Abstract
Durvalumab (Imfinzi®), a therapeutic human monoclonal antibody which binds to and blocks the activity of the immunosuppressive programmed death-ligand 1 (PD-L1) protein, is approved in the USA, EU, Japan and other countries in combination with gemcitabine and cisplatin for adults with advanced biliary tract cancer. In the pivotal phase 3 TOPAZ-1 trial, durvalumab plus gemcitabine and cisplatin significantly prolonged overall survival and progression-free survival compared with placebo plus gemcitabine and cisplatin in adults with advanced biliary tract cancer. Benefit from durvalumab was seen irrespective of primary tumour location, disease status at diagnosis (unresectable or recurrent), or initial levels of PD-L1 expression. The tolerability of durvalumab plus gemcitabine and cisplatin was manageable. Overall, the addition of durvalumab to gemcitabine and cisplatin is a valuable new treatment option for adults with advanced biliary tract cancer.
Plain Language Summary
Biliary tract cancers are a diverse group of cancers that develop in the bile ducts or the gallbladder. Patients with these cancers typically have poor survival. Chemotherapy (gemcitabine plus cisplatin) has been the first-line treatment for biliary tract cancer for over a decade, with no new treatments further improving on its overall survival benefit until recently. Durvalumab (Imfinzi®) belongs to a class of drugs known as checkpoint inhibitors; these drugs activate the immune system to help fight cancer. In the phase 3 TOPAZ-1 trial, the addition of durvalumab to first-line chemotherapy prolonged the overall survival compared with placebo plus chemotherapy in adults with advanced biliary tract cancer. The tolerability of durvalumab in combination with chemotherapy was manageable. Thus, durvalumab plus gemcitabine and cisplatin is a valuable new treatment option for adults with advanced biliary tract cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.24210324. |
A therapeutic fully human monoclonal antibody against PD-L1 |
Significantly prolongs overall survival and progression-free survival compared with placebo when added to gemcitabine and cisplatin |
Manageable tolerability profile; most common adverse reactions included fatigue and gastrointestinal disorders |
1 Introduction
Biliary tract cancers are a diverse group of malignancies that include intrahepatic, extrahepatic (perihilar and distal) cholangiocarcinoma and gallbladder carcinoma [1]. Where surgery is an option, it is the only curative treatment option in patients with biliary tract cancer [2]. However, up to 80% of patients who undergo surgery with curative intent experience relapse within 3 years [2]. Systemic chemotherapy with the combination of gemcitabine and cisplatin has been the first-line treatment of choice since 2010 for patients with advanced biliary tract cancer, with no other first-line treatment option improving on overall survival compared with this treatment regimen until recently [3]. The median overall survival of patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin is ≈ 10–12 months, and progression-free survival is ≈ 5–8 months [4,5,6]. More effective pharmacological options that can prolong overall survival in patients with biliary tract cancer are needed.
Expression of the T cell-suppressor protein programmed death-ligand 1 (PD-L1) in biliary tract cancer is associated with advanced disease stage, lymph node involvement, metastases and poor overall survival [7]. Immunotherapies that block the interaction between PD-L1 and its cognate receptors can activate an antitumour immune response (Sect. 2). Additionally, chemotherapies can enhance the effect of immunotherapies by helping release tumour antigens, disrupting tumour-mediated immunosuppression and sensitising tumours to immune attack [8]. A combined chemotherapy and immunotherapy approach may therefore be effective in patients with advanced biliary tract cancer.
Durvalumab (Imfinzi®) is an intravenously administered, therapeutic fully human monoclonal antibody against PD-L1 [9]. It is currently approved in several countries globally, including in the USA [10], EU [11] and Japan [12] for use in combination with gemcitabine and cisplatin in adults with advanced biliary tract cancer (Sect. 6). Durvalumab is also approved in other indications, including locally advanced and metastatic non-small cell lung cancer, extensive-stage small cell lung cancer and unresectable hepatocellular carcinoma [10,11,12]; these will not be further discussed in this article. This article reviews the clinical data relevant to durvalumab’s use in patients with advanced biliary tract cancer, with a brief description of its pharmacological properties.
2 Pharmacodynamic Properties of Durvalumab
Durvalumab is a human immunoglobulin G1 kappa monoclonal antibody that binds to human PD-L1 with high affinity and specificity, and thus acts as a potent antagonist of the PD-L1 signalling pathway [9]. It is engineered to avoid triggering antibody-dependent cellular cytotoxicity responses. The binding of durvalumab to PD-L1 blocks the interactions between PD-L1 and its receptors [programmed cell death 1 (PD-1) and cluster of differentiation 80], resulting in improved T cell activity and T cell proliferation. This enhanced T cell response generates an antitumour response. In mouse xenograft models co-implanted with human tumour cells and T cells, durvalumab significantly reduced tumour growth [9]. The efficacy of PD-L1 blockade is at least additive with chemotherapy. Combining anti-mouse PD-L1 antibodies with chemotherapy (e.g. oxaliplatin) significantly improves the survival of mice in colon cancer models compared with chemotherapy or antibodies alone [9]. In early clinical trials, durvalumab as a monotherapy [13] or in combination with chemotherapy [14] demonstrated antitumour activity in patients with solid tumours, including advanced biliary tract cancer (Sect. 4) [14]. While promoting anticancer effects, durvalumab's mechanism of action can also potentially lead to immune-mediated adverse events (IMAEs) (Sect. 5.1).
Antibodies against durvalumab can develop in patients receiving durvalumab [10, 11]. Of the 240 patients in the phase 3 TOPAZ-1 study who received durvalumab plus gemcitabine and cisplatin, two patients (0.8% of patients) developed anti-drug antibodies and two patients (0.8%) developed neutralising antibodies to durvalumab [10]. There was an insufficient number of patients with anti-drug antibodies and neutralising antibodies to determine their effect on pharmacodynamics, pharmacokinetics, efficacy and/or safety [10]. In other studies with durvalumab (as a single agent or in combination with other therapies), including in other indications, no clinically relevant effects on pharmacokinetics or safety was identified with anti-drug antibodies and neutralising antibodies against durvalumab [10, 11].
3 Pharmacokinetic Properties of Durvalumab
The pharmacokinetics of durvalumab were studied in patients receiving a range of dosages (0.1–20 mg/kg) administered once every two, three or four weeks as a monotherapy [10, 11]. Exposure to durvalumab was more than dose-proportional at doses < 3 mg/kg and dose proportional at doses ≥ 3 mg/kg. Steady state was reached in ≈ 16 weeks. The geometric mean steady state volume of distribution of durvalumab was 5.4–5.6 L in patients receiving > 10 mg/kg every two weeks [10, 11].
The clearance of durvalumab decreases with time, with a geometric mean steady state clearance of 8 mL/h at day 365 of receiving treatment (≈ 23% mean maximal reduction from baseline values [10]) [10, 11]. This decrease is not considered clinically relevant. The terminal half-life of durvalumab, based on clearance at baseline, is ≈ 18–21 days [10, 11]. Durvalumab is primarily eliminated through catabolism via the reticuloendothelial system or target-mediated disposition [11].
There were no clinically significant differences in durvalumab pharmacokinetics in patients based on age (18–96 years), body weight (31–175 kg), gender or race [10, 11]. Serum levels of albumin (4–57 g/L), lactate dehydrogenase (18–15,800 U/L), soluble PD-L1 (67–3470 pg/mL), tumour type (non-small cell lung, small cell lung, biliary tract and hepatocellular cancers) or Eastern Cooperative Oncology Group (ECOG) performance status did not affect the pharmacokinetics of durvalumab. Mild to moderate impairment in kidney (creatinine clearance 30–89 mL/min) and liver (bilirubin ≤ upper limits of normal (ULN) and aspartate aminotransferase (AST) > ULN, bilirubin > 1.0 to 3.0 × ULN and any AST level) function did not significantly alter the pharmacokinetics of durvalumab. The effects of severe impairment in kidney (creatinine clearance 15–29 mL/min) and liver (bilirubin > 3.0 × ULN and any AST level) function are unknown [10, 11]. However, as durvalumab is not eliminated through the kidneys or liver, impairment is unlikely to affect durvalumab exposure [11].
Coadministration of durvalumab with cisplatin and gemcitabine does not significantly alter the pharmacokinetics of any drug [10, 11]. Although not formally tested, no pharmacokinetic drug-drug interactions are expected, as durvalumab is primarily eliminated through catabolism [11].
4 Therapeutic Efficacy of Durvalumab
Dose-finding studies for durvalumab identified a dosing regimen of 10 mg/kg every 2 weeks to be efficacious and have an acceptable safety profile [15]. Population pharmacokinetic models support the use of flat-dosing regimens, with patients who received durvalumab 750 mg every 2 weeks or 1500 mg every 4 weeks having similar median steady-state exposures of the drug and no increased exposure to extreme concentration values compared with the dosing regimen of 10 mg/kg every 2 weeks [16]. Proof of concept for durvalumab’s activity in adults with biliary tract cancer came from a phase 2 trial, in which 72% of patients receiving concomitant durvalumab plus gemcitabine and cisplatin achieved an objective response [14]. The efficacy of durvalumab in combination with gemcitabine and cisplatin in adults with advanced biliary tract cancer was further evaluated in the randomized, double-blind, placebo-controlled, multinational phase 3, TOPAZ-1 trial [17]. Discussion in this section focusses on the TOPAZ-1 trial.
Patients in TOPAZ-1 were adults (≥ 18 years of age) with histologically confirmed locally advanced, unresectable or metastatic adenocarcinomas of the biliary tract [including intrahepatic or extrahepatic (perihilar and distal) cholangiocarcinoma and gallbladder carcinoma] [17]. The study included patients with previously untreated disease that was unresectable or metastatic at initial diagnosis as well as those who had recurrence of the disease at least 6 months after curative surgery and, if given, had completed adjuvant therapy for a minimum of 6 months. Patients had an ECOG performance status of 0 or 1, had ≥ 1 lesion per Response Evaluation Criteria in Solid Tumour (RECIST) v1.1 and had no prior exposure to immunotherapy. Patients with ampullary carcinomas, a history of allogeneic organ transplantation, known autoimmune disorders or inflammatory disorders (with some exceptions), were among those excluded from TOPAZ-1 [17].
Patients were stratified by disease status (unresectable or recurrent) and primary tumour location (intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma or gallbladder carcinoma) and randomized to receive intravenous infusions of durvalumab 1500 mg or placebo plus gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2 (Table 1) [17]. Durvalumab and placebo were administered on day 1, and gemcitabine and cisplatin were administered on days 1 and 8 of each 21-day cycle for 8 cycles, thereafter patients received durvalumab 1500 mg or placebo monotherapy once every 4 weeks until disease progression or other prespecified discontinuation criteria were met. Patients who were clinically stable at initial disease progression could continue to receive study treatment at the discretion of the investigator and patient [17].
The primary endpoint was overall survival, defined as time between randomization and death due to any cause. All efficacy endpoints were assessed in the full analysis set, which included all patients who were randomized to a treatment group [17]. The final analysis was planned after a total of 496 deaths had occurred, and an interim analysis was planned after ≈ 397 deaths. At the data cut-off for the interim analysis (11 August 2021), 424 patients had died (198 in the durvalumab group and 226 in the placebo group). The median duration of follow-up was 16.8 months and 15.9 months in the durvalumab and placebo groups, respectively [17].
Baseline characteristics were generally well balanced across the treatment groups [17]. Patients had intrahepatic cholangiocarcinoma (55.9% of patients), gallbladder carcinoma (25.0%) or extrahepatic cholangiocarcinoma (19.1%) as the primary tumour type. While a small proportion of patients (19.1%) had recurrent tumours, most patients (80.7%) had unresectable tumours at initial diagnosis. The majority (86.0%) of tumours were metastatic, with only a small proportion (13.9%) being locally advanced at diagnosis. More than half of patients (58.7%) had a tumour area positivity (TAP) score of ≥ 1% (i.e. tumours with ≥ 1% of the area occupied by cancer and/or immune cells with PD-L1 staining), and less than a third (30.1%) had a TAP score of < 1% [17].
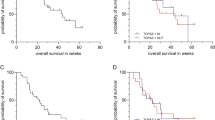
In adults with advanced biliary tract cancer, first-line treatment with durvalumab plus gemcitabine and cisplatin resulted in improved overall survival [17]. At the interim analysis cut-off, the addition of durvalumab to gemcitabine and cisplatin significantly prolonged median overall survival compared with the addition of placebo (Table 1). The estimated rate of overall survival in patients receiving durvalumab plus gemcitabine and cisplatin was higher at 12, 18 and 24 months compared with placebo plus gemcitabine and cisplatin (Table 1). The Kaplan-Meier curves for overall survival showed a growing divergence in favour of durvalumab, starting at ≈ 6 months; the hazard ratio (HR) was 0.91 for the first 6 months and further improved to 0.74 after 6 months (until interim analysis data cut-off). This finding was confirmed by Epanechnikov Kernel-smoothed hazard functions [17].
Furthermore, the addition of durvalumab to gemcitabine and cisplatin improved treatment outcomes as assessed by the investigator using RECIST v1.1 criteria [17]. Median progression-free survival was significantly prolonged in patients receiving durvalumab plus gemcitabine and cisplatin compared with placebo plus gemcitabine and cisplatin (Table 1). The confirmed objective response rate was higher in the durvalumab than in the placebo group (Table 1). In the durvalumab and placebo groups, respectively, there were 7 (2.1% of patients) and 2 (0.6%) complete responses, and 84 (24.6%) and 62 (18.1%) partial responses [17]. Of the patients who had an objective response, the median time to response was 1.6 months in the durvalumab group and 2.7 months in the placebo group and the median duration of response was 6.4 months and 6.2 months in each group. A sustained response of ≥ 12 months was observed in 26.1% of patients in the durvalumab group and 15.0% of patients in the placebo group [17].
The observed benefits in overall survival and progression-free survival with durvalumab plus gemcitabine and cisplatin remained generally consistent across clinically relevant subgroups based on disease status (unresectable or recurrent), primary tumour location (intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma and gallbladder carcinoma), TAP scores (≥ 1% or < 1%), stage at diagnosis (locally advanced or metastatic), ECOG performance status (0 or 1), age (≥ 65 years or < 65 years), sex (male or female), race (Asian or non-Asian) and region (Asia or rest of world) [17]. An exploratory analysis of TOPAZ-1 suggests that durvalumab plus gemcitabine and cisplatin may provide overall survival benefit in patients with tumours that have clinically actionable genetic alterations. While prevalence was low for many of the alterations, median overall survival hazard ratios were < 1 for all clinically actionable mutant subtypes (IDH1, BRAF, BRCA1/2 mutations, and FGFR2 rearrangements) except ERBB2 alterations [18].
Efficacy of durvalumab was maintained in an updated analysis with an additional 6.5 months of follow-up (cut-off 25 February 2022; median follow-up 23.4 months and 22.4 months in the durvalumab and placebo groups) [19]. The overall survival benefit of durvalumab plus gemcitabine and cisplatin was slightly improved compared with the interim analysis (HR 0.76 from 0.80), with overall survival benefit seen in responders (HR 0.69, 95% CI 0.46–1.04) as well as in patients with stable disease (HR 0.77, 95% CI 0.62–0.96). The two-year overall survival rate was 23.6% and 11.5% of patients in the durvalumab and placebo groups, respectively [19].
Health-related quality of life was assessed using the European Organisation for Research and Treatment of Cancer (EORTC) 30-Item Core Quality of Life Questionnaire (QLQ-C30) and the EORTC 21-Item Cholangiocarcinoma and Gallbladder Cancer Quality of Life Questionnaire (QLQ-BIL21), and patient-reported adverse events were assessed using Patient-Reported Outcome – Common Terminology Criteria for Adverse Events [17]. No detriment to patient quality of life and no clinically meaningful differences in mean change from baseline of time to deterioration or patient-reported outcomes was reported with the addition of durvalumab to gemcitabine and cisplatin compared with the addition of placebo [20].
Data from the real-world setting support the effectiveness of durvalumab plus gemcitabine and cisplatin [21]. In a prospective real-world study in 17 sites in Italy in patients (n = 145) with advanced biliary tract cancer who received durvalumab plus gemcitabine and cisplatin, with a median follow-up of 8.5 months, patients had a median overall survival of 12.9 months, a median progression-free survival of 8.9 months and an overall response rate in 34.5% of patients [21].
5 Tolerability of Durvalumab
The tolerability of durvalumab plus gemcitabine and cisplatin was manageable in adults with advanced biliary tract cancer in the TOPAZ-1 trial [17] and was consistent with its safety profile in other indications [22]. In TOPAZ-1, adverse events were reported up to 90 days after the last dose of study treatment and were assessed in all patients who had at least one dose of durvalumab (n = 338) or placebo (n = 342) [17]. The median duration of study treatment was 7.3 months in patients receiving durvalumab plus gemcitabine and cisplatin and 5.8 months in those receiving placebo plus gemcitabine and cisplatin. The median relative dose intensities were similar between durvalumab and placebo groups [17].
In TOPAZ-1, the most common adverse reactions (incidence ≥ 20%) in patients receiving the addition of durvalumab or placebo to gemcitabine and cisplatin were fatigue (42% and 43% of patients in the durvalumab and placebo groups, respectively), nausea (40% and 34%), constipation (32% and 29%), decreased appetite (26% and 23%), abdominal pain (24% and 23%), rash (23% and 14%), and pyrexia (20% and 16%) [10].
Treatment-related adverse events (TRAEs) occurred in 92.9% and 90.1% of patients in the durvalumab and placebo groups [17]. Grade 3 or 4 TRAEs occurred in 62.7% and 64.9% of patients in the respective groups; the most common (incidence ≥ 5% in any group) grade 3 or 4 TRAEs were mainly haematological (Fig. 1). Serious TRAEs occurred in 15.7% and 17.3% of patients in the durvalumab and placebo groups. Treatment discontinuations due to TRAEs occurred in 8.9% and 11.4% of patient in the durvalumab and placebo groups. There were two deaths (hepatic failure and ischaemic stroke) in the durvalumab group and one death (polymyositis) in the placebo group which were considered potentially treatment related [17]. No new safety signals were reported in the updated analysis (data cut-off 25 February 2022), and the rate of adverse events across treatment arms remained similar [19].
The most common (≥ 5% of patients) grade 3 or 4 treatment-related adverse events in patients receiving durvalumab or placebo in combination with gemcitabine and cisplatin in TOPAZ-1 [17]. GC gemcitabine plus cisplatin, pts patients
In a real-world setting, the incidence of adverse events was comparable to those reported in TOPAZ-1 but with a slightly different safety profile, with the most common adverse events of any grade consisting of more haematological rather than gastrointestinal adverse events [21].
5.1 Adverse Events of Special Interest
IMAEs are of special interest given durvalumab’s mechanism of action (Sect. 2) and have been reported in other studies with durvalumab (as a single agent or in combination with other therapies) [10]. In TOPAZ-1, IMAEs occurred in 12.7% of patients receiving durvalumab plus gemcitabine and cisplatin and in 4.7% of patients receiving placebo plus gemcitabine and cisplatin [17]. Grade 3 or 4 IMAEs occurred in 2.4% and 1.5% of patients in the durvalumab and placebo groups [17]. The most common IMAEs with durvalumab included hypothyroid events, dermatitis/rash, hepatic events and adrenal insufficiency [23]. The median time to onset of IMAEs was 108 days and the median time to resolution of IMAEs was 54 days in the durvalumab group. There were three (0.9% of patients) treatment discontinuations due to IMAEs in the durvalumab group and four (1.2%) in the placebo group. Patients receiving durvalumab plus gemcitabine and cisplatin who had an IMAE still benefitted from treatment, with an HR for overall survival of 0.66 compared with placebo plus gemcitabine and cisplatin [23].
Durvalumab has been associated with severe IMAEs in other indications. These events include immune-mediated pneumonitis, hepatitis, colitis, endocrinopathies, adrenal insufficiencies, type 1 diabetes mellitus, hyper/hypopituitarism, nephritis, myocarditis, and pancreatitis [10, 11]. Patients should be informed about the risks and be advised to contact a healthcare provider at the first signs of any IMAEs; early identification and management are essential for the safe use of durvalumab. For grade 3 or 4 IMAEs, treatment with corticosteroids is recommended, as well as temporarily withholding durvalumab or treatment discontinuation. See local prescribing information for further details [10, 11].
6 Dosage and Administration of Durvalumab
Durvalumab in combination with gemcitabine and cisplatin is indicated for the treatment of adults with locally advanced or metastatic biliary tract cancer in the USA [10], as the first-line treatment of adults with unresectable or metastatic biliary tract cancer in the EU [11], and in unresectable biliary tract cancer in Japan [12]. In the USA and EU, durvalumab is administered as an intravenous infusion over 60 min once every 3 weeks in combination with gemcitabine and cisplatin for 8 cycles, and then once every 4 weeks as a single agent until disease progression or unacceptable toxicity [10, 11]. The recommended dosage of durvalumab is 1500 mg. In patients who weigh < 30 kg (USA) or ≤ 36 kg (EU), the recommended dosage is 20 mg/kg [10, 11]. Durvalumab should be administered before chemotherapy but on the same day [10].
In other indications with durvalumab (as a single agent or in combination with other therapies) [10], severe or life-threatening infusion-related reactions have been reported. Prophylactic medications and a reduced rate of infusion should be used in patients who have grade 1 or 2 infusion-related reactions; durvalumab should be discontinued in patients who have a grade 3 or 4 reaction [10, 11].
Drugs that block the PD-1/PD-L1 signalling pathway can cause fatal or serious complications, including hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease after reduced intensity conditioning, and steroid-requiring febrile syndrome in patients who have had allogenic haemopoietic stem cell transplants [10]. The benefit of durvalumab treatment should be carefully weighed against the risk in these patients [10].
There are no available data on use of durvalumab during pregnancy. Based on its mechanism of action and evidence from animal models durvalumab may cause embryo-foetal toxicity [10, 11]. Women who are pregnant should be advised on the potential risk of durvalumab to the foetus. Women of childbearing potential should use effective contraception during treatment and 3 months after the final dose of durvalumab treatment [10, 11].
Adverse events should be managed by temporarily withholding durvalumab or treatment discontinuation [10, 11]. Durvalumab dose reduction and escalation are not recommended [10, 11]. Local prescribing information should be consulted for detailed information, including recommended treatment modifications for adverse events, and warnings and precautions.
7 Place of Durvalumab in the Management of Advanced Biliary Tract Cancer
Durvalumab plus gemcitabine and cisplatin is the first treatment regimen in the first-line setting to improve upon the overall survival of adults with advanced biliary tract cancer compared with the standard of care since 2010 [3]. According to the European Society for Medical Oncology Clinical Practice Guideline, durvalumab plus gemcitabine and cisplatin should be considered for first-line treatment of advanced biliary tract cancer [2]. In the National Comprehensive Cancer Network biliary tract cancer guidelines, durvalumab plus gemcitabine and cisplatin is the preferred first-line treatment for patients with unresectable or metastatic biliary tract cancer, as well as in patients who have disease recurrence > 6 months after surgery with curative intent and > 6 months after completion of adjuvant therapy, if given [24].
In TOPAZ-1, the addition of durvalumab to gemcitabine and cisplatin significantly prolonged overall survival (primary endpoint) and progression-free survival while also improving the objective response rate compared with the addition of placebo (Sect. 4). Furthermore, the deterioration in patient-reported outcomes and quality of life in patients receiving durvalumab was not more rapid than placebo (Sect. 4). The overall survival benefit seen with the addition of durvalumab appeared to improve over time (Sect. 4). This trend towards improvement over time was also reported for durvalumab in other indications [22]. A longer-term follow-up would allow a more precise assessment of treatment benefit, which is essential for conducting a meaningful pharmacoeconomic analysis of durvalumab.
Biliary tract cancers exhibit heterogeneity, which can affect the effectiveness of treatment options [3]. Durvalumab provided overall and progression-free survival benefits in a diverse range of patients with advanced biliary tract cancer, regardless of the tumour location, disease status at diagnosis (unresectable or recurrent), or TAP score (Sect. 4). Additionally, durvalumab appeared to remain efficacious across different genetic profiles of biliary tract cancer (Sect. 4), although further studies with larger sample sizes are necessary to assess the prognostic value of these genetic markers.
The tolerability of durvalumab plus gemcitabine and cisplatin was manageable (Sect. 5), with no new safety signals compared with its use in other indications [22]. The most common adverse reactions with this combination were fatigue, nausea, constipation, decreased appetite, abdominal pain, rash and pyrexia. The addition of durvalumab to gemcitabine and cisplatin was not associated with more serious or severe TRAEs compared with the addition of placebo (Sect. 5). As with the use of durvalumab in other indications, patients should be closely monitored for immune-related reactions, and these should be managed according to severity (Sect. 5.1).
In conclusion, durvalumab plus gemcitabine and cisplatin prolongs overall survival while maintaining a manageable tolerability profile in adults with advanced biliary tract cancer. The available evidence suggests that durvalumab plus gemcitabine and cisplatin represents a valuable new treatment option for these patients.
Data Selection durvalumab: 69 records identified
Duplicates removed | 1 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 29 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 16 |
Cited efficacy/tolerability articles | 10 |
Cited articles not efficacy/tolerability | 13 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Keywords were durvalumab, Imfinzi, MEDI-4736, biliary tract cancer, cholangiocarcinoma, gallbladder cancer, TOPAZ-1. Records were limited to those in English language. Searches last updated 11 Oct 2023 | |
Change history
29 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11523-023-01021-0
References
Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet. 2021;397(10272):428–44.
Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–40.
Roth GS, Neuzillet C, Sarabi M, et al. Cholangiocarcinoma: what are the options in all comers and how has the advent of molecular profiling opened the way to personalised medicine? Eur J Cancer. 2023;179:1–14.
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–74.
Kim BJ, Hyung J, Yoo C, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017;80(1):209–15.
Ma K, Wei X, Dong D, et al. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol Lett. 2017;14(1):250–6.
Luo Q, Zhang L, Luo C, et al. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019;454:191–203.
Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–62.
AstraZeneca Pharmaceuticals LP. IMFINZI® (durvalumab) injection, for intravenous use: US prescribing information. 2022. https://www.fda.gov/. Accessed 11 Oct 2023.
AstraZeneca AB. IMFINZI (durvalumab): EU summary of product characteristics. 2023. https://www.ema.europa.eu/. Accessed 11 Oct 2023.
AstraZeneca Co. Ltd. Imfinzi® intravenous infusion 120mg / Imfinzi® intravenous Infusion 500mg: Japanese prescribing information. 2022. https://www.pmda.go.jp/. Accessed 11 Oct 2023.
Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9): e172411.
Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7(6):522–32.
Lutzky J, Antonia SJ, Blake-Haskins A, et al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors [abstract no. 3001]. J Clin Oncol. 2014;32(Suppl. 15):3001.
Baverel PG, Dubois VFS, Jin CY, et al. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther. 2018;103(4):631–42.
Oh D-Y, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):1–11.
Valle JW, Qin S, Antonuzzo L, et al. Impact of mutation status on efficacy outcomes in TOPAZ-1: a phase III study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+GC) in advanced biliary tract cancer (BTC) [abstract no. 68O plus presentation]. Ann Oncol. 2022;33(Suppl. 9):S1457.
Oh DY, He AR, Qin S, et al. Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+GC) in patients (pts) with advanced biliary tract cancer (BTC) [abstract no. 56P plus poster]. Ann Oncol. 2022;33(Suppl. 7):S565–S6.
Burris HA, III, Okusaka T, Vogel A, et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer [abstract no. 4070 plus poster]. J Clin Oncol. 2022;40(Suppl. 16):4070.
Rimini M, Fornaro L, Lonardi S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int. 2023;43(8):1803–12.
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Antonuzzo L, Takahashi H, Park JO, et al. Immune-mediated adverse event (imAE) incidence, timing and association with efficacy in the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+GC) in advanced biliary tract cancer (BTC) [abstract no. 57P plus poster]. Ann Oncol. 2022;33(Suppl. 7):S566-S7.
National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: biliary tract cancers (v2.2023). 2023. www.nccn.org/. Accessed 11 Oct 2023.
Acknowledgements
During the peer review process the manufacturer of durvalumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
S. Fung and Y. Y. Syed are salaried employee of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: E. Oneda Department of Clinical Oncology, Fondazione Poliambulanza, Brescia, Italy; C. Yoo Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fung, S., Syed, Y.Y. Durvalumab: A Review in Advanced Biliary Tract Cancer. Targ Oncol 18, 965–972 (2023). https://doi.org/10.1007/s11523-023-01007-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01007-y