the Ethics Committee of Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Prot. ID CE 4635). All authors have approved the final article.
Informed consent
Where applicable, informed consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Autorino, R., Gui, B., Panza, G. et al. Radiomics-based prediction of two-year clinical outcome in locally advanced cervical cancer patients undergoing neoadjuvant chemoradiotherapy. Radiol med 127, 498–506 (2022). https://doi.org/10.1007/s11547-022-01482-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01482-9