Abstract
As climate changes, maintenance of yield stability requires efficient selection for drought tolerance. Drought-tolerant cultivars have been successfully but slowly bred by yield-based selection in arid environments. Marker-assisted selection accelerates breeding but is less effective for polygenic traits. Therefore, we investigated a selection based on phenotypic markers derived from automatic phenotyping systems. Our trial comprised 64 potato genotypes previously characterised for drought tolerance in ten trials representing Central European drought stress scenarios. In two trials, an automobile LIDAR system continuously monitored shoot development under optimal (C) and reduced (S) water supply. Six 3D images per day provided time courses of plant height (PH), leaf area (A3D), projected leaf area (A2D) and leaf angle (LA). The evaluation workflow employed logistic regression to estimate initial slope (k), inflection point (Tm) and maximum (Mx) for the growth curves of PH and A2D. Genotype × environment interaction affected all parameters significantly. Tm(A2D)s and Mx(A2D)s correlated significantly positive with drought tolerance, and Mx(PH)s correlated negatively. Drought tolerance was not associated with LAc, but correlated significantly with the LAs during late night and at dawn. Drought-tolerant genotypes had a lower LAs than drought-sensitive genotypes, thus resembling unstressed plants. The decision tree model selected Tm(A2D)s and Mx(PH)c as the most important parameters for tolerance class prediction. The model predicted sensitive genotypes more reliably than tolerant genotype and may thus complement the previously published model based on leaf metabolites/transcripts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural drought stress occurs when water supply by precipitation and soil water reserves does not meet the crop’s water demand (West et al. 2019). Drought is driven by precipitation and by evapotranspiration rates, which increase with air temperature. Models on global climate change predict rising air temperature and altered precipitation patterns, thus increasing the likelihood of drought events (Harrison et al. 2014). Yield limitation by drought will threaten food security (Basu et al. 2016; Farooq et al. 2009). Water-efficient and drought-tolerant crops that produce stable yields under reduced water supply would alleviate the problem. Potato (Solanum tuberosum) is highly water efficient (Aliche et al. 2018), making potato together with cassava and maize the most important staple crops in water-limited agroenvironments. However, Solanum tuberosum ssp. tuberosum is highly sensitive to soil water deficits, which result in stomatal closure and reduce photosynthesis at relatively high leaf water potentials (Aliche et al. 2018). The genepools of both the Andean relative of Solanum tuberosum (Schafleitner et al. 2007) and modern tetraploid Solanum tuberosum ssp. tuberosum cultivars (Aliche et al. 2018; Sprenger et al. 2015) contain genes that increase yield stability under arid conditions. However, breeding for drought tolerance is challenging, as tolerance is a multigene trait, with a substantial interaction between genotype and environment, stress timing and stress intensity (Tardieu 2012). Furthermore, drought response interacts with other abiotic stressors like heat (Millet et al. 2016). Direct selection for yield in arid environment was effective, but is very time-consuming due to the need for repeated yield trials in the target environment (Richards et al. 2014). This triggered the quest for secondary traits that are easily measureable, highly heritable and genetically correlated with yield (Bänziger et al. 2006; Lafitte et al. 2003). Obviously, DNA markers are highly heritable and easily measurable already in seedlings. DNA markers comprise single QTL-markers for marker-assisted selection (MAS) or multigene information for genomic selection. QTL-marker-based selection has been very successful for the selection of traits that rely on single genes, especially disease resistance in potato and submergence resistance in rice (Oladosu et al. 2020; Slater et al. 2014; Xu et al. 2006). In segregating rice populations, QTL explain a high percentage of yield variation under drought conditions (Venuprasad et al. 2009). However, there are only limited examples of successful breeding for drought tolerance or yield based on QTL markers alone (Beyene et al. 2019; Hammer et al. 2006; Rauf et al. 2016). Genomic selection is highly successful in breeding for complex traits, but model parameterisation requires huge datasets containing both genomic and phenotypic information (Hayes et al. 2009). While sequencing costs have dropped, phenotyping remains a bottleneck (Furbank & Tester 2011; Rauf et al. 2016). This bottleneck is especially tight in drought tolerance models, as training datasets have to be produced on many genotypes in the target environment or in managed field trials. As an alternative to genomic markers for yield prediction, morphological, physiological or biochemical markers have been suggested (Lafitte et al. 2003; Rauf et al. 2016). If these markers are less sensitive to the genetic background than DNA markers (de Roos et al. 2009), the transfer of the prediction models between populations will be facilitated. In maize, metabolite profiling on 289 inbred lines revealed a strong association between lignin precursor metabolites and plant height or dry matter yield (Riedelsheimer et al. 2012). In commercial potato cultivars, drought tolerance, estimated as yield stability, was predicted from leaf metabolite and transcript data with an accuracy of more than 90% independent of the agroenvironment (Sprenger et al. 2018). We used this model in a subsequent validation experiment to select for and against drought tolerance from a segregating population (Haas et al. 2020). To mimic selection in arid environments, we selected additional drought-tolerant lines based on yield data from three drought trials. The subsequent drought tolerance quantification in 10 multi-environment trials revealed an overrepresentation of tolerant lines in both populations selected for superior tolerance. The transcript/metabolite marker-based selection allowed selection in an earlier stage and independent of drought treatment. However, it was inefficient in selecting against sensitive genotypes (Haas et al. 2020). A second layer of selection based on morphological or physiological markers may solve the problem. Among the morphological traits associated with yield and drought tolerance are canopy development parameters, the stay-green trait and the canopy temperature (Aliche et al. 2018; Bojacá et al. 2011; Jensen et al. 2010; Prashar et al. 2013). Automatic phenotyping platforms allow quantifying these traits in a limited set of phenotypes with high temporal resolution in controlled environments (Berger et al. 2012; Furbank and Tester 2011; Granier et al. 2006; Junker et al. 2015). RGB-images taken under standardised light conditions provide estimates of plant height, shoot and leaf area (Berger et al. 2012; Granier et al. 2006; Junker et al. 2015). The required standardisation impedes the use of the method under field conditions, where light intensities and qualities change rapidly. On the other end of the scale, remote sensing by satellite-based infrared and microwave sensors assess vegetation cover and standardised precipitation index to predict drought events (Jindo et al. 2021; West et al. 2019), employing the close correlation between infrared reflection and canopy cover (Haverkort et al. 1991). The spatial resolution has been improved to a few metre, but the correlation between remotely measured vegetation indices and yield are still weak (Kempenaar et al. 2017; West et al. 2019). Thus, close range measurements are still phenotyping options for yield prediction. Therefore, we decided to use close-range sensors that moved every 4 h above the canopy of the test plot on an automobile irrigation beam (Fieldscan, Phenospex). The system measured canopy development in a polytunnel under naturally variable environmental conditions, in which potatoes were cultivated under optimal and reduced irrigation. As light conditions were highly variable, we decided against RGB imaging and for the commercial, laser-scanner based system Planteye (Phenospex). We phenotyped a population of potato lines segregating for drought tolerance for several weeks with the aim to identify morphological markers for drought tolerance.
Material and Methods
Drought Stress Trial
LIDAR measurements were performed in two drought stress experiments (B4 and B6, see Supplemental Table S1). These experiments were part of a series of 14 container and field trials, in which the drought tolerance of 64 Solanum tuberosum ssp. tuberosum genotypes was determined. The test population comprised 60 genotypes selected from two crosses between the drought-tolerant cultivar A and two drought-sensitive potato cultivars E and R, the drought-tolerant check cultivar Desiree and the three parent cultivar (details of the pedigree see Supplemental Table S2 and (Haas et al. 2020)). The maturity group of the population was medium.
The experiments were performed in the polytunnel of the Max Planck Institute of Molecular Plant Physiology in Potsdam-Golm, Germany (52° 23′ 55″ N 13° 03′ 56″ E) in spring 2015 (B4, Id 72247) and 2016 (B6, Id 76240) (details see Supplemental Table S1). Micro-propagated cuttings were pre-cultivated as described in Sprenger et al. (2015) and transferred to 30-l big-bags filled with a peat-based potato substrate fertilised with 30 g Novatec classic per bag (see Fig. 1a). The design was a randomised split-plot design, with one block for optimal water supply (40–60% of field capacity, control) and one block for reduced water supply (stress). In 2015, 6 pots per treatment and genotype were grouped in two randomised subplot of three pots. In 2015, 5 pots per treatment and genotyped were fully randomised. Two weeks after transfer, the water supply to the stress block was reduced to 50% of the volume received by the control block by reducing the frequency of water supplies compared to the control (details see Table 1 and Fig. 2d). An injector-based line-irrigation system (model CNL 8 l/h, combined with arrow dripper system Cobra-LF, Netafim) supplied water up to three times per week. PlantCare soil moisture sensors measured the soil moisture every hour (see Fig. S1). At the end of the experiments, the shoots were removed and the tubers harvested, weighed and counted. The tuber starch content was determined with a starch balance (Type E6100, MEKU). All yield data are available at Edal (Köhl 2018).
Automatic phenotyping on potato genotypes subjected to drought stress in a screenhouse experiment at the MPI-MP (Potsdam-Golm). Experimental setup with plants growing in big-bags, irrigation with drip-irrigation system. b Fieldscan (Phenospex) with PlantEye laserscanner. c and d optimally watered (left) and drought-stressed plants (right) before (C. 19.05.16) and after (D. 30.06.16) onset of shoot lodging (notice shoots on the pavement)
Meteorological conditions and water supply in experiments 2015 and 2016. Day = day of the year. a Daily median of the vapour pressure deficit of the air (VPD) in time interval 10:00–14:00 plotted against day of the year. b Daily minimum and maximum of air temperature. c Daily sum of photosynthetically available radiation. d Cumulative water supply to control (c) and drought stressed (s) plants
Tuber starch yield (SY) was determined as the product of starch content and tuber mass for each pot. After correction for outliers, relative tuber starch yield (RelSY) was calculated for each pot by dividing starch yield by the mean starch yield obtained for the respective genotype under optimal water supply. The drought tolerance index DRYMp (see Eq. 1) was calculated for each genotype (G) and experiment (E) by normalising RelSY of each replicate to the median of the three parent cultivars (Haas et al. 2020).
The stress index SI was calculated for each experiment as follows (0 = no stress):
Microclimate Measurements
Light intensity, air temperature and humidity (Fig. 2) were measured continuously and logged with a P22 data logger (UP Umweltanalytische Produkte) (Haas et al. 2020). After an outlier control, cumulative thermal sums were calculated as the sum of daily thermal sum from the day of planting to the actual day. The daily thermal sum was calculated as the mean of the daily minimum (Tmin) and maximum (Tmax) temperature, with a base temperature of 6 °C and maximum temperatures above 30 °C set to 30 °C (Haas et al. 2020; Jefferies & MacKerron 1987).
The daily midday vapour pressure deficit VPD was calculated as median of the VPD in the time interval 10–14 CET and summarised as the cumulative VPD from planting date to the actual day as described in Haas et al. (2020). Original data are available at Edal (Köhl 2018).
LIDAR Measurements
The LIDAR measurements were performed with two PlantEye (Model F400, Phenospex, Heerlen) infrared (IR) laser scanners, which were moved over the plant canopy by a Fieldscan (Phenospex) system (see Fig. 1b) every 4 h (6 measurements/24 h). During the measurement, the Fieldscan moved the laser scanners with a speed of 35 mm/s. The measurements were performed between 22 and 60 days from planting (DFP) in 2015 and between 5 and 76 DFP in 2016. The PlantEye’s Hortcontrol software (Phenospex) separated the continuous 3D point cloud into distinct 3D images based on the timestamps of the LIDAR measurements and the spatial design data. The Hortcontrol software estimates the features plant height (PH), 3D leaf area (A3D), projection of the leaf area on the ground (A2D) and leaf angle (LA) from the 3D image, links the estimates to the metadata and stores the data with the date and time information in a PostgreSQL database. The metadata contained treatment information and the unique plant identifier that links to the pedigree information in the plant database of the MPI-MP (Köhl et al. 2008). Original LIDAR data are available at Köhl et al. (2022).
Evaluation of Laser Scanner Data
Supplemental figure S2a depicts the schematic overview over the evaluation workflow for LIDAR. We downloaded data from the PlantEye database as csv file, uploaded them to SAS (version 9.4, SAS-Institute) and joined them with the plant pedigree and tolerance information. SAS scripts for data evaluation are available on request from the corresponding author.
Quality Control and Descriptive Statistics
Chronological plant age was calculated as the difference between the measurement date minus the planting date. For visual quality control, we plotted the raw data of the features PH, A3D, A2D and LA against the time of measurement for each genotype to determine the time range, in which we could obtain reliable data (see the “Results” section, quality control). Observations with a LA < 0 and visible outliers in any of the three features were excluded from further analysis. The data evaluation window was restricted to the time interval 27 to 50 DAP in 2015 and 20 to 50 DAP in 2016.
After quality control, the descriptive parameters’ daily mean (av), standard deviation (std), median (md), maximum (max), minimum (min) and range (r) were calculated for the features PH, A3D, A2D and LA (Proc Means). The effect of treatment, genotype, treatment × genotype and plant age on these parameters was tested by analysis of covariance (ANCOVA) with plant age as covariate (Proc Glm). As the age effect on LA was very different from that on the features PH, A3D and A2D, we employed different approaches for the subsequent data analysis.
Non-Linear Regression
Non-linear regression was performed on the relationship between daily medians (md) of the features PH or A2D and plant age, as these curves resembled saturation curves. To select against observations affected by plant lodging, maxima of mdPH and mdA2D and the plant age, at which the maximum value was achieved, were calculated. Observations, at which the plant age was higher than this threshold, were removed from the dataset when mdPH < 0.85 max (mdPH) (right censoring). Linear (Proc Reg) and non-linear regression (Proc Nlin) were calculated for the parameters md(PH) and md(A2D) of each replicate plant. The following non-linear regression equations (Archontoulis & Miguez 2014) were tested on a subset of data from 2015.
E3 beta function:
E4 Weibull regression:
E5 Verhulst logistic regression:
E6 Richard’s logistic regression:
Verhulst logistic regression (E5) was calculated for all replicates and yielded the parameters Mx(PH), k(PH), Tm(PH) for plant height and Mx(A2D), k(A2D) and Tm(A2D) for A2D. The parameter Mx estimates the maximum value of the feature height or area. The inflection point (Tm) is the plant age, at which the slope of the curve starts decreasing. The slope k estimates the initial growth rate. Figure 4f illustrates all parameters. In an additional quality control step after regression, we excluded those datasets where the difference between the maximum height that was calculated from the daily median (max) and the maximum height estimated by logistic regression (Mx) was larger than 200 mm. After the outlier control, an analysis of variance was performed for the effects of genotype, treatment and their interaction on all six parameters.
Correlation Analysis
For correlation analysis, we calculated the median of the regression parameters of the features PH and A2D and the parameter average (av) and standard deviations of the feature LA for each of the 64 genotypes, two treatments and both experiment. A Spearman correlation analysis (Proc Corr) was calculated for these parameters and the median drought tolerance index DRYMp calculated from starch yield data in three big-bag experiments (DRYMp_B), three pot experiments (DRYMp_P) and seven field experiments (DRYMp_F) (Haas et al 2020).
Drought Tolerance Assessment Based on Tuber Starch Yield
Drought tolerance was assessed based on tuber starch yields. Tuber yields and starch yields were significantly higher in 2015 than in 2016 (Fig. 3), presumably due to the increased heat stress in 2016 (Table 1). In both years, the drought stress significantly decreased tuber fresh weight and starch yield, resulting in drought stress indices (SI) of 0.6 in 2015 and of 0.54 in 2016. The significant genotype × treatment interaction on yield in both years indicated a significant genetic variation for drought tolerance in the population. The drought tolerance index DRYMp (see the “Material and methods” section, Eq. 1) was calculated for each genotype based on the starch yield data of 2015 or 2016 (Fig. 3c). The comparison of the DRYMp distribution showed large differences between lines and considerable variation between years. The differences between years may result from insufficient sample size for tolerance assessment in a single experiment (Köhl et al. 2021). Based on the data from experiments F3 to F8 (see Table S1), Köhl et al. (2021) showed that a minimum of three experiments is required to achieve a stable tolerance assessment. Therefore, we calculated a general DRYMp for each genotype for the three test environments from the combined data of the three (big-bag, pot) or seven (field) experiments; the distribution of which are shown in Fig. 3d. These general DRYMp values were the response variables in the analysis to identify and validate phenotyping parameters for their power to predict drought tolerance.
Characterisation of drought tolerance in potato population. a Distribution of tuber fresh weight per plant (FW) and (b) tuber starch yield per plant (SY) of 64 potato genotypes cultivated under optimal (c) or reduced (s) water supply in the big-bag test system. c Drought tolerance index DRYMp calculated for 64 potato genotypes based on the starch yield data obtained in the big-bag experiments 2015 and 2016. Pedigree of the genotypes see Supplemental Table S2. d Distribution of drought tolerance index DRYMp of 64 potato genotypes in 3 big-bag, 7 field and 3 pot experiments (details see Supplemental Table S1)
Analysis of Diurnal Leaf Movement
The leaf angle is the angle between the leaf plane and a horizontal plane. Low leaf angles indicate a horizontal leaf position. Leaf angle data were analysed separately for 2015 and 2016. To establish whether the diurnal pattern of leaf movement can be used as a predictor for drought tolerance, median leaf angles were calculated for six diurnal time intervals (DTI): 1LN (22:30–2:30 UTC), 2DW (2:30–6:30), 3AM (6:30–10:30), 4PM (10:30–14:30), 5DK (14:30–18:30) and 6EN (18:30–22:30). During the experiment, the sunrise varied between 5:28 CEST (3:28 UTC) and 4:44 CEST (2:44 UTC), the sunset between 20:39 and 21:29 CEST (18:39 and 19:29 UTC). Analysis of variance (Proc Glm) was performed on the effect of age, genotype, treatment and their interactions on leaf angle in the six DTI for both experiments separately. As the analysis revealed a strong age effect, age intervals of age < 30 DFP (vegetative), 30 DFP ≤ age ≤ 45 DFP (flowering) and age > 45 DFP (tuber filling) were defined. Spearman correlation analysis was calculated for DRYMp (big-bag) and the mean leaf angle of each genotype separately for the six DTI, three age intervals, both treatments and both years. As the timing of dawn and dusk shifted within DTI 2EN and 6DW, respectively, age effects are confounded with light intensity effects for these DTI.
Multivariate Analysis
Principal component analysis (Proc Prinqual) was calculated separately for 2015 and 2016 with Proc Prinqual. The following variables were included: genotype and treatment means and medians of the regression parameters for plant height and projected leaf area, the median values of leaf angle in different DTI and age classes, the mean of the median values of leaf angle, plant height, leaf area 2D and leaf area 3D on day 29, 39 and 49 after sowing (see Supplemental Table S4 for abbreviations).
For the decision tree analysis (Proc Hpsplit), four of these variables (U654PMC, U655DKC, U304PMS, U304PMC) were dropped because of missing values in several genotypes. The tolerance classes were defined based on the mean DRYMp of each genotype calculated from 2015 to 2016 data. The adjusted thresholds yielded three tolerance classes of similar size: sensitive (S, 21 genotypes, DRYMp < 0), intermediate (I 21 genotypes, 0 < DRYMp < 0.095) and tolerant (T 20 genotypes DRYMP > 0.095).
Results
Experimental Setup and Climate Conditions
Canopy measurements by a laser scanner were performed in potato drought stress trials (Fig. 1 and Supplemental Table S1) on 64 genotypes segregating for drought tolerance (details see (Haas et al. 2020)). During the experiment, plants grew in big-bags with a substrate volume similar to the soil volume per plant in the field trials. Drought stress was imposed by increasing the time interval between subsequent irrigations, thus mimicking the situation in the field, where drought stress arises from infrequent water supply by rain (Köhl et al. 2021).
Micrometeorological conditions are shown in Fig. 2 and summarised in Table 1 for the three time intervals 1 = vegetative growth, 2 = onset of flowering to end of measurements and 3 end of measurements to haulm destruction. Time interval 2 corresponded to the beginning of tuber production. Both experiments 2015 and 2016 were similar with respect to light sums and median VPD. However, the median of minimum and maximum air temperatures was substantially higher in phase 2 of the trial in 2016 compared to 2015. Furthermore, there were more hot days (T > 30 °C) in the vegetative phase of 2016. The substantially higher air temperature and earlier heat stress delayed development and reduced tuber yield in 2016 compared to 2015 (Fig. 3a).
Data Evaluation Workflow for LIDAR Data
Quality Control
When visually checking raw data of the features plant height (PH) and projection of the leaf area on the ground (A2D) (Fig. 4b, c), we noticed that data were very noisy at the beginning of the experiment (days from planting DFP < 25 days) and after DFP > 55. Early noise resulted from difficulties in distinguishing the plant from the background, while lodging and intermingling of shoots affected estimates in older plants (compare Fig. 1c and d). Therefore, the data evaluation window was left and right-censored. When focusing on the raw data from eight plants of a single genotype within this window (Fig. 4d), a diurnal rhythm of the plant height estimate became apparent. This rhythm resulted from the diurnal movement of the leaves (Fig. 5a), which changed from a horizontal position during the day to a more vertical position during the night. This process affected the position of the uppermost point of the plant and thus the height estimate. To get parameter estimates unaffected by leaf movement, we calculated the daily median of plant height, leaf area (A3D and A2D) and leaf angle. We chose the median over the mean to reduce the outlier effects. The plot of median plant height against plant age (Fig. 4e) yielded a saturation curve that represented the finite growth of potato shoots. Shoots stopped elongating, when the shoot apical meristem converted into an inflorescence. Afterwards, shoot lodging resulted in a sudden decrease of shoot height. Height increased again, when the shoots bended upwards or side shoots elongated. For each plant, we estimated the threshold, above which these processes affected feature estimates, and adjusted the right censoring age accordingly (see the “Material and Methods” and “Non-linear regression” sections).
Workflow for the evaluation of growth-related data from repeated laser scanner imaging. a Scanner image. b Raw data of plant height (PH) plotted against plant age DFP (days from planting) for plants from four genotypes cultivated under control conditions (blue) or drought stress (red). c Raw data of projected leaf area A2D plotted against plant age for plants from four genotypes cultivated under control conditions (blue) or drought stress (red). d Detail of plant height against plant age of genotype 22497 showing the diurnal variation in plant height. e Daily median of plant height plotted against leaf age for plants of genotypes 22497. f Logistic regression for the effect of age for a single plant of genotype 22497, indicating the logistic regression parameters initial slope (k), age at half maximum plant height = inflection point (tm) and maximum plant height Mx (hmax)
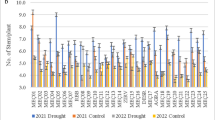
Effect of drought stress on the growth parameters distribution of plant height (PH) and leaf area 2D (A = A2D). Logistic regression of plant height or leaf area on plant age yielded the parameters maximum (Max), inflection point Tm and slope k for 64 genotypes cultivated under control conditions (C) or drought stress (S)
The analysis of variance on quality-controlled data revealed a highly significant effect of genotype, treatment and plant age on the daily median of plant height (PH), leaf area 2d (A2D), leaf area 3d (A3D) and leaf angle (Supplemental Table S3). Significant effects of genotype and the genotype × treatment interaction indicate that genotypes differ morphologically and in their morphological response to drought stress. The effect of age was highest on PH, intermediate on A3D and A2D, and lowest on LA. PH and A2D increased rapidly in young plants and reached a saturation value at about 50 DFP. In contrast, leaf angle changed linearly with plant age. Different evaluation strategies are thus required for the age-dependent features PH and A2D than for LA. Further analysis revealed very close correlations (Pearson coefficient > 0.97) between the features A2D and A3D. We therefore concentrate the growth curve analysis on A2D and PH.
Logistic Regression
We performed regression analysis on the quality-controlled data set of daily median of A2D (md(A2D)) and PH (md(PH)) to obtain growth parameter estimates for each of the 764 or 768 plants grown in 2015 or 2016, respectively. Linear regression analysis results in high r2 values, but overestimated median PH for DFP < 30 and DFP > 35. We therefore tested four of the regression functions suggested by Archontoulis and Miguez (2014), namely beta function (Yin et al. 2003), Weibull regression (Weibull 1951), logistic regression (Verhulst 1838) and Richard’s logistic regression (Richard1959) on a subset of 48 time series (equations, see the “Material and methods” section). The model on Weibull and beta function failed to converge for 47 out of 48 time series. Logistic regression achieved the highest percentage of converging models (47 out of 48). For the complete 2015 data set, logistic regression converged for 711 and failed for 53. Among the data sets, which failed to converge, genotype 899646 was overrepresented. This genotype showed a delayed early development. Hence, the regression for this genotype would have required a later time window. For 2016 data, regression converged for 738 out of 768 dataset. Among the dataset, for which regression failed to converge, stress-treated plants of genotype 22497 and 899717 were overrepresented. We excluded the check cultivar 22497 from further analysis as a genetic and morphological outgroup.
Relationship Between Growth and Drought Tolerance
We analysed the effect on genotype and treatment on the regression parameters initial slope k, inflection point Tm and maximum Mx (see the “Material and methods” section and Fig. 4f) by ANOVA (Table 2). Mx(PH) and Mx(A2D) were significantly affected by genotype, treatment and their interaction in 2015 and 2016. In both years, Mx(PH) and Mx(A2D) were significantly higher in control than in drought stressed plants (Fig. 5). Under control conditions, Mx(A2D) was very similar in both years. In contrast, Mx(PH) was much lower under stress conditions in 2015 than in 2016. The inflection point Tm(PH)cs was at about 32 DAP in both years. Tm(PH) was significantly lower in stressed than in control plants in 2015, but significantly higher in 2016. Tm(A2D) was not significantly affected by the treatment and very similar in both years. However, the genotype explained a large part of the variation in Tm(A2D), as indicated by the very large distribution range of Tm(A2D) in Fig. 5d. Interestingly, median Tm for leaf area was lower than for plant height, indicating that the plants reach the maximal projected leaf area before they reach the maximum height. The genotype also affected the initial slope of plant height (k(PH)) and leaf area (k(A2D)) growth significantly (Fig. 5e and f). The k(A2D) showed a skewed distribution and a group of outliers with a very high initial growth rate, suggesting genotypic variation in early plant development. The ANOVA on log-transformed k yielded similar result as the analysis on untransformed data (Table 2). K(PH) was lower in 2016 than in 2015, which matched the difference in Mx(PH) between the years.
The significant genotype × treatment effect for the Mx and Tm of A2D and PH suggest that the growth response to drought differed between genotypes. This makes these growth parameters potential candidates as drought tolerance markers. We tested this by analysing their correlation to the drought tolerance index DRYMp (see Eq. 1) for the three test environments (Table 3). Mx(PH)s correlated negatively with DRYMp(big-bag) in both years. Tm(PH)c in 2016 correlated negatively with DRYMp(big-bag) and DRYMp(pot), but the correlation was not found for Tm estimated from the 2015 data. For leaf area A2D, we found positive correlation between the Mx(A2D)s and the DRYMp in all three test environments. The inflection point Tm(A2D)s correlated significantly positive with DRYMp(big-bag) and DRYMp(pot), but not with DRYMp(field). Generally, a higher number of significant (p < 0.05) correlations were found between parameters derived from stressed plant (14) than parameters derived from control plants (8). This means that the predictive value of the parameter depends on the environment. The highest number of significant correlation (11 for stress measurements, 3 for control measurements) was found with DRYMp quantified in the big-bag experiments, which was the test system, in which the laser scanner measurements were done. The extrapolation from parameters measured in the big-bag system to the performance in the field works less well.
Leaf Movement
A simple analysis of the correlation between mean and standard deviation of the leaf angle with DRYMp yielded no reproducible correlation (Table 3). The leaf angle (LA) changed significantly with plant age (F1,4384 = 68.58 p < 0.0001) and was strongly affected by the diurnal cycle (Fig. 6a). During the night, leaf angles were 20–25°, whereas the leaf angle decreased to about 15° during the day light period. Leaf movement was furthermore significantly affected by treatment (F = 95, p < 0.001) (Fig. 6c). We analysed this pattern by calculating the leaf angle distribution for all genotypes in different time intervals of the diurnal cycle for control and stressed plants. Table 4 shows the results of an ANOVA on the effects of age (DFP), genotype (G) and treatment (E). The treatment affected the leaf angle most distinctly in the light phase between 8:30 and 20:30 CET(DTI 3AM to 5DK), when leaf angles of control plants were about 5° lower than in stressed plants. In stressed plants, the leaf angle remained almost constant during the diurnal cycle. During the night, leaf angles were higher in control than in stressed plants in 2015, while both treatments caused similar angles in 2016. The lower nocturnal leaf angle of control plants in 2016 may be related to the higher number of heat days in 2016, which could have stressed the control plants. The ANOVA furthermore revealed a strong age effect on the nocturnal leaf angle, especially at the end of the night (6EN), consistently found in both years. The genotype affected the mean leaf angle in all time intervals and this effect was different for stress and control plants. However, the genotype effect was weaker than the age and the treatment effect, which decreases the predictive value of the absolute leaf angle measurements for drought tolerance. To take the age effect on the leaf angle into account, we calculated the mean leaf angles for each genotype, treatment and diurnal time interval for the vegetative, flowering and tuber filling phase and correlated the mean leaf angle to the drought tolerance (Table 5). In control plants, correlations between the leaf angle and DRYMp were weak and not reproducible between years. In stress plants, however, leaf angles of U45 plants correlated significantly with DRYMp in DTI 1LN, 2DW, 3AM and 6EN. The most significant correlations were found in both years during the late night (1LN) and at dawn (2DW). The correlation is negative, which means that a high DRYMp was linked to a low leaf angle. In tolerant genotypes, leaf angles under stress were thus more similar to control conditions than in sensitive genotypes.
Effect of diurnal cycle and water supply on the leaf angle of potato. a Raw data of leaf angle plotted against time at 25.05.16 for control (black) and drought stressed (grey) plants. Leaf angles were estimated from laser scanner images for plants from 64 genotypes. The vertical lines indicate sunrise and sunset. c Diurnal change of leaf angle depicted against plant age for plants cultivated under control (black) or drought stress (grey) conditions, genotype 2249, experiment 2016, F(treatment) = 95 p < 0.001. b, d Comparison of the effect of water supply (control dark grey, drought stress light grey) on the leaf angle distribution in different intervals of the diurnal cycle in 2015 (c) and 2016 (d): 1LN: 22:30–2:30 UTC. 2DW: 2:30–6:30. 3AM: 6:30–10:30. 4PM: 10:30–14:30. 5DK: 14:30–18:30. 6EN: 18:30–22:30. Statistical analysis see Table 4
Multivariate Analysis
After analysing the relationship between drought tolerance and single canopy features, we investigated whether we can predict drought tolerance from a combination of morphological features. We combined regression parameters and—as a proxy for manual measurement—genotypic means of features at fixed ages. For the decision tree analysis, we defined three tolerance classes of similar size based on combined tolerance data from two experiments. The distribution of the genotypes was similar in the PCA plots in both years (Supplemental Fig. S3). Furthermore, loadings of both components correlated significantly between years (Supplemental Table S4). However, the lack of a clear separation between the three tolerance classes in the PCA plot (Supplemental Fig. S3) suggested that multiple regression models were unlikely to yield a tolerance prediction model based on the canopy features. Thus, we decided for decision tree models (Fig. S4) that allow identifying different combinations of canopy features linked to sensitivity or tolerance. The decision tree sorted 83% (2015) or 81% (2016) of the genotypes in the correct tolerance class. In both years, the identification was much better for class S (error rate 5%) than for the other two classes (see confusion matrix in Fig. 7a, b). The inflection point of the leaf area growth curve under stress (Tm(A2D)s) was the most important factor for classification. Genotypes with a low Tm(A2D)s were more likely to be drought-sensitive (Fig. 7c, d). The second decision node was linked to plant height under control conditions. The 2015 model predicted genotypes with a high slope of plant height growth as sensitive, and both the 2015 and the 2016 model linked high plant height at 39 DFP to class S (Fig. 7e,f). The third factor represented in both models was the leaf angle in the vegetative age interval (U30). In 2015, low pre-noon leaf angles under control conditions indicated tolerance. In 2016, high nocturnal leaf angles under stress conditions indicated tolerance.
Drought tolerance classification of potato genotypes based on a decision tree with canopy parameters. Model-based confusion matrix with actual (rows) and predicted (columns) tolerance classes S (sensitive), I (intermediate) and T (tolerant) and error rate E for 2015 (a) and 2016 (b). Distribution of canopy growth parameter Tm(A2D)s in 2015 (c) and 2016 (d) by tolerance class. Distribution of PH(39 DFP)c 2015 (e) and 2016 (f) by tolerance class
Altogether, the decision tree analysis detected sensitive genotypes more reliably than tolerant genotypes. The most predictive factors were the shoot height at the end of the juvenile phase and the inflection point Tm of leaf area growth under stress. Sensitive genotypes grew high shoots under control conditions and slowed down leaf area development under stress earlier than tolerant genotypes.
Discussion
In this study, we aimed to identify morphological markers for drought tolerance that ultimately could serve as selection markers in potato breeding under field conditions. We employed continuous LIDAR measurement of shoots’ growth on 64 potato genotypes with known drought tolerance (Haas et al. 2020). Two independent drought tolerance trials conducted under variable climate conditions enabled testing the reproducibility of the prediction model.
Parameter Extraction
A general challenge in continuous phenotyping is the identification of those features and parameters that explain or predict plant response to environmental challenges in large datasets—a needle in the haystack task. We can approach the problem by unbiased analysis based on single data points or by hypothesis-driven extraction of descriptive statistical parameters like maximum or growth rate of shoot height. We decided for the second approach to circumvent the overfitting problem that occurs when > 150 k data points combine with tolerance data on about 64 genotypes. Furthermore, the exact climatic conditions, under which phenotyping measurements were done, are not reproducible. In each year, climate conditions at a given plant age will be different. To address the problem, we classified the metadata to achieve reproducible groups. Water status was classified as optimal and drought stress instead of using soil water contents or VPD values. The age at the measurement was classified based on the developmental stage into vegetative (pre-flowering), flowering/tuber initiation and tuber filling. Six diurnal time intervals (DTI) grouped the time of measurement as described in the “Material and methods” section.
Quality Control
Measurements of shoot features were highly variable in young plants and after flowering (Fig. 4b, c). Small plant were hard to distinguish from the background. After flowering, height measurements were affected by lodging of the main stem and subsequent development of side branches. When side shoots developed, neighbouring plant started to intermingle, so that measurements could no longer be linked to a single plant. We therefore decided to censor the measurements based on plant age. The left-censoring was done with a fixed age, as the culture was started with similar sized plantlets. However, subsequent development and flowering date varied between genotypes and between treatments. Poor water availability slows down canopy development of potato and affects fitting of canopy growth curves (Khan et al. 2019a). We avoided confounding treatment effects with fitting effects by estimating the age for the right-censoring from the data set. In pot trials, the problem of intermingling shoots could be addressed by increasing the distance between pots. The delayed canopy closure would, however, increase the differences to the conditions in the field, in which agricultural practice fixes the planting density. However, in the field, multi-row plots rather than single plants could represent genotypes and be the object of phenotyping.
Non-Linear Regression
In their analysis of genotype and environment effects on the canopy development in field-grown potato, Khan et al. (2019a) used non-linear regression employing the Gauss-method to estimate the development and decline of the canopy. From the regression parameters, they calculated derived parameters describing the canopy growth stages development (P1), time of maximum cover (P2) and time of decline (P3). For the last stage, they reported high standard errors for estimates although they avoided problems from intermingling shoots of different genotypes by growing plants in 6-row-plots. In potato, the estimation of canopy parameters from LIDAR seems to be generally more difficult than in other crops, as the complexity of the potato canopies increases with age (Liu et al. 2019; Su et al. 2019; ten Harkel et al. 2020). As our method was restricted to observations in phase P1 and P2, we decided to use non-linear-regression methods for saturation curves. We tested different methods suggested by Archontoulis and Miguez (2014) on a subset of left- and right-censored data. The method that yielded the highest percentage of converging regressions was the logistic regression (Verhulst 1838), which describes the growth curve by the parameters initial slope k, inflection point Tm and maximum Mx.
The next question is whether growth parameters in the early phase P1 and part of P2 are likely to allow predictions on drought tolerance or yield. An early analysis reduces the influence of factors like Phytophtora infestation and shoot lodging or interacting stressors like heat, and it speeds up the selection process. Furthermore, early development is not affected by maturity class of the potato genotype (Khan et al. 2019a). However, early development can be substantially affected by starting material, e.g. tuber size and quality (Maris 1986) and by N-availability (Khan et al. 2019a). Both factors can, however, be controlled for in managed screening trials. The lower heritability of growth parameters in P1 compared to P2 indicates that finding prediction parameters in early development will be challenging.
Value of the Identified Traits
Subsequently, we will investigate whether the parameter PH, A3D, A2D and LA meet the criteria postulated for useful secondary selection traits (see Introduction (Lafitte et al. 2003)). By analysis of covariance with plant age as a covariate, we tested for genotype (genetic variation) and drought treatment (environmental) effects. Significant genotype effects on the traits are a prerequisite for its use as a marker. They were found for all four parameters. The study population predominantly comprised lines derived from two crosses between sensitive and tolerant genotypes plus the parent cultivars and a check cultivar. Thus, the population was a good proxy for a breeding population as its variability was smaller than the variability in the entire genepool of potato cultivars. In contrast, the strong treatment effects on many morphological traits were disadvantageous as this limits the predictive value of the trait to specific environmental conditions. We dealt with the situation by determining the association between drought tolerance and the secondary trait separately for traits measured under control (c) or under stress (s) conditions (Table 5).
Plant Height
Shoot height is an important component of vegetation models that predict canopy development, interception of radiation and evapotranspiration (Li et al. 2020; Papadavid et al. 2011; ten Harkel et al. 2020). It thus may be a candidate secondary trait for yield prediction. In our study, the genotype significantly affected the daily median of plant height as well as the regression parameters k, Tm and Mx in both years (Table 2, Supplemental Table S3). Research on potato cultivars revealed a significant variation for shoot height between potato cultivars within potato maturity classes and a positive correlation between shoot height and (starch) yield (Moll et al. 1987). However, the QTL identified for plant height in a tetraploid mapping population was linked to maturity, which would suggest an indirect effect of height on yield (Bradshaw et al. 2004). A QTL study in diploid potato found two major QTL for plant height, one of which overlapped with the QTL for tuber yield after drought stress recovery (Anithakumari et al. 2012). In our analysis, drought stress significantly reduced shoot height (Supplemental Table S3). Substantial height differences between years suggest that other factors like heat stress affect plant height too (Fig. 5). Heat stress increases plant height in greenhouse experiments (Deblonde & Ledent 2001; Tang et al. 2018; Yuan et al. 2003). As heat and drought stress often appear in combination, their opposite effect on plant height will render the interpretation of plant height measurements as marker for drought tolerance challenging. The correlation analysis revealed a negative correlation between drought tolerance in big-bag trials and Mx(PH) under stress conditions and a positive correlation between k(PH)s and drought tolerance. Both correlations were also significant under control conditions in 2016 but not in 2015. Thus, drought-tolerant genotypes were shorter but elongated faster under stress.
In conclusion, we found genetic variation for plant height in potato and a correlation with drought tolerance for the growth parameters k and Mx. Environmental factors affected the relationship between genotype and plant height, thus limiting the use of the traits for the prediction of drought tolerance.
Leaf Area
Leaf area and leaf area index (LAI) are important features in vegetation models as parameters to estimate intercepted solar radiation (Haverkort et al. 1991) and to predict growth and yield (Ray et al. 2006). The leaf area duration integrates the LAI curve, thus taking into account the effects of early leaf development and delayed senescence (Jefferies & MacKerron 1993; Khan et al. 2019a). LAI and canopy cover are thus among the parameters estimated for potato crops from satellite data to predict yield (Clevers et al. 2017). The total leaf area depends on leaf initiation rate, the duration of the leaf initiation phase and on the leaf expansion growth. Leaf growth is extremely sensitive to low soil water potentials or high evaporative demand (Hammer et al. 2006). In maize, already mild water stress affects cell expansion growth and causes the abortion of reproductive organs. Under water stress (low soil water potential, high VPD), leaf elongation rates (LER) oscillate diurnally with lowest rates at and after solar noon (Caldeira et al. 2014). The oscillation of LER has been linked to diurnal changes in root hydraulic conductivity in response to xylem water potential. The sensitivity of the LER amplitude to water stress corresponds to the drought sensitivity of the genotype (Caldeira et al. 2014). In maize, the relationship between plant water status and leaf growth depends on the genotype (Caldeira et al. 2014; Sadok et al. 2007; Zhao et al. 2018). The QTL for the slopes of the response curve of the leaf elongation rate to water status co-localises with the QTL for anthesis-silking interval, which is a major predictor for yield under drought in maize (Bänziger et al. 2006; Bolanos & Edmeades 1996; Welcker et al. 2007). This suggests that data on leaf area development may be informative for the prediction genotype-specific drought tolerance. In potato, water stress has been shown to impede leaf development by reducing leaf number and decreasing leaf expansion growth (Hill et al. 2021). The latter is more sensitive to soil drying in potato than in other crops and this sensitivity may cause the low drought tolerance of potato (Weisz et al. 1994). A genotype-dependent response of root hydraulic conductance to water status may also be behind the genotype effect on diurnal leaf movement in potato: drought affected the leaf movement less in tolerant than in sensitive genotypes.
In our analysis, genotype significantly affected the daily median of A2D and A3D (Supplemental Table S3) as well as the regressions parameters k(A2D), Tm(A2D) and Mx(A2D). The genotype effect was strongest for k(A2D), which means that the genotypes differed markedly in the initial canopy growth rate. This contrasts with the finding of Khan et al. (2019a), who found only weak effects of the genotype on the initial phase of canopy development, but considerable genotypic variation in the duration of the phase of maximum canopy cover (DP2). However, the population of Khan et al. segregated for maturity, while ours did not. We thus would not expect variation in the duration of DP2, but we cannot estimate the duration of the canopy from our dataset. In our study, the drought treatment affected median leaf area in a genotype-dependent (Supplemental Table S3). Drought treatment significantly reduced Mx(A2D) (Table 2) compared to control conditions in both years (Fig. 5) but had only a weak effect on the slope k(A2D) and the inflection point Tm(A2D).
Drought tolerance in big-bag trials correlated positively with Mx(A2D) and Tm(A2D) under stress conditions, but not under optimal water supply. Under stress, tolerant genotypes developed new leaves for a longer time (higher Tm) and thus achieved a higher leaf area. Yield under drought correlates with leaf area duration; thus, selection for the ability to sustain leaf growth on dry soils may improve yield stability (Jefferies & MacKerron 1993). In drought tolerance screens, vegetation indices that related to leaf area index and above ground biomass were major determinants of yield already on day 25 and 40 (Schafleitner et al. 2007). When LAI data estimated from MODIS satellite data were tested as yield predictor for potato, positive correlations were found especially in the middle of the vegetation period (Johnson 2016). The decline of correlations towards the end of the vegetation period may, however, be linked to larger measurement errors due to lodging in towards the end of the growth period. A path coefficient analysis on the effects of different canopy traits on tuber dry yield revealed a strong and positive effect of the integral Asum and of the radiation use efficiency RUET (Khan et al. 2019b). Asum is the area under the canopy cover against time curve; the radiation use efficiency RUET is the ratio of the cumulative intercepted light sum and the tuber dry weight (Khan et al. 2019b). The same study reports a negative correlation between Asum and RUET, and concludes that vigorous vegetative growth impedes tuber bulking (Khan et al. 2019b). Thus, sustained leaf development under drought stress seems to be important for yield stability up to an optimal level.
Leaf Angle
The leaf angle LA strongly affects interception of direct radiation by the leaf and the entire canopy, and it alters the aerodynamic resistance within and above the canopy (Lhomme et al. 1992; Li et al. 2015). Photosynthesis and yield thus strongly depend on the LA (Emmel et al. 2020). Furthermore, productivity increases in modern rice and maize cultivars have been linked to more upright growing leaves (Tian et al. 2011; van Zanten et al. 2010). The median LA distribution or its daily range may thus indicate photosynthetic performance or water status and hence are potential secondary traits for the selection for yield under drought conditions (Bolanos & Edmeades 1996; Kamphorst et al. 2020; Martynenko et al. 2016; Sah et al. 2020). QTL for LA have been mapped in maize and rice (Huang et al. 2010; Ku et al. 2010; Tian et al. 2011). For potato, our study found significant genotypic variation on the median LA (Table 4). Furthermore, LA changed periodically within the diurnal cycle. In potato, LA was high during the night, decreased after sunrise and increased again in the afternoon (Fig. 6). Similar leaf movements have been found in other species and were linked to optimised photosynthesis, but also to a reduction of transpiration and the avoidance of photoinhibition and heat stress (Biskup et al. 2007; Briglia et al. 2019; Rakocevic et al. 2018; van Zanten et al. 2010; Werner et al. 2001). The movement can be caused by hyponastic growth or turgor changes (van Zanten et al. 2010). Water supply affects LA and the diurnal change of LA. In grapevine, LA correlated linearly to the leaf water potential (Briglia et al. 2020) (Briglia et al. 2019). In soybean, LA correlates to soil water potential (Rosa et al. 1991). Water stress also changes the diaheliotropic movement of soybean leaflets (Rakocevic et al. 2018). The LA increases under drought stress in maize and in potato (Kamphorst et al. 2020; Xu et al. 2021). Drought stress reduces the diurnal variation of LA in potatoes (Xu et al. 2021). We found no association between drought tolerance and LAc under optimal water supply, but a correlation with the LAs during late night and at dawn (Table 5). Drought-tolerant genotypes had lower LAs than drought-sensitive genotypes, thus resembling unstressed plants (Fig. 6).
Multivariate Model
The determination of k(PH)s and Tm(A2D)s requires repeated measurements under drought-stress conditions, which decreases their value as screening markers for breeding populations. Thus, we employed a decision tree analysis to establish whether growth parameters from stressed plants can be replaced by parameters from unstressed plants. To find out whether we can replace repeated measurements by single measurements, we included median values of PH and A from single days as proxy for single measurements. Furthermore, we enquired, which time of the life cycle is most informative by picking single measurements from three different developmental states. The decision analysis was performed separately for 2015 and 2016 on three tolerance classes contain 20 genotypes each (see the “Material and methods” section). Both decision trees used the parameter Tm(A2D)s as the first decision criterion, identifying plants with a shorter time of leaf area development under stress as sensitive. The mean of the parameter Tm(A2D) was similar in both years (Fig. 5). This increases the value of the parameter for selection, as it seems to be independent of meteorological conditions. The second decision node grouped plants that rapidly increased PH under control conditions into the sensitive group. The association in our study may, however, be affected by the fact that the tolerant parent A had lower shoot heights than the sensitive parents R and E (Haas et al. 2020). In maize, Su et al. (2019) reported a negative association between drought tolerance and plant height. In contrast, Bänziger et al. (2006) list plant height as a confounding factor in drought tolerance screens.
Altogether, our decision tree based on phenotypic markers classified sensitive genotypes more reliably than intermediate or tolerant genotypes. It thus may complement our metabolite/transcript based model, which identified tolerant genotypes very well, but failed to detect drought-sensitive genotypes (Haas et al. 2020). An independent selection experiment or the test of the association between reduced leaf area development and drought sensitivity in an independent population could verify the assumption that joining both models will improve selection for drought tolerance.
For breeding purposes, shoot parameters have to be quantified under field conditions. Satellite-based sensors provide repeated canopy scans. However, Kempenaar et al. (2016) found poor correlations between satellite data derived biomass indices and final yield. Jindo et al. (2021) therefore suggested using remote sensing data to estimate the parameters for crop simulation models rather than to estimate yield from single vegetation indices. Thus, instead of directly using phenotypic markers for selection, it might be more useful to employ data derived from phenotyping to feed into genomic models. The genomic prediction model for the anthesis-silk-interval (ASI) is a very elegant example (Bänziger et al. 2006; Bolanos & Edmeades 1996). The sensitivity of ASI to VPD and soil water potential can be predicted from the sensitivity of leaf expansion under controlled conditions (Chapuis et al. 2012; Welcker et al. 2007). Based on this relationship and the prediction of phenological stages of genotypes under field conditions, environmental conditions during the drought-sensitive ASI could be determined from local climate sensors in field trials. This allowed the quantification of drought stress during the sensitive period of each genotype in various field trials to generate the genotypic sensitivity curves. This approach provided phenotypic drought tolerance information of sufficiently large maize genotype populations to generate a whole genomic prediction model for drought sensitivity factors in maize (Millet et al. 2019).
Future work for our phenotypic traits would need to identify the sensitive period that determines the inflection point Tm for leaf area development Tm(A2D) and subsequently to identify those environmental factors that influence Tm(A2D).
Conclusion
The analysis of the genotype and treatment effects on morphological features and the correlation analysis with the drought tolerance index DRYMp revealed phenotypic differences related to drought tolerance. Under drought stress, drought-tolerant genotypes grew shorter (lower Mx(PH)s) shoots faster (higher k(PH)s) and they increased leaf area for a longer time (higher Tm(A2D)s) and thus achieved a higher projected leaf area (higher Mx(A2D)s). Furthermore, tolerant plants maintained a lower leaf angle at dawn under drought stress, thus resembling unstressed plants. The parameter Tm(A2D) was an especially promising predictor as it was only weakly affected by climate conditions.
Data availability
All data are available at E!DAL (Köhl et al. 2022). Material and SAS scripts used for evaluation are available from the corresponding author.
References
Aliche EB, Oortwijn M, Theeuwen TPJM, Bachem CWB, Visser RGF, van der Linden CG (2018) Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric Water Manag 206:20–30. https://doi.org/10.1016/j.agwat.2018.04.013
Anithakumari AM, Nataraja KN, Visser RGF, van der Linden CG (2012) Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol Breeding 30:1413–1429. https://doi.org/10.1007/s11032-012-9728-5
Archontoulis SV, Miguez FE (2014) Nonlinear regression models and applications in agricultural research. Agron J 107:786–798. https://doi.org/10.2134/agronj2012.0506
Bänziger M, Setimela PS, Hodson D, Vivek B (2006) Breeding for improved abiotic stress tolerance in maize adapted to southern Africa. Agric Water Manag 80:212–224. https://doi.org/10.1016/j.agwat.2005.07.014
Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000Research 5: F1000 Faculty Rev-1554. https://doi.org/10.12688/f1000research.7678.1
Berger B, de Regt B, Tester M (2012) High-throughput phenotyping of plant shoots. Methods Mol Biol 918:9–20. https://doi.org/10.1007/978-1-61779-995-2_2
Beyene Y, Gowda M, Olsen M, Robbins KR, Pérez-Rodríguez P, Alvarado G, Dreher K, Gao SY, Mugo S, Prasanna BM, Crossa J (2019) Empirical comparison of tropical maize hybrids selected through genomic and phenotypic selections. Front Plant Sci. 10.https://doi.org/10.3389/fpls.2019.01502
Biskup B, Scharr H, Schurr U, Rascher U (2007) A stereo imaging system for measuring structural parameters of plant canopies. Plant Cell Environ 30:1299–1308. https://doi.org/10.1111/j.1365-3040.2007.01702.x
Bojacá CR, García SJ, Schrevens E (2011) Analysis of potato canopy coverage as assessed through digital imagery by nonlinear mixed effects models. Potato Res 54:237. https://doi.org/10.1007/s11540-011-9189-y
Bolanos J, Edmeades GO (1996) The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crop Res 48:65–80. https://doi.org/10.1016/0378-4290(96)00036-6
Bradshaw JE, Pande B, Bryan GJ, Hackett CA, McLean K, Stewart HE, Waugh R (2004) Interval mapping of quantitative trait loci for resistance to late blight Phytophthora infestans (Mont.) de Bary, height and maturity in a tetraploid population of potato (Solanum tuberosum subsp tuberosum). Genetics 168:983–995. https://doi.org/10.1534/genetics.104.030056
Briglia N, Montanaro G, Petrozza A, Summerer S, Cellini F, Nuzzo V (2019) Drought phenotyping in Vitis vinifera using RGB and NIR imaging. Scientia Horticulturae 256. https://doi.org/10.1016/j.scienta.2019.108555
Caldeira CF, Jeanguenin L, Chaumont F, Tardieu F (2014) Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nature Communications 5. https://doi.org/10.1038/ncomms6365
Chapuis R, Delluc C, Debeuf R, Tardieu F, Welcker C (2012) Resiliences to water deficit in a phenotyping platform and in the field: how related are they in maize? Eur J Agron 42:59–67. https://doi.org/10.1016/j.eja.2011.12.006
Clevers JGPW, Kooistra L, Van den Brande MMM (2017) Using Sentinel-2 Data for retrieving LAI and leaf and canopy chlorophyll content of a potato crop. Remote Sens 9:405. https://doi.org/10.3390/rs9050405
de Roos APW, Hayes BJ, Goddard ME (2009) Reliability of genomic predictions across multiple populations. Genetics 183:1545–1553. https://doi.org/10.1534/genetics.109.104935
Deblonde PMK, Ledent JF (2001) Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur J Agron 14:31–41. https://doi.org/10.1016/s1161-0301(00)00081-2
Emmel C, D’Odorico P, Revill A, Hörtnagl L, Ammann C, Buchmann N, Eugster W (2020) Canopy photosynthesis of six major arable crops is enhanced under diffuse light due to canopy architecture. Glob Change Biol 26:5164–5177. https://doi.org/10.1111/gcb.15226
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C (eds), Sustainable Agriculture. Springer Netherlands, Dordrecht, pp 153–188. https://doi.org/10.1007/978-90-481-2666-8_12
Furbank RT, Tester M (2011) Phenomics - technologies to relieve the phenotyping bottleneck. Trends Plant Sci 16:635–644. https://doi.org/10.1016/j.tplants.2011.09.005
Granier C, Aguirrezabal L, Chenu K, Cookson S, Dauzat M, Hamard P, Thioux J, Rolland G, Bouchier-Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol 16:623–635. https://doi.org/10.1111/j.1469-8137.2005.01609.x
Haas M, Sprenger H, Zuther E, Peters R, Seddig S, Walther D, Kopka J, Hincha DK, Köhl KI (2020) Can metabolite- and transcript-based selection for drought tolerance in Solanum tuberosum replace selection on yield in arid environments? Frontiers Plant Sci 11. https://doi.org/10.3389/fpls.2020.01071
Hammer G, Cooper M, Tardieu F, Welch S, Walsh B, van Eeuwijk F, Chapman S, Podlich D (2006) Models for navigating biological complexity in breeding improved crop plants. Trends Plant Sci 11:587–593. https://doi.org/10.1016/j.tplants.2006.10.006
Harrison MT, Tardieu F, Dong ZS, Messina CD, Hammer GL (2014) Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob Change Biol 20:867–878. https://doi.org/10.1111/gcb.12381
Haverkort AJ, Uenk D, Veroude H, Van De Waart M (1991) Relationships between ground cover, intercepted solar radiation, leaf area index and infrared reflectance of potato crops. Potato Res 34:113–121. https://doi.org/10.1007/bf02358105
Hayes BJ, Bowman PJ, Chamberlain AJ, Goddard ME (2009) Invited review: Genomic selection in dairy cattle: progress and challenges. J Dairy Sci 92:433–443. https://doi.org/10.3168/jds.2008-1646
Hill D, Nelson D, Hammond J, Bell L (2021) Morphophysiology of potato (Solanum tuberosum) in response to drought stress: paving the way forward. Front Plant Sci 11. https://doi.org/10.3389/fpls.2020.597554
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Li W, Lin Z, Buckler ES, Qian Q, Zhang Q-F, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42: 961–967. http://www.nature.com/ng/journal/v42/n11/abs/ng.695.html#supplementary-information
Jefferies RA, MacKerron DKL (1987) Thermal time as a non-destructive method of estimating tuber initiation in potatoes. J Agric Sci 108:249–252. https://doi.org/10.1017/S0021859600064339
Jefferies RA, MacKerron DKL (1993) Responses of potato genotypes to drought. II. Leaf area index, growth and yield. Ann Appl Biol 122:105–112. https://doi.org/10.1111/j.1744-7348.1993.tb04018.x
Jensen CR, Battilani A, Plauborg F, Psarras G, Chartzoulakis K, Janowiak F, Stikic R, Jovanovic Z, Li G, Qi X, Liu F, Jacobsen S-E, Andersen MN (2010) Deficit irrigation based on drought tolerance and root signalling in potatoes and tomatoes. Agric Water Manag 98:403–413. https://doi.org/10.1016/j.agwat.2010.10.018
Jindo K, Kozan O, Iseki K, Maestrini B, van Evert FK, Wubengeda Y, Arai E, Shimabukuro YE, Sawada Y, Kempenaar C (2021) Potential utilization of satellite remote sensing for field-based agricultural studies. Chemical and Biological Technologies in Agriculture 8:58. https://doi.org/10.1186/s40538-021-00253-4
Johnson DM (2016) A comprehensive assessment of the correlations between field crop yields and commonly used MODIS products. Int J Appl Earth Obs Geoinf 52:65–81. https://doi.org/10.1016/j.jag.2016.05.010
Junker A, Muraya MM, Weigelt-Fischer K (2015) Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00770
Kamphorst SH, Goncalves GMB, do Amaral AT, de Lima VJ, Leite JT, Schmitt KFM, dos Santos DR, Santos JS, de Oliveira FT, Correa CCG, Rodrigues WP, Campostrini E (2020) Screening of popcorn genotypes for drought tolerance using canonical correlations. Agronomy-Basel 10: 1519. https://doi.org/10.3390/agronomy10101519
Kempenaar C, Been T, Booij J, van Evert F, Michielsen J-M, Kocks C (2017) Advances in variable rate technology application in potato in The Netherlands. Potato Res 60:295–305. https://doi.org/10.1007/s11540-018-9357-4
Kempenaar C., Kocks C.G., Been T.H., van Evert F.K., Nysten S. and Westerdijk K. (2016) Towards data-intensive, more sustainable farming: advances in predicting crop growth and use of variable rate technology in arable crops in the Netherlands. ICPA conference 2016.
Khan MS, Struik PC, van der Putten PEL, Jansen HJ, van Eck HJ, van Eeuwijk FA, Yin XY (2019a) A model-based approach to analyse genetic variation in potato using standard cultivars and a segregating population. I. Canopy cover dynamics. Field Crops Research 242: 107581. https://doi.org/10.1016/j.fcr.2019a
Khan MS, Yin X, van der Putten PEL, Jansen HJ, van Eck HJ, van Eeuwijk FA, Struik PC (2019b) A model-based approach to analyse genetic variation in potato using standard cultivars and a segregating population. II. Tuber bulking and resource use efficiency. Field Crops Research 242: 107582. https://doi.org/10.1016/j.fcr.2019b
Köhl K (2018) Selection and validation experiment comparing phenotypic and marker-assisted selection for drought tolerance in Solanum tuberosum ssp. tuberosum. https://doi.org/10.5447/ipk/2020/18
Köhl KI, Basler G, Luedemann A, Selbig J, Walther D (2008) A plant resource and experiment management system based on the Golm Plant Database as a basic tool for omics research. Plant Methods 4:11. https://doi.org/10.1186/1746-4811-4-11
Köhl KI, Haas M, Mulugeta Aneley G (2022) LIDAR-based phenotyping of potato shoot development under drought stress. https://doi.org/10.5447/ipk/2022/12
Köhl KI, Mulugeta AG, Haas M, Peters R (2021) Confounding factors in container-based drought tolerance assessments in Solanum tuberosum. Agronomy 11:865. https://doi.org/10.3390/agronomy11050865
Ku L, Zhao W, Zhang J, Wu L, Wang C, Wang P, Zhang W, Chen Y (2010) Quantitative trait loci mapping of leaf angle and leaf orientation value in maize (Zea mays L.). J Theoretical Applied Genetics 121:951–959. https://doi.org/10.1007/s00122-010-1364-z
Lafitte HR, Blum A, Atlin G (2003) Using secondary traits to help identify drought-tolerant genotypes. In: Fisher K, Lafitte R, Fukai S, Atlin G, Hardy B (eds), Breeding rice for drought-prone environments. IRRI, Los Baños, pp 37–48
Lhomme JP, Katerji N, Bertolini JM (1992) Estimating sensible heat flux from radiometric temperature over crop canopy. Bound-Layer Meteorol 61:287–300. https://doi.org/10.1007/BF02042936
Li B, Xu XM, Zhang L, Han JW, Bian CS, Li GC, Liu JG, Jin LP (2020) Above-ground biomass estimation and yield prediction in potato by using UAV-based RGB and hyperspectral imaging. ISPRS J Photogramm Remote Sens 162:161–172. https://doi.org/10.1016/j.isprsjprs.2020.02.013
Li C, Li Y, Shi Y, Song Y, Zhang D, Buckler ES, Zhang Z, Wang T, Li YJPo (2015) Genetic control of the leaf angle and leaf orientation value as revealed by ultra-high density maps in three connected maize populations. PLOS One 10: e0121624. https://doi.org/10.1371/journal.pone.0121624
Liu J, Skidmore AK, Wang T, Zhu X, Premier J, Heurich M, Beudert B, Jones S (2019) Variation of leaf angle distribution quantified by terrestrial LiDAR in natural European beech forest. ISPRS J Photogramm Remote Sens 148:208–220. https://doi.org/10.1016/j.isprsjprs.2019.01.005
Maris B (1986) The effect of seed tuber weight on characters in the first and the second clonal generation of potato populations. Euphytica 35:465–482. https://doi.org/10.1007/bf00021855
Martynenko A, Shotton K, Astatkie T, Petrash G, Fowler C, Neily W, Critchley AT (2016) Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. Springerplus 5:1393. https://doi.org/10.1186/s40064-016-3019-2
Millet EJ, Kruijer W, Coupel-Ledru A, Alvarez PS, Cabrera-Bosquet L, Lacube S, Charcosset A, Welcker C, van Eeuwijk F, Tardieu F (2019) Genomic prediction of maize yield across European environmental conditions. Nat Genet 51:952–956. https://doi.org/10.1038/s41588-019-0414-y
Millet EJ, Welcker C, Kruijer W, Negro S, Coupel-Ledru A, Nicolas SD, Laborde J, Bauland C, Praud S, Ranc N, Presterl T, Tuberosa R, Bedo Z, Draye X, Usadel B, Charcosset A, Van Eeuwijk F, Tardieu F (2016) Genome-wide analysis of yield in Europe: allelic effects vary with drought and heat scenarios. Plant Physiol 172:749–764. https://doi.org/10.1104/pp.16.00621
Moll A, Heerkloss B, Griess H, Moller KH (1987) Relationship between morphological characters, rate of photosynthesis and yield of potato genotypes of different maturity classes. 1. Crop height and stem characters. Potato Res 30:89–101. https://doi.org/10.1007/bf02357687
Oladosu Y, Rafii MY, Arolu F, Chukwu SC, Muhammad I, Kareem I, Salisu MA, Arolu IW (2020) Submergence tolerance in rice: review of mechanism, breeding and, future prospects. Sustainability 12:1632
Papadavid G, Hadjimitsis D, Toulios L, Michaelides S (2011) Mapping potato crop height and leaf area index through vegetation indices using remote sensing in Cyprus. J Appl Remote Sensing 5. https://doi.org/10.1117/1.3596388
Prashar AYY, McNicol JW, Bryan GJ, Jones HG (2013) Infra-red thermography for high throughput field phenotyping in Solanum tuberosum. PLoS ONE 8:e65816. https://doi.org/10.1371/journal.pone.0065816
Rakocevic M, Muller M, Matsunaga FT, Neumaier N, Farias JRB, Nepomuceno AL, Fuganti-Pagliarini R (2018) Daily heliotropic movements assist gas exchange and productive responses in DREB1A soybean plants under drought stress in the greenhouse. Plant J 96:801–814. https://doi.org/10.1111/tpj.14069
Rauf S, Al-Khayri JM, Zaharieva M, Monneveux P, Khalil F (2016) Breeding strategies to enhance drought tolerance in crops. In: Al-Khayri JM, Jain SM, Johnson DV (eds), Advances in plant breeding strategies: agronomic, abiotic and biotic stress traits. Springer International Publishing, Cham, pp 397–445. https://doi.org/10.1007/978-3-319-22518-0_11
Ray SS, Das G, Singh JP, Panigrahy S (2006) Evaluation of hyperspectral indices for LAI estimation and discrimination of potato crop under different irrigation treatments. Int J Remote Sens 27:5373–5387. https://doi.org/10.1080/01431160600763006
Richards RA, Hunt JR, Kirkegaard JA, Passioura JB (2014) Yield improvement and adaptation of wheat to water-limited environments in Australia—a case study. Crop Pasture Sci 65:676–689. https://doi.org/10.1071/cp13426
Riedelsheimer C, Lisec J, Czedik-Eysenberg A, Sulpice R, Flis A, Grieder C, Altmann T, Stitt M, Willmitzer L, Melchinger AE (2012) Genome-wide association mapping of leaf metabolic profiles for dissecting complex traits in maize. Proc Natl Acad Sci USA 109:8872–8877. https://doi.org/10.1073/pnas.1120813109
Rosa LM, Dillenburg LR, Forseth IN (1991) Responses of soybean leaf angle, photosynthesis and stomatal conductance to leaf and soil-water potential. Ann Bot 67:51–58. https://doi.org/10.1093/oxfordjournals.aob.a088099
Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F (2007) Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant, Cell Environ 30:135–146. https://doi.org/10.1111/j.1365-3040.2006.01611.x
Sah RP, Chakraborty M, Prasad K, Pandit M, Tudu VK, Chakravarty MK, Narayan SC, Rana M, Moharana D (2020) Impact of water deficit stress in maize: phenology and yield components. Sci Rep 10:2944. https://doi.org/10.1038/s41598-020-59689-7
Schafleitner R, Gutierrez R, Espino R, Gaudin A, Pérez J, Martínez M, Domínguez A, Tincopa L, Alvarado C, Numberto G, Bonierbale M (2007) Field screening for variation of drought tolerance in Solanum tuberosum L. by agronomical, physiological and genetic analysis. Potato Res 50:71–85. https://doi.org/10.1007/s11540-007-9030-9
Slater AT, Cogan NOI, Hayes BJ, Schultz L, Dale MFB, Bryan GJ, Forster JW (2014) Improving breeding efficiency in potato using molecular and quantitative genetics. Theor Appl Genet 127:2279–2292. https://doi.org/10.1007/s00122-014-2386-8
Sprenger H, Erban A, Seddig S, Rudack K, Thalhammer A, Le MQ, Walther D, Zuther E, Köhl KI, Kopka J, Hincha DK (2018) Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotechnol J 16:939–950. https://doi.org/10.1111/pbi.12840
Sprenger H, Rudack K, Schudoma C, Neumann A, Seddig S, Peters R, Zuther E, Kopka J, Hincha DK, Walther D, Köhl K (2015) Assessment of drought tolerance and its potential yield penalty in potato. Funct Plant Biol 42:655–667. https://doi.org/10.1071/FP15013
Su Y, Wu F, Ao Z, Jin S, Qin F, Liu B, Pang S, Liu L, Guo Q (2019) Evaluating maize phenotype dynamics under drought stress using terrestrial LIDAR. Plant Methods 15:11. https://doi.org/10.1186/s13007-019-0396-x
Tang R, Niu S, Zhang G, Chen G, Haroon M, Yang Q, Rajora OP, Li X-Q (2018) Physiological and growth responses of potato cultivars to heat stress. Botany 96:897–912. https://doi.org/10.1139/cjb-2018-0125
Tardieu F (2012) Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J Exp Bot 63:25–31. https://doi.org/10.1093/jxb/err269
ten Harkel J, Bartholomeus H, Kooistra L (2020) Biomass and crop height estimation of different crops using UAV-based LIDAR. Remote Sensing 12. https://doi.org/10.3390/rs12010017
Tian F, Bradbury PJ, Brown PJ, Hung H, Sun Q, Flint-Garcia S, Rocheford TR, McMullen MD, Holland JB, Buckler ES (2011) Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat Genet 43:159–162. https://doi.org/10.1038/ng.746
van Zanten M, Pons TL, Janssen JAM, Voesenek LACJ, Peeters AJM (2010) On the relevance and control of leaf angle. Crit Rev Plant Sci 29:300–316. https://doi.org/10.1080/07352689.2010.502086
Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Cruz MTS, Amante M, Kumar A, Atlin GN (2009) Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor Appl Genet 120:177–190. https://doi.org/10.1007/s00122-009-1168-1
Weisz R, Kaminski J, Smilowitz Z (1994) Water-deficit effects on potato leaf growth and traspiration - utilizing fraction extractable soil-water for camparison with other crops. Am Potato J 71:829–840. https://doi.org/10.1007/bf02849378
Welcker C, Boussuge B, Bencivenni C, Ribaut JM, Tardieu F (2007) Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of Anthesis-Silking Interval to water deficit. J Exp Bot 58:339–349. https://doi.org/10.1093/jxb/erl227
Werner C, Ryel RJ, Correia O, Beyschlag W (2001) Structural and functional variability within the canopy and its relevance for carbon gain and stress avoidance. Acta Oecologica-International Journal of Ecology 22:129–138. https://doi.org/10.1016/s1146-609x(01)01106-7
West H, Quinn N, Horswell M (2019) Remote sensing for drought monitoring & impact assessment: progress, past challenges and future opportunities. Remote Sens Environ 232:111291. https://doi.org/10.1016/j.rse.2019.111291
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442:705–708
Xu S, Atherton J, Riikonen A, Zhang C, Oivukkamäki J, MacArthur A, Honkavaara E, Hakala T, Koivumäki N, Liu Z (2021) Structural and photosynthetic dynamics mediate the response of SIF to water stress in a potato crop. J Remote Sensing of Environment 263:112555
Yin XY, Goudriaan J, Lantinga EA, Vos J, Spiertz HJ (2003) A flexible sigmoid function of determinate growth. Ann Bot 91:361–371. https://doi.org/10.1093/aob/mcg029
Yuan B-Z, Nishiyama S, Kang Y (2003) Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric Water Manag 63:153–167. https://doi.org/10.1016/S0378-3774(03)00174-4
Zhao XQ, Fang P, Zhang JW, Peng YL (2018) QTL mapping for six ear leaf architecture traits under water-stressed and well-watered conditions in maize (Zea mays L.). Plant Breeding 137:60–72. https://doi.org/10.1111/pbr.12559
Acknowledgements
We thank D. Zerning, L. Westphal and M. Walenko for skilled technical assistance, and J. Bergstein, A. Svens and B. Maltry for photographic work. We thank the breeders of the GFPi for the donation of planting material and R. Peters, S. Seddig, R. Hofferbert, J. Strahwald and B. Truberg for many enlightening discussions. We thank R. Peters for critically reading the manuscript. The contribution of H. Lindqvist-Kreuze and A. Khan (both International Centre for Potato, Peru), who initiated and coordinated the project ‘Accelerating the Development of Early-Maturing-Agile Potato for Food Security through a Trait Observation and Discovery Network’, is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by grant 107945 ‘Accelerating the Development of Early-Maturing-Agile Potato for Food Security through a Trait Observation and Discovery Network’ of the Federal Ministry for Economic Cooperation and Development and by grants No. 22011208 and 22010913 of the Fachagentur Nachwachsende Rohstoffe (Federal Ministry of Food and Agriculture), Germany, and the Gemeinschaft zur Förderung der Privaten Deutschen Pflanzenzüchtung (GFPi).
Author information
Authors and Affiliations
Contributions
Conduction of the phenotyping experiments, data collection and analysis were performed by Manuela Haas, Gedif Mulugeta Aneley and Karin Köhl. The first draft of the manuscript was written by Karin Köhl and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mulugeta Aneley, G., Haas, M. & Köhl, K. LIDAR-Based Phenotyping for Drought Response and Drought Tolerance in Potato. Potato Res. 66, 1225–1256 (2023). https://doi.org/10.1007/s11540-022-09567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-022-09567-8