Abstract
For the past two decades, the USA has been embroiled in a growing prescription drug epidemic. The ripples of this epidemic have been especially apparent in the state of Maine, which has fought hard to mitigate the damage caused by addiction to pharmaceutical and illicit opioids. In this study, we construct a mathematical model of the opioid epidemic incorporating novel features important to better understanding opioid abuse dynamics. These features include demographic differences in population susceptibility, general transmission expressions, and combined consideration of pharmaceutical opioid and heroin abuse. We demonstrate the usefulness of this model by calibrating it with data for the state of Maine. Model calibration is accompanied by sensitivity and uncertainty analysis to quantify potential error in parameter estimates and forecasts. The model is analyzed to determine the mechanisms most influential to the number of opioid abusers and to find effective ways of controlling opioid abuse prevalence. We found that the mechanisms most influential to the overall number of abusers in Maine are those involved in illicit pharmaceutical opioid abuse transmission. Consequently, preventative strategies that controlled for illicit transmission were more effective over alternative approaches, such as treatment. These results are presented with the hope of helping to inform public policy as to the most effective means of intervention.
Similar content being viewed by others
1 Introduction
Since the late 1990s, the opioid epidemic has affected millions of Americans per year and as recently as 2018 was killing more than a hundred Americans per day (HHS 2019). April 2021 marked the first time that drug overdose deaths surpassed 100,000 in a 12-month period, with opioid overdoses comprising the majority (CDC 2021a). Opioid analgesic prescriptions picked up speed in the late 1990s in response to growing advocacy for the treatment of patient pain. Pain would eventually be recognized as the fifth vital sign thanks to the efforts of various federal and medical institutions (Walid et al. 2008). As a result, opioid prescriptions would erupt in the next decade, increasing by over 35% (Kenan et al. 2012). In this short amount of time, the opioid epidemic would balloon to an imposing size. Rates of nonmedical opioid use and abuse followed a similar trajectory as prescriptions (Zacny et al. 2003), with overdose deaths in tow. Eventually, research would help elucidate the addictive potential of opioid analgesics (Kosten and George 2002), as well as establish links between opioid dosage and overdose risk (Dunn et al. 2010; Zacny et al. 2003). Neurobiological findings suggested that environmental, social, and even genetic factors are implicated in increasing this risk (Sehgal et al. 2012; Kosten and George 2002). This and other findings led to more stringent practices in opioid prescription, reaching a peak in 2012 and declining in subsequent years, a trajectory that has persisted to the present day (Guy Jr. et al. 2017; CDC 2021b).
The opioid epidemic remains an issue of central importance despite the downward trend in prescriptions, even growing in complexity due to the popularity of synthetic opioids and the COVID-19 pandemic. A large part of this complexity has to do with the interplay between pharmaceutical and nonpharmaceutical opioids—the latter including drugs such as heroin and fentanyl. In addition, the COVID-19 pandemic has worsened the situation in many ways (Haley and Saitz 2020; Ochalek et al. 2020; Rabin 2021; Slavova et al. 2020). Healthcare facilities, strained by the pandemic, have found it difficult to reconcile treatment services for those afflicted with opioid abuse. Since 2020, opioid overdose mortalities have increased by nearly 30% (Rabin 2021). In some areas, during the same four-month window from 2019 to 2020, the number of nonfatal opioid overdose visits doubled (Ochalek et al. 2020).
To address the renewed ferocity of the opioid epidemic, it is imperative that steps are taken to best mitigate its continued growth. To help inform this process, we propose a novel mathematical model of the opioid epidemic. We demonstrate the usefulness of this model by calibrating it using publicly accessible data of the opioid epidemic in the state of Maine. This framework is a step forward in understanding the opioid epidemic. The most significant contributions are the simultaneous consideration of pharmaceutical opioid and heroin abuse, the interactions of different opioid abusers, the use of general incidence rates to accommodate social contagions, and accounting for differences in susceptibility to opioid abuse. We hope this will serve not only as a tool to inform opioid policy and epidemic management, but also as a megaphone to draw attention to data needs that, if resolved, will assist in further research and understanding.
To preface our study of the opioid epidemic in Maine, the general framework we present distinguishes between the dynamics of pharmaceutical and nonpharmaceutical opioids. Although the abuse dynamics of these classes are inextricably linked, they differ substantially in isolation. Pharmaceutical opioid abuse results from prescriptions and diversion, while nonpharmaceutical opioid abuse manifests predominantly in an illicit manner. Diverted prescription drugs are those that are illegally channeled and made available illicitly. Pharmaceutical opioids are those that can be obtained through a prescription, while nonpharmaceutical drugs include everything else. Although we discuss these opioid classes in general, it may be more useful (e.g., because of data limitations) to model one opioid in particular from each class, which is the approach we take here. That is, rather than consider nonpharmaceutical opioid abuse broadly, we confine our study to heroin abuse specifically. We model drug abuse spread drawing ideas from susceptible-infectious disease models. In this case, nonabusing “susceptible” individuals may be “infected” by drug abusers.
Previous mathematical model studies have looked at only a single class of opioids, such as prescription opioids (Battista et al. 2019; Caldwell et al. 2019) or heroin/illicit opioids (White and Comiskey 2007; Liu and Zhang 2011; Samanta 2011; Cole and Wirkus 2022). Unfortunately, most mathematical models in the literature do not account for the transition of individuals between pharmaceutical and nonpharmaceutical opioid abuse (Sharareh et al. 2019). The importance of considering multiple types of opioids cannot be understated, owing to the link between illicit and prescription sources of opioid addiction. For example, one recent survey found that nearly 80% of heroin abusers were first introduced to opioids via prescription drugs (Cicero et al. 2014). The authors of (Jones et al. 2015) found that individuals with past-year opioid pain reliever abuse or dependence were most likely to use heroin. Research has shown that a transition to illicit opioids following prescription use typically occurs because illicit sources tend to be cheaper and more readily available (Compton et al. 2016). Thus, the interplay between pharmaceutical and nonpharmaceutical opioids in drug abuse patterns in a population is critical.
The work of Pitt et al. (Pitt et al. 2018) considered both prescription opioid and heroin use disorders, but individuals in the latter compartment do not contribute to drug abuse propagation in their model. Nonlinear interactions in a mathematical model of the opioid epidemic should be considered because of how social contagions such as drug abuse spread in a population, specifically how behavior propagation inherently relies on interactions between individuals of different drug abuse statuses (Behrens et al. 2000). This is a component in most mechanistic models but is neglected in other modeling studies, such as more policy- and prediction-oriented models (e.g., (Chen et al. 2019; Ballreich et al. 2020; Alexander et al. 2021)); hence, why mechanistic models have been referred to as the more practical modeling tool (Sharareh et al. 2019).
To our knowledge, besides the work presented here, (Phillips et al. 2021) is the only work to date that considers both pharmaceutical and nonpharmaceutical opioids as well as nonlinear transmission between drug abusers. The authors of (Phillips et al. 2021) distinguish between susceptibles, prescription opioid users, prescription opioid addicted, heroin/fentanyl addicted, and a “stably” recovered compartment. Our proposed model expands on this work by incorporating demography-based susceptibility, exploring incidence rates beyond the conventional assumption of mass-action, and using more granular data in model calibration. Our consideration of more general incidence rates raises interesting questions regarding policy, and we hope our validation of model assumptions using uncertainty quantification can serve as a template for future modelers. The importance of these contributions will be argued in the sections to come, but with this work we hope to contribute to the growing body of mechanistic models that consider the transitions and interplay between pharmaceutical and nonpharmaceutical opioid abuse.
The rest of this paper is structured as follows: The following section introduces a general opioid modeling framework and fits it to Maine opioid mortality data. The model is analyzed in Sect. 3, including parameter uncertainty and model forecasts in Sect. 3.1, and a sensitivity analysis in Sect. 3.2. An investigation of control strategies within the context of the findings of previous sections is presented in Sect. 3.3. Finally, we conclude by interpreting our results, highlighting study limitations, and suggesting areas of future work in Sect. 4.
2 An Opioid Epidemic Modeling Framework
We compartmentalize the population into “susceptible” individuals, “infected” or active opioid abusers, and “in recovery/treatment.” An individual is susceptible if they are not currently abusing opioids but are at risk of doing so, while infected refers to a status of current abuse. We define current abuse as any opioid usage in the past month, following the definition used by the National Household Survey on Drug Abuse (SAMHSA 2002). Following the approach of (Battista et al. 2019), who in turn refer to (Vowles et al. 2015), we take abuse to mean “nonmedical use with the potential for harm.” This definition includes all harmful use of opioids, specifically opioid misuse and abuse, which will be used interchangeably henceforth. Individuals in recovery are undergoing treatment/rehabilitation and consequently are not actively abusing opioids.
Susceptible and infected individuals are further broken up into separate compartments; susceptible individuals are classified as being either low-risk (\(S_{\textrm{LR}}\)) or high-risk (\(S_{\textrm{HR}}\)). This designation allows the model to account for factors that contribute to a higher risk of initiating pharmaceutical opioid abuse, including but not limited to comorbid psychopathology and history of substance use disorder (see (Sehgal et al. 2012) and the references therein). Low-risk susceptible individuals can only initiate pharmaceutical opioid abuse through a naturally developed addiction resulting from prescription use, which occurs at rate \(\epsilon _{\textrm{LR}}\). High-risk susceptibles are k times more likely than low-risk susceptibles to initiate pharmaceutical opioid abuse via the same mechanism and are also capable of developing a use disorder via diverted drugs or contact with opioid abusers. We ignore movement between susceptible risk compartments. While it is true that the risk classification of an individual can change in practice (i.e., direct movement from low-risk to high-risk or vice versa), modeling this phenomenon heavily depends on the predictor(s) of choice. Because of this and the difficulties associated with measuring said predictors, we opt to make the simplifying assumption that individuals can only change their risk classification from low risk to high risk (i.e., indirectly after moving through the other compartments).
Infected individuals are classified by the type of opioid they abuse; these are either pharmaceutical opioids (\(A_\textrm{P}\)) or heroin (\(A_\textrm{H}\)). Pharmaceutical opioids are those that can be obtained through a prescription. We ignore comorbid opioid abuse, i.e., simultaneous abuse of pharmaceutical opioids and heroin. Individuals abusing pharmaceutical opioids need not have obtained these drugs with a prescription. In general, we choose to distinguish between pharmaceutical opioids and heroin because of the stark differences between either drug in terms of overdose mortality, means of addiction, and acquisition. Genetic determinants in drug abuse have been found to account for up to \(60\%\) of an individual’s risk (Kreek et al. 2005). Therefore, opioid prescription inherently risks the development of an abuse disorder. The parameter \(\epsilon _{\textrm{LR}}\) is the product of the proportion of the population with a prescription and this natural abuse rate. Individuals that leave recovery/treatment enter the high-risk susceptible compartment. This way, the mechanisms of relapse and recovery are built into the dynamics of our model. This is in contrast to other models that include nonlinear movement out of treatment and into an abuse compartment (Battista et al. 2019; White and Comiskey 2007). Individuals can pursue treatment to cease active abuse, in which case they enter the recovered compartment (R). Some proportion of these individuals are still susceptible to opioid abuse and will thus re-enter the high-risk susceptible compartment.
Movement into and between abusing compartments occurs by different processes, represented by either linear or nonlinear expressions in the model. Movement is linear when it takes place absent the influence of other individuals. The linear movements in our model are as follows:
-
(i)
individuals entering the low-risk susceptible compartment via a recruitment rate term (\(\Lambda \)) and exiting all compartments through nonoverdose death (\(\mu \));
-
(ii)
progression to pharmaceutical opioid abuse from the low-risk susceptible compartment (\(\epsilon _{\textrm{LR}}\)) or the high-risk susceptible compartment (\(k \epsilon _{\textrm{LR}}\) with \(k > 1\)) as a result of a prescription;
-
(iii)
pharmaceutical opioid and heroin abusers seeking treatment (\(\eta _\textrm{P}\) and \(\eta _\textrm{H}\), respectively);
-
(iv)
pharmaceutical opioid and heroin abusers exiting abuse compartments due to overdose death (\(\mu _\textrm{P}\) and \(\mu _\textrm{H}\), respectively);
-
(v)
pharmaceutical opioid and heroin abusers ceasing drug abuse for reasons other than death and treatment (\(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\), respectively, which may differ as active drug use fluctuates for many reasons beyond treatment and overdose mortality (Cicero et al. 2014)); and
-
(vi)
recovering individuals exiting treatment and re-entering the high-risk susceptible compartment according to the treatment discharge rate (\(\tau \)).
Nonlinear movements are assumed when mechanisms involve the influence of other individuals. This is the social contagion aspect of our model, as drug abusers can “transmit” opioid abuse to other individuals (Behrens et al. 2000). We assume that only high-risk susceptibles are capable of nonlinear recruitment into the two abuse compartments. That is, low-risk susceptibles do not initiate opioid abuse as a result of interactions with drug abusers. The nonlinear modes of transmission are functions of a general susceptible and infected population, as well as a parameter vector we denote by \(\varvec{\theta }\). These functions and their roles are as follows: initiation of abuse as a result of diverted pharmaceutical opioids, \(f_1(S_{\textrm{HR}}, \ A_\textrm{P}, \ \varvec{\theta })\); recruitment directly into the heroin abuse compartment, \(f_2(S_{\textrm{HR}}, \ A_\textrm{H}, \ \varvec{\theta }\)); and progression from pharmaceutical opioid to heroin abuse, \(f_3(A_\textrm{P}, \ A_\textrm{H}, \ \varvec{\theta })\). In the case of a social contagion, contact need not be physical and multiple contacts or infected individuals may be required for transmission, thus the transmissibility of the contagion need not be linearly proportional to prevalence in the population. A variety of incidence rates that take this into consideration were tested when fitting the model (see the next section and Supplementary Material A for fitting details). The incidence rate producing the best fit was \(f_i(X,Y,\varvec{\theta }) = \beta _i X \left( \frac{Y}{N}\right) ^{q_i}\). Here, individuals in Y infect those in X, N is the total number of individuals in the population, \(q_i > 0\) represents the number of infected individuals required for transmission to occur, and \(\beta _i > 0\) is a transmission parameter. For a social contagion, it may not be the case that transmission is bilinear (i.e., \(q_i=1\)). For example, a single heroin abuser may influence a disproportionate number of susceptibles in the community. For \(q_i>1\), transmission is slower than that assuming standard incidence, as susceptible individuals require more contacts in order to become infected. Employing the incidence rate above, the full system of ODEs to model the opioid epidemic is given as follows:
with initial conditions \(S_{\textrm{LR}}(0)=S_{\textrm{LR},0} \ge 0\), \(S_{\textrm{HR}}(0) =S_{\textrm{HR},0}\ge 0\), \(A_\textrm{P}(0)=A_{\textrm{P},0}\ge 0\), \(A_\textrm{H}(0) = A_{\textrm{H},0}\ge 0\), \(R(0) = R_0 \ge 0\) satisfying
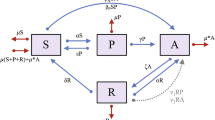
where N is the total population, assumed to be constant by choosing \(\Lambda = \mu N + \mu _\textrm{P} A_\textrm{P} + \mu _\textrm{H} A_\textrm{H}\). Parameter descriptions are given in Table 1. Note that there is no direct movement back into the pharmaceutical opioid abuse compartment after an individual has progressed to abusing heroin. However, movement between the two abuse compartments is still possible via intermediate transitions through the high-risk susceptible compartment, e.g., an individual abuses pharmaceutical opioids, seeks treatment, recovers with high-risk status, and progresses to heroin abuse. A schematic of the model is shown in Fig. 1, with nonlinear transmission terms denoted by \(f_i\). Under the assumptions listed above, standard arguments yield that (1) admits a unique solution, local to the initial conditions, in the invariant physical domain
A schematic of the model in (1). Dashed lines indicate mortality rates
2.1 Fitting the Model to the Opioid Epidemic in Maine
We apply the framework presented in (1) to the opioid epidemic for the adult (18+) population in Maine during the years 2012–2019. As stated above, we restrict consideration of nonpharmaceutical opioids to heroin, due to data availability. For this case study, we took a history of substance use disorder to be the predictor distinguishing low- and high-risk susceptibles. This choice of predictor is based on its faculty in determining an individual’s risk in abusing opioids, as shown in (Sehgal et al. 2012). Primarily due to limited data, we assume that all individuals entering the system (i.e., that turn 18 years of age) are low risk. The initial condition for \(S_{\textrm{HR}}\) is the estimated proportion of Maine adults who had a history of dependence or abuse of illicit drugs or alcohol during 2012, or around \(8.5\%\), as recorded by the National Survey on Drug Use and Health (NSDUH) (SAMHSA 2014a). Initial conditions for the abuse compartments \(A_\textrm{P}\) and \(A_\textrm{H}\) were calculated according to the mortality data obtained from CDC WONDER (CDC 2020). This estimate was obtained by dividing the number of overdoses to either opioid class in January 2012 by their respective overdose mortality rate. For example, if 20 individuals died of a pharmaceutical opioid overdose in January 2012, then the initial condition used is \(A_{\textrm{P},0} = 20/\mu _\textrm{P}\). The initial recovered population was set to zero, \(R_0 = 0\), so that \(S_{\textrm{LR},0} = N - S_{\textrm{HR},0} - A_{\textrm{P},0} - A_{\textrm{H},0} - R_0\).
There are several parameter values drawn from the literature. These include the treatment discharge rate, \(\tau \), the natural death rate, \(\mu \), the progression rate from pharmaceutical opioid use to abuse, \(\epsilon _{\textrm{LR}}\), and the number of times more likely a high-risk individual is than a low-risk individual of progressing from pharmaceutical opioid use to abuse, k. For the treatment discharge rate \(\tau \), we assume \(90\%\) of individuals will relapse in their first year following recovery based on the findings of (Smyth et al. 2010; Bailey et al. 2013) [(see also the discussion in (Battista et al. 2019)]. The remaining \(10\%\) of abusers remain in R and are assumed to be in recovery, but are not immune from further opioid abuse. The rate of movement from pharmaceutical opioid use to abuse, \(\epsilon _{\textrm{LR}}\), is a product of two factors: (i) the probability of having an opioid prescription and (ii) the rate at which an individual with an opioid prescription develops an abuse disorder. Piper et al. (Piper et al. 2016) found that \(21.9\%\) of Mainers possess an opioid prescription. Since this includes individuals younger than 18 years of age (and thus outside the scope of our model), the prescribed proportion is assumed constant at \(20\%\) of the adult population. The authors of (Edlund et al. 2010) estimated k to be 2.87 for nonopioid substance use disorders, but this parameter varies depending on the database used and could be much higher (see, e.g., (Edlund et al. 2014)). In the same study, the authors found that individuals with an opioid substance use disorder were 5.55 times more likely than those without to initiate opioid abuse/dependence (Edlund et al. 2010). We decided on the more conservative estimate mentioned because nonopioid substance use disorders (including, but not limited to, those involving alcohol and marijuana) constitute the majority of those substance use disorders observed, according to NSDUH estimates.
Overdose rates for both abuser compartments were determined from the CDC WONDER database mentioned above. Approximate annual abuser counts in Maine were obtained from the State Estimates of Mental Health and Substance Use, reported by NSDUH (SAMHSA 2014a, b, 2015, 2017a, b, 2018, 2019). This database includes estimates for the number of individuals abusing pharmaceutical opioids and heroin in any given year. To determine the former, an estimate for the prevalence of individuals with a pain reliever use disorder was used as a proxy. For the years in which this prevalence was not provided in the report, values were inferred based on the assumption that a certain proportion of the population nonmedically using prescription pain relievers (as estimated by NSDUH) will develop a substance use disorder. To estimate the annual number of individuals abusing heroin, the NSDUH prevalence estimate for individuals using heroin in the past year was used. Finally, NSDUH prevalence data were used in combination with CDC WONDER overdose data to estimate the average monthly overdose rates, \(\mu _\textrm{H}\) and \(\mu _\textrm{P}\).
Treatment parameters, \(\eta _\textrm{P}\) and \(\eta _\textrm{H}\), were determined from annual treatment admission data, reported yearly in the Substance Use Trends in Maine State Epidemiological Profile (DHHS 2015, 2016, 2017, 2018, 2019). When admitted to treatment, an individual identifies a primary and secondary drug as a reason for seeking treatment. The yearly report includes categories, such as heroin/morphine, based on the drug identified by admitted patients. These category amounts were tallied together in accordance with the distinction of pharmaceutical opioids/heroin employed by CDC WONDER and used for the calculation of either treatment rate. The treatment data used for model calibration here includes primary and secondary admissions and is therefore vulnerable to duplicate reporting. Tertiary reasons for admission are reported some years but were not included to compensate for this overcounting. Treatment parameter values were obtained by calculating the average treatment rates for the years in which data were available and converting these averages to monthly rates. The total population, N, is taken to be the average Maine adult population during 2012–2019.
The remaining eight parameters (\(\beta _{1}\), \(\beta _{2}\), \(\beta _{3}\), \(q_1\), \(q_2\), \(q_3\), \(\sigma _{P}\), \(\sigma _\textrm{H}\)), as well as the choice of incidence rate functional form, \(f_i(X,Y,\varvec{\theta }) = \beta _i X \left( \frac{Y}{N}\right) ^{q_i}\), were fitted to Maine data. Model calibration was done with monthly overdose death data from CDC WONDER (CDC 2020) using overdose data recorded for the state of Maine from 2012–2019 for both pharmaceutical opioid overdoses (ICD-10 codes T40.2-T40.4) and heroin overdoses (ICD-10 code T40.1); causes of death included those of any intent except for suicide (ICD-10 codes X40-44, X85, and Y10-14). This data set, containing \(96 \times 2=192\) data points corresponding to pharmaceutical opioid overdoses and heroin overdoses over a 96 month period (2012–2019), was then smoothed by taking a rolling (nearest neighbor) average to reduce inherent noise: the raw data point \(x_i=x(t_i)\) at month \(t_i\) is re-scaled as \(x_i=(x_{i-1} + x_i + x_{i+1})/3\), \(i=2,\ldots ,95\) (with \(x_1=(x_{1}+x_{2})/2\) and \(x_{96}=(x_{95}+x_{96})/2\) for the boundary cases). Model fitting was done with lsqcurvefit in MATLAB using the trust-region reflective algorithm with a generalized least squares (GLS) scheme following the approach in (Banks et al. 2016a) using second-order differencing to calculate the pseudo-measurement errors (Banks et al. 2017) (outlined in detail in Supplementary Material A, along with the other incidence rates we considered).
A summary of parameter values and their respective sources are given in Table 1. Initiation of heroin abuse from either the high-risk susceptible or pharmaceutical opioid abuser compartments is well approximated by standard incidence, as \(q_2, \ q_3 \approx 1\). This is not the case with pharmaceutical opioid abuse as a result of diversion, since \(q_1 > 1\). This means that, compared to other mechanisms of abuse, fewer heroin abusers are needed in order to have sustained transmission of heroin abuse in the community. The model fit is plotted in Fig. 2 using the parameters from Table 1.
3 Model Analysis
We next analyze the opioid epidemic model in (1). As mentioned in Sect. 2, it follows from standard ODE theory that the opioid epidemic model in (1), with initial conditions satisfying (2) admits a unique solution local to said initial conditions, invariant to the physically meaningful domain (3). The model in (1) admits an abuse-free equilibrium that is given by
under the restrictive assumption that \(\epsilon _{\textrm{LR}}=0\) (i.e., the absence of opioid prescriptions or the use of completely nonaddictive opioids), in line with other works in the literature (e.g., (Phillips et al. 2021; Battista et al. 2019)). In such a case, a standard stability analysis applied to (1) fails because of the form of the model (even if \(q_i=1\) is assumed for all i); hence, the basic reproduction number (Van den Driessche and Watmough 2002; van den Driessche and Watmough 2008) cannot be calculated. Though we emphasize again that this scenario is less interesting because of the restrictive (and unrealistic) requirement that \(\epsilon _{\textrm{LR}}=0\). From numerical investigations, there also seems to be an abuse endemic equilibrium where \(A_\textrm{P}^*>0\) and \(A_\textrm{H}^*=0\), though it is difficult to obtain explicitly in closed-form. Because of the time horizon of interest in this work and because the basic reproduction number (and stability analysis) is not a viable avenue here, we proceed with an uncertainty analysis and forecast (Sect. 3.1), sensitivity analysis (Sect. 3.2), and an investigation of control strategies to manage the opioid epidemic (Sect. 3.3).
3.1 Parameter Uncertainty and Model Forecasts
In this part, we seek to quantify model uncertainty in our parameter estimates and predict future trajectories of the epidemic in the years 2020–2025. Uncertainty in our parameter estimates (i.e., those listed in Table 1) is measured by the coefficient of variation (CV), following the work of (Sutton et al. 2008) and (Banks et al. 2016b). (Full details of the uncertainty analysis are given in Supplementary Material A.) In brief, a conventional GLS fitting of the eight parameters produced high correlations between parameters. To help alleviate this issue, parameter subset selection was performed in order to fix the least number of parameters at their nominal values and still achieve the greatest reduction in parameter correlations. It is important to note that fixing parameter values essentially changes the model we are analyzing. We found that fixing \(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\) produced the best results, and the GLS algorithm was re-executed on the reduced parameter set to arrive at the new best-fit values, \(\widehat{\varvec{\theta }}_\textrm{FIX}\). Even after parameter subset selection, parameter correlations remained. Unsurprisingly, these were the \(q_i\) and \(\beta _i\) parameter pairs, owing to the functional form of the transmission terms in (1). The associated standard errors (S.E.’s) and coefficients of variation, which characterize estimate uncertainty, are given in Table 2. The latter coefficient is obtained by dividing the S.E. by the parameter estimate, allowing us to compare the relative uncertainty for each parameter—the larger the CV, the more uncertain the estimate. From Table 2, we see that the estimate of \(\beta _3\) is the most uncertain. In fact, if we consider the \(\beta _i\) and \(q_i\) parameters together according to the transmission expressions in which they appear, one sees that the fitted parameters of the incidence rate controlling recruitment into heroin abuse from pharmaceutical opioid abuse (\(\beta _3\) and \(q_3\)) are the most uncertain. Thus, within our modeling framework, mechanisms of heroin abuse following pharmaceutical opioid abuse are least informed by monthly overdose death data, relative to the other avenues of transmission in (1).
To determine parameter distributions, 500 iterations of a bootstrap algorithm were performed, following the methods in (Banks et al. 2016b) (see Supplementary Material A for algorithm details). Briefly, sampling of the data is done with replacement and synthetic noise is added. A new fit is then produced using the same GLS routine as before, and the subsequent parameter values were stored. From the bootstrap results, \(95\%\) confidence intervals were calculated (see Table 2). The GLS best-fit parameter values for the reduced parameter set described above, \(\widehat{{\theta }}_{FIX}\), and the bootstrap estimate, \(\varvec{\theta }_\textrm{BOOT}\) (obtained by taking the mean of all parameter distributions), are also given in Table 2. Note that the bootstrap algorithm was performed with \(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\) fixed as well.
Forecasts of the model are calculated using \(\varvec{\widehat{\theta }}_\textrm{GLS}\) (i.e., values from Table 1), as shown in Fig. 3 for the case of pharmaceutical opioid overdose deaths, alongside the approximate 95% confidence intervals (i.e., two standard deviations). The forecasts are compared to overdose data released for the year 2020 (the 12 black dots in Fig. 3); the agreement between the 2020 data and our model is encouraging, but it should be noted that the COVID-19 pandemic has disrupted treatment facilities, complicated data reporting, altered drug testing practices, and, in some cases, exacerbated the severity of the opioid epidemic (Haley and Saitz 2020; Ochalek et al. 2020; Rabin 2021; Slavova et al. 2020). Recalling that the total population is fixed, and noting that variations in \(\Lambda \) are relatively minor over the time horizon of interest, the oscillations appearing in Fig. 3 are due to individuals moving between the recovery/high-risk susceptible compartments and active opioid abuse. (The onset of these oscillations can also be seen in Fig. 2.)
As mentioned before, fixing certain parameters changes the model but, in doing so, correlations between parameters can be reduced to allow for more accurate parameter estimates. In practice, the rates at which individuals cease opioid abuse for reasons other than treatment or overdose are much simpler to quantify than the (more important) transmission parameters \(\beta _i\) and \(q_i\). In this section we have demonstrated how this can be leveraged to arrive at parameter estimates with less uncertainty: namely, efforts should be undertaken to estimate \(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\) from real-world data. That being the case, the analysis in the remainder of this paper will concern the original eight fitted parameters, \(\widehat{\varvec{\theta }}_{\textrm{GLS}}\).
Monthly overdose deaths due to pharmaceutical opioids from the model in (1) (solid blue curve), with 96 data points (red dots) and 12 forecast points (black dots) overlaid, and \(2 \sigma \) confidence intervals (shaded area). The black dotted vertical line delineates the beginning of the forecast period. (CDC WONDER data policy is to suppress data points of 9 or fewer, indicated by the red dashed line.) (Color figure online)
3.2 Sensitivity Analysis
Next we use sensitivity analysis for the purpose of identifying key trends and mechanisms in (1). The relative local sensitivity of the ith state variable \(x_i\) (here, \(\varvec{x} = (S_{\textrm{LR}}, \ S_{\textrm{HR}}, \ A_\textrm{P}, \ A_\textrm{H},\ R)\)) with respect to the jth parameter \(\theta _j\) is given by
where \(\theta _{0,j}\) is the baseline value of the jth parameter. This function is calculated for each state variable and parameter combination. To quantify overall relative sensitivities for means of comparison, we calculate the following indices:
These indices are, respectively, the average absolute relative sensitivity of the pharmaceutical opioid abuser population, the heroin abuser population, the high-risk susceptible population, and the recovered population to each parameter over the 96-month period 2012–2019.
The calculated indices for each parameter are given in Table 3, which can be grouped together based on their effect on the total number of abusers. These results show that the most influential parameters are the exponents \(q_1\), \(q_2\), followed by \(q_3\) and the transmission parameters \(\beta _1\) and \(\beta _{2}\), and, interestingly, the linear rates of movement \(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\). The exponents \(q_1\) and \(q_2\) control the number of exposures needed for a susceptible individual to initiate pharmaceutical opioid and heroin abuse, respectively. For example, in the incidence rate given by \(\beta _{1}S_{\textrm{HR}}(A_\textrm{P}/\textrm{N})^{q_1}\), a value of \(q_1 = 1.44\) indicates that between one and two abusers in \(A_\textrm{P}\) are required to recruit a high-risk susceptible individual. In this case, transmission is slower than would be the case if we had assumed mass-action incidence (i.e., \(q_1 = 1\)). Either transmission parameter draws from the high-risk susceptible compartment, or those individuals with a history of substance use disorder. Slight changes to these parameters can significantly impact the size of the epidemic, especially in later years. This final point is emphasized by the plots in Figs. 4a, b, which show the relative rate of change of either abuser compartment with respect to \(q_1\) and \(q_2\), respectively, versus time. In the case of \(q_1\), the negative relative sensitivity depicted in Fig. 4a indicates that any perturbation to \(q_1\) produces an opposite change to either opioid abuser compartment. That is, increasing \(q_1\) decreases the number of individuals in \(A_\textrm{P}\) and \(A_\textrm{H}\) over time. The opposite phenomenon is observed for the parameter \(q_2\), as shown in Fig. 4b; increasing or decreasing \(q_2\) will produce a proportional change in the number of opioid abusers over time. These facts are important when considering control strategies, discussed in the next section.
The sensitivity indices in Table 3 indicate that nonlinear recruitment from the high-risk susceptible compartment into heroin abuse (\(\beta _{2}\)) significantly affects the number of abusers in either opioid compartment, more so than similar transmission parameters governing alternative methods of movement between compartments. Initiation of heroin abuse from the high-risk susceptible compartment circumvents pharmaceutical opioid abuse completely, and so it is interesting that \(A_\textrm{P}\) is most sensitive to this mechanism of nonlinear transmission. This is a consequence of how we model social contagion: the term \(A_\textrm{H}/\textrm{N}\) grows at the expense of \(A_\textrm{P}/\textrm{N}\) as more heroin abusers encourages additional movement into \(A_\textrm{H}\). This result is explored more fully in Sect. 3.3 when control strategies are considered. The relative sensitivity of either abuser compartment to \(\beta _2\) and \(\beta _1\) is plotted in Figs. 4c, d. Local changes to \(\beta _2\) produce an inverse response in either abuser population, since reduced heroin abuse recruitment from the high-risk susceptible compartment is compensated by a larger pharmaceutical opioid abuser population. In contrast, local changes to \(\beta _1\) produce proportional changes to either abuser population. This is important, as limiting recruitment into pharmaceutical opioid abuse (specifically by limiting diversion) achieves the desired effect of reducing abuser populations, and is not compensated by alternative drug abuse mechanisms. However, model responses are much more sensitive to changes in the former scenario. This result is explored in Sect. 3.3.
It should be noted that the most highly sensitive parameters are all related to the recruitment of individuals from the high-risk susceptible compartment in (1). Our high-risk group—defined as those with a history of substance use disorder—contributes significantly to the total number of opioid abusers, underlining the potential of control strategies that target groups most at risk to abuse. More broadly, all of the incidence rate parameters (both \(\beta _i\) and \(q_i\) parameters) are highly influential in our model. The fact that these rates account for addiction via illicit routes, such as pharmaceutical opioid diversion and heroin abuse, illustrates the importance of controlling these mechanisms of transmission over, say, treatment (represented by the parameters \(\eta _\textrm{P}\) and \(\eta _\textrm{H}\), which have comparatively little effect on abuser numbers). Our finding that prevention is more effective than treatment echoes that of similar modeling studies (Sharareh et al. 2019).
Perhaps most curious is the sensitivity of the state variables to the parameter \(\sigma _\textrm{H}\), representing linear movement out of heroin abuse and into the high-risk susceptible compartment. Recall that this parameter is included to capture cessation of heroin abuse for all reasons excluding treatment and death. The fact it features prominently in Table 3 (sometimes even more so than illicit routes of transmission) highlights the significance of these alternative means of stopping abuse. Various interpretations are possible. For example, individuals may cease heroin abuse of their own accord or because of changes in drug availability. Regarding the latter, the nonlinear mechanisms we have relied on do not consider availability or cost as factors in abuse, which are likely important (Cicero et al. 2014). It is known that the availability of either drug is a factor in observed abuse patterns (Cicero et al. 2014). A third possible interpretation has to do with relapse. As our model is currently constructed, individuals that cease heroin abuse for reasons other than death or treatment need not do so permanently. Therefore, this might suggest that repeated abuse of either opioid class contributes crucially to epidemic size over time. The large sensitivity indices for \(\sigma _\textrm{P}\) in Table 3 can be explained by similar reasoning. Relative sensitivity indices for \(\sigma _\textrm{P}\) and \(\sigma _\textrm{H}\) are shown in Figs. 3e, f. Perturbations to \(\sigma _\textrm{H}\) produce proportional changes in both abuser compartment, as more individuals are funneled into pharmaceutical opioid abuse after ceasing their heroin abuse. Importantly, a positive perturbation to \(\sigma _\textrm{P}\) will lead to a decrease in drug abuser populations as this directly reduces the number of pharmaceutical opioid abusers at any point in time.
Finally, from the plots given in Fig. 4, local extrema in relative sensitivities appear to align with the local extrema in overdose deaths (and thus the total number of abusers) shown in Fig. 3. This implies that changes to parameter values (such as by the implementation of control strategies) are most influential during peaks in abuser numbers; in contrast, changes to parameter values are less effective during years in which the number of abusers is small.
We also note the parameters identified to be comparatively noninfluential, locally speaking. These include the treatment parameters (\(\tau \), \(\eta _\textrm{P}\), and \(\eta _\textrm{H}\)), the rate at which individuals with a prescription naturally develop addiction (\(\epsilon _\textrm{LR}\)), and the overdose mortality parameters (\(\mu _\textrm{P}\) and \(\mu _\textrm{H}\)). We found that treatment is less effective than preventive measures at reducing the overall number of opioid abusers. The insensitivity of abuser numbers to treatment-seeking rates, \(\eta _\textrm{P}\) and \(\eta _\textrm{H}\), might have to do with our choice of \(\tau \), or the proportion of abusers that return to the high-risk compartment and are thus susceptible to opioid abuse following treatment. We assume that \(90\%\) of individuals will relapse after 1 year in treatment (Smyth et al. 2010; Bailey et al. 2013); the inefficiency of treatment in ensuring permanent recovery means that treatment overall is less effective than preventing (illicit) drug abuse in the first place. The parameter \(\epsilon _\textrm{LR}\) provides for an interesting comparison between mechanisms of initiating pharmaceutical opioid abuse. The sensitivity indices for \(\epsilon _\textrm{LR}\) in Table 3 suggest that, although developing a pharmaceutical opioid abuse disorder simply from having a prescription is significant, it is overall less important than illicit sources of pharmaceutical opioid abuse. Since the parameter \(\epsilon _\textrm{LR}\) includes the proportion of Mainers with an opioid prescription, this additionally means that reducing opioid prescriptions is relatively ineffective. Furthermore, although we do not consider the number of overdoses in our local sensitivity analysis, the results in Table 3 indicate that adjusting the overdose mortality parameters (such as might be the case if naloxone or other overdose reversing treatments are made more available) have very little effect on overall abuser numbers. The benefits and drawbacks of these programs have been debated in the literature (Doleac and Mukherjee 2018; Jones et al. 2017; McClellan et al. 2018), but our findings suggest that any such changes are negligible compared to other methods of control. This question is raised again in the following section.
3.3 Control Strategies: Managing an Opioid Epidemic
To help understand what these results mean in practice, we model a variety of control strategies over a 5-year period (2020–2025). The initial conditions for 2020 are those produced using the parameter values in Table 1, \(\varvec{\widehat{\theta }}_{{GLS}}\), at the end of the fitting period (December 2019). The performance of each case scenario is measured using four metrics:
-
(i)
average number of pharmaceutical opioid (PO) abusers;
-
(ii)
average number of heroin abusers;
-
(iii)
total number of PO overdoses; and
-
(iv)
total number of heroin overdoses.
Each control strategy is modeled by changing the relevant baseline parameter values in Table 1 by a certain percentage. For example, to model the effects of wider naloxone distribution on the quantities of interest above, we reduce the overdose mortality rates (\(\mu _\textrm{H}\) and \(\mu _\textrm{P}\)) by an appropriate percentage. The percentage used to model a control strategy depends on the sensitivity of the total abuser population to the parameter of interest, i.e., the fourth column in Table 3. Highly sensitive parameters are perturbed by \(1\%\), moderately sensitive parameters are perturbed by \(5\%\), and least sensitive parameters are perturbed by \(10\%\). The direction of change in each parameter perturbation is based on what would be reasonably expected to produce a positive result, or a decrease in the number of abusers/overdoses. For example, mortality rates are decreased while treatment rates are increased. Although these percentages are arbitrary, our hope with these experiments is to illustrate how common control strategies compare to one another in a controlled and informative manner for the management of an opioid epidemic. Control strategy results are shown in Table 4.
Pharmaceutical opioid and heroin abuser solutions for the model in (1) using nominal (blue) and control strategy (dashed red) parameters; values for the latter are taken from Table 4. Each row of figures compares abuser trajectories for a different parameter, these are: a, b \(q_1\), c, d \(\beta _2\), and e, f \(q_3\) (Color figure online)
We found that the only control strategies producing positive results for the full 5-year period target parameters governing certain pathways of pharmaceutical opioid abuse. Specifically, positive results were obtained by reducing illicit (nonlinear) recruitment into \(A_\textrm{P}\) (reducing \(\beta _1\) and/or increasing \(q_1\)), or by increasing the linear quit rate, \(\sigma _\textrm{P}\). Recall that illicit recruitment into pharmaceutical opioid abuse occurs as a result of opioid diversion. Reducing opioid diversion can be accomplished in many ways, such as by the creation or expansion of prescription drug monitoring programs (Rhodes et al. 2019; Surratt et al. 2013), educating patients on proper disposal of prescribed medications (Johnson et al. 2011; Rose et al. 2016; Spoth et al. 2013), and drug drop-off/take-back programs (Stewart et al. 2015), to name but a few. An example of the latter has already been successful in Maine (Stewart et al. 2015). Recommendations for reducing diversion are widely available (see, e.g., (Volkow and McLellan 2011; Compton et al. 2015)), and can be tailored to a variety of circumstances. Various studies have found the primary sources of diversion are those with existing prescriptions, typically friends and family (Cicero et al. 2011; Daniulaityte et al. 2014; Hulme et al. 2018) and pain patients (Inciardi et al. 2009, 2007), including the elderly. These findings and those of our model suggest that patient education on opioid dependence and proper disposal is likely an effective strategy of controlling opioid diversion and abuse in general. Similar results can be obtained by limiting transmission to high-risk individuals. This nonlinear recruitment, as it appears in our model, can be reduced by intervention programs that aim to make individuals more reluctant to initiate pharmaceutical opioid abuse. Research has shown the effectiveness of these strategies in reducing the likelihood of future misuse (Spoth et al. 2013) and potentially reducing overdose deaths (Johnson et al. 2011).
The success of control strategies targeting pharmaceutical opioid diversion can be explained as follows. In our model, heroin abuse relies primarily on nonlinear recruitment, i.e., recruitment involving the faculty of other opioid abusers. The pool of individuals that may initiate heroin abuse is comprised of high-risk individuals (individuals with a history of substance use disorder) and pharmaceutical opioid abusers, where the latter appears to be the majority in practice (Cicero et al. 2014; Mars et al. 2014). Preventing pharmaceutical opioid abuse therefore indirectly mitigates heroin abuse levels. This was the case when the parameter \(q_1\) was increased, or the transmission parameter controlling recruitment via diversion of high-risk susceptibles into pharmaceutical opioid abuse. Increasing this parameter by \(1\%\) produced significant decreases in the number of abusers as well as overall overdoses. Plots comparing the nominal to control strategy scenarios are shown in Figs. 5a, b. Abuser populations no longer oscillate but monotonically decrease over the course of the five year forecast period.
Focusing solely on reducing heroin abuse only tackles the downstream escalation of opioid abuse and does not address the component played by pharmaceutical opioids, possibly worsening long-term outcomes. This occurred when reducing the transmission parameter \(\beta _2\), which would reduce movement of high-risk susceptibles directly into the heroin abuse compartment. As can be seen in Figs. 5c, d, while the heroin abuser population at first declines, all progress is immediately lost as individuals instead move to pharmaceutical opioid abuse. Other model parameters demonstrated the same trend of producing short-term (possibly one-sided) benefits but ultimately yielding worse outcomes in the long term. We suspect this is a consequence of how we model social contagion. For example, increasing treatment efficacy by reducing the relapse rate (i.e., reducing \(\tau \)) produces positive results in the first year. Increasing treatment efficacy reduces the number of heroin abusers dramatically, as the treatment rate is higher for heroin abusers than pharmaceutical opioid abusers (\(\eta _\textrm{H} > \eta _\textrm{P}\)). However, as the number of heroin abusers falls after the first year, high-risk individuals will progress to abusing pharmaceutical opioids rather than heroin, given that the former is the more “popular” option. The same phenomenon occurred when the proportion of Mainers with prescriptions was reduced (i.e., reducing \(\epsilon _{\textrm{LR}}\)). Closer analysis reveals the increase is due to individuals initiating pharmaceutical opioid abuse via diverted (illicit) means. Reducing the rate of illicit recruitment into heroin abuse from \(A_\textrm{P}\) (i.e., reducing \(\beta _3\) or increasing \(q_3\)) actually increased the number of individuals abusing pharmaceutical opioids in the long term. Once again, opioid diversion more than compensated for the decrease in heroin abuse. Thus, it is clear that reduction in certain avenues of pharmaceutical opioid or heroin abuse is compensated by an increase in illicit abuse of diverted pharmaceutical opioids. In some instances this is particularly extreme, as can be seen in the example of \(q_3\), in which a small perturbation produced a dramatic change in the number of pharmaceutical opioid abusers. Figure 5e, f shows how this happens: increasing the number of heroin abusers required to recruit pharmaceutical opioid abusers causes the rapid collapse of \(A_\textrm{H}\), but in return the pharmaceutical opioid abuser population swells to more than double those expected when using nominal parameter values.
Such counter-intuitive results are far from baseless. For example, various studies have found connections between increases in regulation of prescription opioids and increases in illicit opioid use (Coffin et al. 2020; Fischer et al. 2020). The authors in (Coffin et al. 2020) observed that discontinuing opioid prescriptions resulted in individuals seeking illicit outlets for their drug abuse in the form of (diverted) nonprescribed opioids and heroin. Modeling studies have also revealed this to be the case for certain control strategies (Sharareh et al. 2019). The question then becomes how opioid abuse can be controlled given this complicated balance. Our findings suggest that controlling (illicit) pharmaceutical opioid abuse is the most effective strategy. Finally, it is worth remarking that reducing overdose rates had an insignificant impact on the average monthly number of abusers for either opioid class. The fact that this has been observed in practice (Jones et al. 2017; McClellan et al. 2018) is encouraging.
4 Conclusions
The opioid epidemic remains a debilitating public health crisis, claiming the lives of tens of thousands of Americans each year and growing (CDC 2022b). To assist in informing policy going forward, we introduced a novel mathematical framework calibrated using publicly available mortality data from CDC WONDER. We demonstrated its usefulness for the state of Maine for modeling pharmaceutical opioid and heroin abuser populations. Our model includes several features we believe to be critical in any mathematical analysis of the opioid epidemic, including general transmission terms to better contend with social contagions, susceptible compartments differentiated by risk of opioid abuse, and separating opioid abuser compartments by drug type to more closely study interactions between them. The rise and fall of drug deaths, as forecasted by our model (see Fig. 3), mirrors national trends (Sorg 2022) and is predicted to continue into future years. In our model, these oscillations are caused by individuals moving between different abuse compartments and recovery.
Future work should consider other important illicit opioids, especially fentanyl, as well as pharmaceutical opioids. Fentanyl has become more common nationally in recent years (Park et al. 2021) and in Maine has already eclipsed the contributions of other illicit opioids such as heroin in terms of annual overdoses (Sorg 2019, 2020, 2021, 2022). More complicated models could apply the framework here to a larger nonpharmaceutical class. Progress in this direction has already been made (Phillips et al. 2021), but data on fentanyl abuse are in desperate need. By assumption, individuals must progress through pharmaceutical opioid abuse in our model before initiating heroin abuse—as is presently observed a majority of the time (Cicero et al. 2014; Jones et al. 2015; Mars et al. 2014). However, we do not consider drug availability or cost as factors affecting drug patterns. These are likely important considerations (Cicero et al. 2014). In practice, active abusers that typically rely on pharmaceutical opioids switch to heroin if the former becomes harder to obtain. Note that while this has been observed in practice (Dart et al. 2015; Cicero et al. 2012), the more general trends are debated (Compton et al. 2016).
While isolating drug types in a compartment model simplifies its structure, a pertinent question is comorbidity, i.e., abusers who abuse multiple drug types. Comorbid drug disorders are important for individuals with an opioid use disorder (Cicero et al. 2020) and drug death reports for the state of Maine show that comorbid abuse of both pharmaceutical and nonpharmaceutical opioids in overdose victims is no isolated phenomenon (Sorg 2017, 2018, 2019). The addition of a comorbid compartment, however, begs the question of how movement occurs between the different abuser states. These transitions are not well-studied, and we resolved on avoiding comorbidity in favor of the disjoint compartmentalization in our model. It would be enlightening to study these transitions more closely and collect data of comorbid substance abuse for future analysis. In keeping with the topic of more data, movement between susceptible risk groups is another useful consideration. As a consequence of assuming individuals enter the model population as low-risk susceptible (i.e., that they do not have a history of substance use disorder), individuals may only re-enter the high-risk susceptible compartment if they have a history of opioid abuse. Accounting for these details requires data concerning drug abuse patterns at a state level. Interactions between susceptible compartments also depend on the predictor chosen to discriminate individuals in terms of their opioid abuse risk. We chose a history of substance use disorder given data availability and research indicating this as a strong predictor (Sehgal et al. 2012). The predictor used will likely depend on the US state, but we want to emphasize that differences between opioid abuse risk exist and should be included in mathematical models of the opioid epidemic moving forward. Finally, we showed that if data can be obtained concerning the rates that abusers cease drug use for reasons other than death or seeking treatment, the accuracy of (important) parameter estimates can be improved markedly.
The major findings from our analysis are summarized as follows:
-
(i)
According to our model forecast, the trend for the pharmaceutical opioid abuser population is to grow with oscillations; the appearance of oscillations is due to individuals fluctuating between states of active opioid abuse (residing in either abuse compartment) and inactive opioid abuse (residing in recovery or the high-risk susceptible compartment). In practice, these are likely a result of legislation and changing drug availability.
-
(ii)
Illicit opioid abuse transmission, specifically pharmaceutical opioid diversion, is the most important target for epidemic control moving forward. The extent that pharmaceutical opioid abuse is due to diverted opioids is substantiated by Maine drug death reports, which found that a majority of individuals that overdosed on pharmaceutical opioids did so with drugs they did not have a current prescription for (Sorg 2020, 2021). Parameter fitting results further suggested that heroin abuse is more “infectious” than pharmaceutical opioid abuse.
-
(iii)
Maxima in overdose deaths (and thus total number of abusers) align with maxima in relevant parametric sensitivities of the model. Hence, targeted control strategies should be undertaken during overdose death peaks for maximum effect.
-
(iv)
As mentioned previously, controlling pharmaceutical opioid abuse indirectly mitigates heroin abuse given the evidence suggesting most individuals abusing heroin began with abusing pharmaceutical opioids (Cicero et al. 2014; Jones et al. 2015; Mars et al. 2014). We have suggested courses of action based both on our findings and elsewhere, including but not limited to expanding prescription drug monitoring programs, prescribed patient education, and drug drop-off programs.
-
(v)
Our analysis also suggests that linear rates of abuse, such as via natural pathways resulting from opioid prescriptions, are much less influential than strategies targeting illicit transmission. Increasing treatment rates and efficacy were also found to be less effective. Reducing overdose rates produced insignificant changes to the average monthly number of opioid abusers, lending support to existing research (Jones et al. 2017; McClellan et al. 2018) and demonstrating the promise of naloxone in reducing overdose deaths without ill effects.
-
(vi)
Furthermore, we found that certain control strategies have the possibility to worsen opioid abuse in the long term. This is a disputed matter in the literature, and evidence remains mixed (Coffin et al. 2020; Dowell et al. 2016; Fischer et al. 2020; Lee et al. 2021; Rhodes et al. 2019). More complicated relationships than those we have included are likely at play (especially concerning other illicit opioids, such as fentanyl), and studying these are an important next step to better understanding the efficacy of policies confronting the opioid epidemic.
All else being equal, our analysis suggests that controlling diverted pharmaceutical opioids is more effective than alternative strategies, such as controlling heroin abuse or improving access to treatment, at reducing the number of abusers and the total number of overdoses.
Data Availability
Data have been taken from CDC WONDER (https://wonder.cdc.gov/).
Code Availability
Available upon request.
References
Alexander GC, Ballreich J, Mansour O et al (2021) Effect of reductions in opioid prescribing on opioid use disorder and fatal overdose in the United States: a dynamic Markov model. Drug Alcohol Depend 117(4):969–76. https://doi.org/10.1111/add.15698
Bailey GL, Herman DS, Stein MD (2013) Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J Subst Abus Treat 45:302–5. https://doi.org/10.1016/j.jsat.2013.04.002
Ballreich J, Mansour O, Hu E et al (2020) Modeling mitigation strategies to reduce opioid-related morbidity and mortality in the US. JAMA Netw Open 3(11):e2023677–e2023677. https://doi.org/10.1001/jamanetworkopen.2020.23677
Banks HT, Catepacci J, Hu S (2016) Use of difference-based methods to explore statistical and mathematical model discrepancy in inverse problems. J Inverse Ill Posed Probl 24:413–433. https://doi.org/10.1515/jiip-2015-0090
Banks HT, Hu S, Thompson WC (2016) Modeling and inverse problems in the presence of uncertainty. CRC Press, Boca Raton
Banks HT, Bekele-Maxwell K, Everett RA et al (2017) Dynamic modeling of problem drinkers undergoing behavioral treatment. Bull Math Model 79(6):1254–1273. https://doi.org/10.1007/s11538-017-0282-5
Battista NA, Pearcy LB, Strickland WC (2019) Modeling the prescription opioid epidemic. Bull Math Biol 81(7):2258–2289. https://doi.org/10.1007/s11538-019-00605-0
Behrens DA, Caulkins JP, Tragler G et al (2000) Optimal control of drug epidemics: Prevent and treat–but not at the same time? Manage Sci 46(3):333–47. https://doi.org/10.1287/mnsc.46.3.333.12068
Butler C (2020) A mathematical model of the opioid epidemic in the state of Maine. Honors College, University of Maine https://digitalcommons.library.umaine.edu/honors/630/
Caldwell WK, Freedman B, Settles L et al (2019) The Vicodin abuse problem: a mathematical approach. J Theor Biol 108(10):110003. https://doi.org/10.1016/j.jtbi.2019.110003
CDC (2020) Multiple cause of death 1999–2018 on CDC wonder online database, released in 2020. URL: http://wonder.cdc.gov/mcd-icd10.html
CDC (2021a) Drug overdose deaths in the U.S. top 100,000 annually. URL: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.html. Accessed 18 Sep 2022
CDC (2021b) U.S. opioid dispensing rate maps. URL: https://www.cdc.gov/drugoverdose/rxrate-maps/index.html. Accessed 18 Sep 2022
CDC (2022a) CDC WONDER (database). https://wonder.cdc.gov. Accessed 01 Sep 2022
CDC (2022b) Overdose death rates. URL: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed 18 Sep 2022
Chen Q, Larochelle MR, Weaver DT et al (2019) Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open 2(2):e187621–e187621. https://doi.org/10.1001/jamanetworkopen.2018.7621
Cicero TJ, Kurtz SP, Surratt HL et al (2011) Multiple determinants of specific modes of prescription opioid diversion. J Drug Issues 41(2):283–304. https://doi.org/10.1177/002204261104100207
Cicero T, Ellis M, Surratt H (2012) Effect of abuse-deterrent formulation of oxycontin. N Engl J Med 367:187–189. https://doi.org/10.1056/NEJMc1204141
Cicero TJ, Ellis MS, Surratt HL et al (2014) The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiat 71:821–6. https://doi.org/10.1001/jamapsychiatry.2014.366
Cicero TJ, Ellis MS, Kasper ZA (2020) Polysubstance use: a broader understanding of substance use during the opioid crisis. Am J Public Health 110(12):244–50. https://doi.org/10.2105/AJPH.2019.305412
Coffin PO, Rowe C, Oman N et al (2020) Illicit opioid use following changes in opioids prescribed for chronic non-cancer pain. PLOS One 15(5):e0232538. https://doi.org/10.1371/journal.pone.0232538
Cole S, Wirkus S (2022) Modeling the dynamics of heroin and illicit opioid use disorder, treatment, and recovery. Bull Math Biol 84(4):1–49. https://doi.org/10.1007/s11538-022-01002-w
Compton WM, Boyle M, Wargo E (2015) Prescription opioid abuse: problems and responses. Prev Med 80:5–9. https://doi.org/10.1016/j.ypmed.2015.04.003
Compton WM, Jones CM, Baldwin GT (2016) Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 374(2):154–63. https://doi.org/10.1056/NEJMra1508490
Daniulaityte R, Russel F, Carlson RG (2014) Sources of pharmaceutical opioids for non-medical use among young adults. J Pyschoactive Drugs 46(3):198–207. https://doi.org/10.1080/02791072.2014.916833
Dart RC, Surratt HL, Cicero TJ et al (2015) Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372(3):241–248. https://doi.org/10.1056/NEJMsa1406143
DHHS M (2015) Substance abuse trends in Maine: state epidemiological profile 2015. URL: http://www.fpddev.com/staging/ctn/wp-content/uploads/2016/11/SA-Trends-in-ME-2015.pdf. Accessed 25 Sep 2022
DHHS M (2016) Substance abuse trends in Maine: atate epidemiological profile 2016. URL: https://vdocuments.net/substance-abuse-trends-in-maine-maine-seow-epiprofile-2016-finalpdfsubstance.html?page=1. Accessed 25 Sep 2022
DHHS M (2017) Substance abuse trends in Maine: atate epidemiological profile 2017. URL: https://static1.squarespace.com/static/60b00f2b0884962f72a32db5/t/60e5eb0d0990c24a70620288/1625680655311/SEOW+EpiProfile+2017+FINAL+09292017.pdf. Accessed 25 Sep 2022
DHHS M (2018) Substance use trends in Maine: state epidemiological profile 2018. URL: https://static1.squarespace.com/static/60b00f2b0884962f72a32db5/t/60e5e3d47612d607a368e716/1625678806106/SEOW+EpiProfile+2018+with+sub+state+data+11302018.pdf. Accessed 25 Sep 2022
DHHS M (2019) Substance use trends in Maine: State epidemiological profile 2019. URL: https://static1.squarespace.com/static/60b00f2b0884962f72a32db5/t/60e5e3b7a6110f30d7c5f209/1625678777496/SEOW+EpiProfile+2019+Final+092419.pdf. Accessed 25 Sep 2022
Doleac J, Mukherjee A (2018) The moral hazard of lifesaving innovations: naloxone access, opioid abuse, and crime. URL: https://ideas.repec.org/p/iza/izadps/dp11489.html. Accessed 18 Sep 2022
Dowell D, Zhang K, Noonan RK et al (2016) Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff 35(10):1876–83. https://doi.org/10.1377/hlthaff.2016.0448
Dunn KM, Saunders KW, Rutter CM et al (2010) Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 152(2):85–92. https://doi.org/10.7326/0003-4819-152-2-201001190-00006
Edlund MJ, Martin BC, Fan MY et al (2010) Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend 112:90–8. https://doi.org/10.1016/j.drugalcdep.2010.05.017
Edlund MJ, Martin BC, Russo JE et al (2014) The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain 30:557–64. https://doi.org/10.1097/AJP.0000000000000021
Fischer B, Jones W, Tyndall M et al (2020) Correlations between opioid mortality increases related to illicit/synthetic opioids and reductions of medical opioid dispensing–exploratory analysis from Canada. BMC Public Health 20(143):1–7. https://doi.org/10.1186/s12889-020-8205-z
Guy GP Jr, Zhang K, Bohm MK et al (2017) Vital signs: changes in opioid prescribing in the United States 2006–2015. Morb Mortal Wkly Rep 66(26):697–704
Haley DF, Saitz R (2020) The opioid epidemic during the COVID-19 pandemic. JAMA 324(16):1615–7. https://doi.org/10.1001/jama.2020.18543
HHS (2019) The opioid epidemic by the numbers. URL: https://www.hhs.gov/opioids/sites/default/files/2019-11/Opioids%20Infographic_letterSizePDF_10-02-19.pdf. Accessed 18 Sep 2022
Hulme S, Bright D, Nielsen S (2018) The source and diversion of pharmaceutical drugs for non-medical use: a systematic review and meta-analysis. Drug Alcohol Depend 186:242–56. https://doi.org/10.1016/j.drugalcdep.2018.02.010
Inciardi JA, Surratt HL, Kurtz SP et al (2007) Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med 8(2):171–83. https://doi.org/10.1111/j.1526-4637.2006.00255.x
Inciardi JA, Surratt HL, Cicero TJ et al (2009) Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med 10(3):537–48. https://doi.org/10.1111/j.1526-4637.2009.00603.x
Johnson EM, Porucznik CA, Anderson JW et al (2011) State-level strategies for reducing prescription drug overdose deaths: Utah’s prescription safety program. Pain Med 12:66–72. https://doi.org/10.1111/j.1526-4637.2011.01126.x
Jones CM, Logan J, Gladden RM et al (2015) Vital signs: demographic and substance use trends among heroin users–United States, 2002–2013. Morb Mortal Wkly Rep 64(26):719–25
Jones J, Campbell A, Metz V et al (2017) No evidence of compensatory drug use risk behavior among heroin users after receiving take-home naloxone. Addict Behav 71:104–6. https://doi.org/10.1016/j.addbeh.2017.03.008
Kenan K, Mack K, Paulozzi L (2012) Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med 6(2):41–47
Kochanek KD, Murphy SL, Xu J et al (2014) Mortality in the United States, 2013. NCHS Data Brief 178:1–8
Kochanek KD, Murphy SL, Xu J et al (2017) Mortality in the United States, 2016. NCHS Data Brief 293:1–8
Kochanek KD, Xu J, Arias E (2020) Mortality in the United States, 2019. NCHS Data Brief 395:1–8
Kosten T, George T (2002) The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1:13–20. https://doi.org/10.1151/spp021113
Kreek MJ, Nielsen DA, Butelman ER et al (2005) Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8:1450–7. https://doi.org/10.1038/nn1583
Lee B, Zhao W, Yang K et al (2021) Systematic evaluation of state policy interventions targeting the US opioid epidemic. JAMA Netw Open 4(2):e2036687. https://doi.org/10.1001/jamanetworkopen.2020.36687
Liu J, Zhang T (2011) Global behaviour of a heroin epidemic model with distributed delays. Appl Math Lett 24(10):1685–92. https://doi.org/10.1016/j.aml.2011.04.019
Mars SG, Bourgois P, Karandinos G et al (2014) “Every ‘never’ i ever said came true’’: transitions from opioid pills to heroin injecting. Int J Drug Policy 25(2):257–66. https://doi.org/10.1016/j.drugpo.2013.10.004
McClellan C, Lambdin B, Ali M et al (2018) Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav 86:90–5. https://doi.org/10.1016/j.addbeh.2018.03.014
Murphy SL, Kochanek KD, Xu J et al (2015) Mortality in the United States, 2014. NCHS Data Brief 229:1–8
Murphy SL, Xu J, Kochanek KD et al (2018) Mortality in the United States, 2017. NCHS Data Brief 328:1–8
Ochalek TA, Cumpston KL, Wills BK et al (2020) Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA 324(16):1673–4. https://doi.org/10.1001/jama.2020.17477
Park JN, Rashidi E, Foti K et al (2021) Fentanyl and fentanyl analogs in the illicit stimulant supply: results from U.S. drug seizure data, 2011–2016. Drug Alcohol Depend 218:108416. https://doi.org/10.1016/j.drugalcdep.2020.108416
Phillips T, Lenhart S, Strickland WC (2021) A data-driven mathematical model of the heroin and fentanyl epidemic in Tennessee. Bull Math Biol 83(10):1–27. https://doi.org/10.1007/s11538-021-00925-0
Piper BJ, Desrosiers CE, Lipovsky JW et al (2016) Use and misuse of opioids in Maine: results from pharmacists, the prescription monitoring, and the diversion alert programs. J Stud Alcohol Drugs 77(4):556–65. https://doi.org/10.15288/jsad.2016.77.556
Pitt AL, Humphreys K, Brandeau ML (2018) Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health 108(10):1394–1400. https://doi.org/10.2105/AJPH.2018.304590
Rabin RC (2021) Overdose deaths reached record high as the pandemic spread. URL: https://www.nytimes.com/2021/11/17/health/drug-overdoses-fentanyl-deaths.html. Accessed 18 Sep 2022
Rhodes E, Wilson M, Robinson A et al (2019) The effectiveness of prescription drug monitoring programs at reducing opioid-related harms and consequences: a systematic review. BMC Health Serv Res. https://doi.org/10.1186/s12913-019-4642-8
Rose P, Sakai J, Argue R et al (2016) Opioid information pamphlet increases postoperative opioid disposal rates: a before versus after quality improvement study. Can J Anesth 63:31–7. https://doi.org/10.1007/s12630-015-0502-0
Samanta GP (2011) Dynamic behaviour for a nonautonomous heroin epidemic model with time delay. J Appl Math Comput 35:161–178. https://doi.org/10.1007/s12190-009-0349-z
SAMHSA (2002) Office of Applied Studies, results from the 2001 national household survey on drug abuse: volume I. summary of national findings. (Office of Applied Studies, NHSDA Series h-17, DHHS Publication no. SMA 02-3758). Substance Abuse and Mental Health Services Administration
SAMHSA (2014a) 2011–2012 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2011-2012-nsduh-state-prevalence-estimates-pdf-tables
SAMHSA (2014b) 2012–2013 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2012-2013-nsduh-state-prevalence-estimates-pdf-tables-printing
SAMHSA (2015) 2013–2014 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2013-2014-nsduh-state-prevalence-estimates-pdf-tables-printing
SAMHSA (2017a) 2013–2014 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2014-2015-nsduh-state-prevalence-estimates-pdf-tables-printing
SAMHSA (2017b) 2015–2016 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2015-2016-nsduh-state-prevalence-estimates-pdf-tables-printing
SAMHSA (2018) 2016–2017 NSDUH state prevalence estimates. Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2016-2017-nsduh-state-prevalence-estimates
SAMHSA (2019) 2017–2018 national survey on drug use and health: model-based prevalence estimates (50 states and the District of Columbia). Substance Abuse and Mental Health Services Administration, https://www.samhsa.gov/data/report/2017-2018-nsduh-state-prevalence-estimates
Sehgal N, Machikanti L, Smith HS (2012) Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 15:67–92
Sharareh N, Sabounchi SS, McFarland M et al (2019) Evidence of modeling impact in development of policies for controlling the opioid epidemic and improving public health: a scoping review. Subst Abuse Res Treat 13:1–10. https://doi.org/10.1177/1178221819866211
Slavova S, Rock P, Bush HM et al (2020) Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend 214(1):1615–7. https://doi.org/10.1016/j.drugalcdep.2020.108176
Smyth BP, Barry J, Keenan E et al (2010) Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J 103:176–9
Sorg MH (2017) Expanded Maine drug death report for 2016. Health & Public Safety 5. https://digitalcommons.library.umaine.edu/mcspc_healthsafety/5/
Sorg MH (2018) Expanded Maine drug death report for 2017. Health & Public Safety 6. https://digitalcommons.library.umaine.edu/mcspc_healthsafety/6/
Sorg MH (2019) Expanded Maine drug death report for 2018. Health & Public Safety 8. https://digitalcommons.library.umaine.edu/mcspc_healthsafety/8/
Sorg MH (2020) Maine drug death report for 2019. Health & Public Safety 7. https://digitalcommons.library.umaine.edu/mcspc_healthsafety/7/
Sorg MH (2021) Maine drug death report for 2020. Maine Office of Attorney General, https://mainedrugdata.org/wp-content/uploads/2021/06/2020_Annual_ME_Drug_Death_Rpt_Final.pdf
Sorg MH (2022) Maine’s overdose data and the fentanyl epidemic. Margaret Chase Smith Policy Center, URL: https://legislature.maine.gov/doc/7889. Accessed 25 Sep 2022
Spoth R, Trudeau L, Shin C et al (2013) Longitudinal effects of universal preventive intervention on prescription drug misuse: three randomized controlled trials with late adolescents and young adults. Am J Public Health 103(4):665–72. https://doi.org/10.2105/AJPH.2012.301209
Stewart H, Malinowski A, Ochs L et al (2015) Inside Maine’s medicine cabinet: findings from the drug enforcement administration’s medication take-back events. Am J Public Health 105:65–71. https://doi.org/10.2105/AJPH.2014.302207
Surratt HL, O’Grady C, Kurtz SP et al (2013) Reductions in prescription opioid diversion following recent legislative interventions in Florida. Pharmacoepidemiol Drug Saf 23(3):314–20. https://doi.org/10.1002/pds.3553
Sutton KL, Banks HT, Castillo-Chavez CC (2008) Estimation of invasive pneumococcal disease dynamics parameters and the impact of conjugate vaccination in Australia. Math Biosci Eng 5(1):175–204. https://doi.org/10.3934/mbe.2008.5.175
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1–2):29–48. https://doi.org/10.1016/S0025-5564(02)00108-6
van den Driessche P, Watmough J (2008) Further notes on the basic reproduction number. Springer Berlin Heidelberg, Berlin, pp 159–178. https://doi.org/10.1007/978-3-540-78911-6_6
Volkow ND, McLellan TA (2011) Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA 305(13):1346–7. https://doi.org/10.1001/jama.2011.369
Vowles KE, McEntee ML, Julnes PS et al (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156(4):569–76. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
Walid M, Donahue S, Darmohray D et al (2008) The fifth vital sign–What does it mean? Pain Pract 8:417–22. https://doi.org/10.1111/j.1533-2500.2008.00222.x
White E, Comiskey C (2007) Heroin epidemics, treatment and ODE modeling. Math Biosci 208(1):312–24. https://doi.org/10.1016/j.mbs.2006.10.008
Xu J, Kochanek KD, Murphy SL et al (2014) Mortality in the United States, 2012. NCHS Data Brief 168:1–8
Xu J, Murphy SL, Kochanek KD et al (2017) Mortality in the United States, 2015. NCHS Data Brief 267:1–8
Xu J, Murphy SL, Kochanek KD et al (2020) Mortality in the United States, 2018. NCHS Data Brief 355:1–8
Zacny J, Bigelow G, Compton P et al (2003) College on problems of drug dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend 69:215–232. https://doi.org/10.1016/s0376-8716(03)00003-6
Acknowledgements
The work in this article builds on a preliminary version of these modeling, simulation, and analysis efforts carried out in the author’s Honors thesis Butler (2020). The authors would like to thank Marcella Sorg for lending her expertise during the preliminary stages of this project.
Funding
This research was supported by funding from the Margaret Chase Smith Policy Center at the University of Maine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Butler, C., Stechlinski, P. Modeling Opioid Abuse: A Case Study of the Opioid Crisis in New England. Bull Math Biol 85, 45 (2023). https://doi.org/10.1007/s11538-023-01148-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01148-1