Abstract
Background
Expensive novel anticancer drugs put a serious strain on healthcare budgets, and the associated drug expenses limit access to life-saving treatments worldwide.
Objective
We aimed to develop alternative dosing regimens to reduce drug expenses.
Methods
We developed alternative dosing regimens for the following monoclonal antibodies used for the treatment of lung cancer: amivantamab, atezolizumab, bevacizumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, and ramucirumab; and for the antibody-drug conjugate trastuzumab deruxtecan. The alternative dosing regimens were developed by means of modeling and simulation based on the population pharmacokinetic models developed by the license holders. They were based on weight bands and the administration of complete vials to limit drug wastage. The resulting dosing regimens were developed to comply with criteria used by regulatory authorities for in silico dose development.
Results
We found that alternative dosing regimens could result in cost savings that range from 11 to 28%, and lead to equivalent pharmacokinetic exposure with no relevant increases in variability in exposure.
Conclusions
Dosing regimens based on weight bands and the use of complete vials to reduce drug wastage result in less expenses while maintaining equivalent exposure. The level of evidence of our proposal is the same as accepted by regulatory authorities for the approval of alternative dosing regimens of other monoclonal antibodies in oncology. The proposed alternative dosing regimens can, therefore, be directly implemented in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The high expenses for lung cancer drugs limit the access to innovative treatments worldwide. |
Alternative dosing regimens for amivantamab, atezolizumab, bevacizumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, ramucirumab, and trastuzumab deruxtecan have the potential to save up to approximately 30% in drug expenses without predicted loss in effective exposure. |
The level of evidence for these alternative dosing regimens is the same as accepted by regulatory authorities, facilitating their direct implementation. |
1 Introduction
Lung cancer is one of the most common cancers, with more than 2 million new cases and 1.8 million deaths reported in 2020 [1]. In the last decade, the development of novel systemic treatments, such as immune checkpoint inhibitors and targeted therapies, has majorly improved treatment outcomes of locally advanced and metastatic lung cancer [2]. For non-small cell lung cancer, these drugs are now also moving to the early disease setting. However, these advances are associated with a serious price tag. The ever-increasing cancer drug prices are already straining healthcare budgets and they present serious hurdles to treatment access worldwide [3]. It is, therefore, of utmost importance to save treatment costs whenever possible. For cancer drugs, there is often considerable drug waste due to drug spill of partially used vials [4]. Additionally, several monoclonal antibodies are dosed within the linear milligram per kilogram bodyweight (mg/kg) paradigm. Because of the well-established non-linear relationship between body weight and systemic exposure to monoclonal antibodies, a linear mg/kg dosing approach will result in unnecessary pharmacokinetic variability [5, 6]. Therefore, dosing regimens accounting for this non-linear relationship have the potential to partly decrease pharmacokinetic variability.
The use of modeling and simulation to develop alternative dosing regimens for monoclonal antibodies has been embraced by the medical community [7], pharmaceutical companies [8, 9], and regulatory authorities [10]. This approach has, thus far, been employed to develop fixed dosing regimens and prolonged dosing intervals for the purposes of patient and prescriber convenience. This generally results in higher cumulative doses than initially approved [11, 12]. However, modeling and simulation can also be applied to develop cost-effective and clinically equivalent dosing regimens while limiting cumulative drug use.
Using the same approach that led to label changes of marketed monoclonal antibodies approved by the European Medicines Agency and the US Food and Drug Administration (FDA), we present alternative dosing regimens for currently approved monoclonal antibodies and antibody-drug conjugates for the treatment of lung cancer to reduce drug expenses, while maintaining equivalent systemic drug exposure, as a direct indicator for clinical response.
2 Methods
2.1 Drug Selection
The following drugs were included in this study: amivantamab, atezolizumab, bevacizumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, ramucirumab, and trastuzumab deruxtecan. Cemiplimab was excluded from this study, as only a single vial size is available containing exactly the dose to be administered, hampering further dose optimization, in contrast to the other drugs where vial sizes smaller than the approved dose were available.
2.2 Pharmacokinetic Modeling
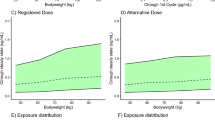
We used the population pharmacokinetic models as developed in representative cancer populations by the license holders and that were published in peer-reviewed scientific articles or in the drug approval data (see Table 1). The model code was verified by two experienced modelers. Then, for each individual drug, we performed a Monte Carlo simulation using the non-linear mixed-effects modeling software package NONMEM Version 7.5 (Icon, Dublin, Ireland) of the dosing regimens used in the pivotal phase III trials for registration. For this purpose, we created 500 virtual patients with representative European demographic data from the International Cancer Research Partnership database from the PopGen virtual human population generator [13]. The median total body weight of this population was 68 kg, with an interquartile range of 57–78 kg (total range 31–128 kg). Covariate distributions were simulated as reported for the populations of the published population pharmacokinetic models from the reported mean or median and corresponding variability. The pharmacokinetic data obtained from our simulations with the pivotal phase III dose were then considered as the reference for alternative dosing regimens. Thereafter, for each drug, we developed a weight band-based dosing regimen with the same dosing interval, based on both the available vial sizes and the described relationship between body size and pharmacokinetics. All simulations were performed in compliance with the recently published FDA guideline describing pharmacokinetic-based criteria to develop alternative dosing regimens for programmed cell death-1- or ligand-1-blocking antibodies [10]. This guideline states that the geometric mean of the area under the concentration–time curve during a dosing interval and the geometric mean concentration at steady state (AUCss and Ctrough,ss) and/or at the end of the first dosing cycle (AUCss,1st and Ctrough,1st) are no more than 20% lower or higher compared with the reference dosing regimen. Furthermore, the maximum concentration (Cmax) at steady state should not increase more than 20% compared with that of the reference dosing regimen.
As a clear dose– and exposure–response relationship has been reported for ipilimumab, leading to less efficacy at a lower exposure and dose and more toxicity at a higher exposure and dose [14, 15], the alternative ipilimumab dose to be developed was chosen to match the reference pharmacokinetics as closely as possible. For all other drugs, an alternative dosing regimen was developed leading to an exposure lower than associated with the reference dose, but within the margins of equivalence (a < 20% difference in relevant pharmacokinetic endpoints). Furthermore, separate alternative dosing regimens were evaluated for nivolumab every 2 weeks (Q2W) and every 3 weeks (Q3W). Initially, nivolumab was approved in a 3-mg/kg Q2W dose [16]. Recently, the combination of nivolumab and ipilimumab with two cycles of chemotherapy was approved based on the results of the CHECKMATE-9LA phase III trial, where nivolumab was dosed in a flat, fixed, 360-mg Q3W dose [17]. As chemotherapy is administered in Q3W cycles, an alternative Q3W dosing regimen was developed as well for nivolumab.
The alternative dosing regimens were based on the established relationship between body size and pharmacokinetics in the selected population pharmacokinetic models, to prevent a systematic bias of drug exposure versus body size. Furthermore, the alternative dosing regimens were based on the available vial sizes to prevent wastage as much as possible. From our simulations, we derived the geometric mean of the AUC during a dosing interval and the Ctrough (and the corresponding geometric coefficient of variation) of the selected drugs at the first cycle and at steady state. Moreover, for both dosing scenarios, the average quantity of drug used per dose administered was calculated, assuming wastage of the remainder of the smallest vial size available when it was partially used. The potential savings compared to dosing regimens in the drug labels were then calculated. The code for all pharmacokinetic models used for modeling and simulation is supplied in the Electronic Supplementary Material of this article as a reference. Table 1 describes the selected drugs, the dosing regimens from the pivotal phase III trial used as the reference dose, the reported approved alternative dosing regimens already developed by the license holders based on modeling and simulation, as well as the available vial sizes and the source for the population pharmacokinetic models.
3 Results
The results of our study are presented in Table 2. All predicted Ctrough or AUC from the alternative dose deviated were within ± 20% from the reference dose, as all ratios for these pharmacokinetic endpoints for the alternative and reference dose fell within the 0.8–1.2 boundaries for pharmacokinetic equivalence. For ipilimumab, no difference in predicted exposure was observed in the alternative dose. Furthermore, the predicted variability in Ctrough and AUC of the alternative dosing regimens was similar to that of the reference dosing regimens, showing that weight-band dosing does not relevantly increase variability in drug exposure. As no AUC or dose on the population was higher than associated with the reference dosing regimen, none of the Cmax of the alternative dosing regimens deviated > 20% from the Cmax from the reference dosing regimens for the population (data not shown).
As observed in Table 2, all alternative dosing regimens resulted in equivalent exposures with a < 20% difference in both the geometric means of Ctrough and AUC during a dosing interval after the first cycle and at steady state (calculated ratios within the predefined 0.8–1.2 boundaries), with maximum average savings in expenses compared with the reference dose that range from 28% (trastuzumab deruxtecan) to 11% (pembrolizumab).
The reductions in the average quantity of drug used in our proposed alternative dosing regimens are driven both by lower doses and rounding of the dose based on weight bands. For pembrolizumab, we propose a dose of either 100 or 150 mg for people weighing < 60 or > 60 kg, respectively. As only 100-mg vials exist for pembrolizumab, the preparation of a 150-mg dose results in a projected wastage of two vials. We predicted savings of approximately 11% for the alternative dose of pembrolizumab compared with the reference dose of 2 mg/kg. Compared to the currently approved 200-mg Q3W and 400-mg Q6W doses, the projected savings increase to 16%. As pembrolizumab is currently one of the most frequently used programmed cell death-1 inhibitors, we think that the actual savings in drug expenses will be higher, as vial sharing will further reduce wastage. The manufacturer of pembrolizumab has recently shown that pembrolizumab infusions are stable for at least 1 week after reconstitution, further facilitating paring of preparations for multiple patients to prevent wastage when appropriate microbiological conditions are met [37]. The 400-mg Q6W dosing regimen for pembrolizumab has been approved based on modeling and simulation with the 2-mg/kg Q3W dosing regimen as the reference. It was shown that in the Q6W dosing regimen, the Ctrough was 12% lower than the Ctrough associated with the initially approved 2-mg/kg Q3W dosing interval [12]. This shows that further dose reductions for a Q6W dosing regimen of pembrolizumab are not possible without violating the proposed criteria for equivalence.
4 Discussion
In our analysis, we calculated the maximum fraction of drug saved by our alternative dosing regimen compared to the reference dosing regimen. However, for durvalumab and nivolumab, the savings compared with the currently approved dosing regimens may be even higher. Although the durvalumab dosing regimen of 10 mg/kg Q2W was used in the phase III clinical study that led to its approval, the license holder has changed the label to a 1500-mg Q4W dosing regimen based on modeling and simulation. This may facilitate additional savings. For patients weighing < 60 kg, we propose a 480-mg Q2W dose (corresponding with a 960-mg cumulative dose during a 4-week interval), which results in 36% savings in drug costs compared with the approved 1500-mg Q4W dose. For the nivolumab 240-mg Q2W and 480-mg Q4W dosing regimens, which were developed by the license holder based on modeling and simulation after initial approval of the 3-mg/kg Q2W dose, the savings generated by our alternative dosing regimen for nivolumab may be up to 50% compared with the fixed-dose regimens, depending on the weight of the patient.
Our simulations were based on a representative European population. In a population with a higher average body weight, the average administered dose will be higher. Nonetheless, our proposed alternative dosing regimens, based on weight bands, may still result in reductions in drug expenses compared with the reference dosing regimens in populations with a higher body weight.
In contrast to the other drugs in our analysis, trastuzumab is an antibody conjugated with the cytotoxic drug deruxtecan, which is a topoisomerase inhibitor [36]. As it is known that the pharmacokinetics of the (release of the) payload of trastuzumab deruxtecan is described by a linear pharmacokinetic process, the pharmacokinetics of the payload is directly proportional to the complete conjugate. Therefore, the pharmacokinetics of the complete conjugate was used as an endpoint for our investigation of alternative dosing regimens [36]. The approved linear (mg/kg) dosing regimen of trastuzumab deruxtecan leads to relative overdosing in patients with a relatively higher weight, owing to the non-linear relationship between antibody clearance and body size [5]. For non-conjugated monoclonal antibodies, if complete receptor occupancy is reached in the approved dose across the complete range of body weights, relative overdosing will not result in toxicity. However, relative overdosing may result in additional toxicity for antibody-drug conjugates, as more of cytotoxic drug will also reach the systemic circulation. In the proposed alternative dose for trastuzumab deruxtecan, we accounted for the non-linear relationship between body size and drug clearance, resulting in less relative overdosing in the higher weight range. Whether this also results in less toxicity should be prospectively verified, but seems plausible. No alternative dosing regimen could be developed for cemiplimab because of the limitations of a single vial size of 350 mg. The flat fixed 350-mg Q3W dose was based on modeling and simulation and based on a 3-mg/kg Q2W dosing regimen [38]. The development of smaller vial sizes (e.g., 50 and 100 mg), either by the manufacturer or by a public initiative, may have the potential to vastly reduce drug expenses.
There are some studies that indicate that nivolumab may be dosed lower than proposed by use here [39, 40], yet results are conflicting and higher doses of nivolumab appear to be required for the treatment of lung cancer [41,42,43]. Although we specifically focused on monoclonal antibodies and antibody-drug conjugates used for the treatment of lung cancer, most drugs in our analysis have broader indications. We propose that the developed alternative dosing regimens may be extrapolated to other indications for which these drugs are used, when the approved doses for lung cancer and the alternative indication are the same.
Our analysis was mainly focused on savings in drug expenses. However, total costs of treatment depend on more than drug expenses only. Some of the reference dosing regimens in our study (e.g., durvalumab) require more frequent (Q2W) administration than also described in the label (Q4W). More frequent administration may result in additional costs associated with preparation and administration of the drug. Furthermore, with regard to patient convenience, it may be argued that less frequent dosing may be more desirable as hospital visits will be reduced. Our analysis can be used to carefully weigh all these considerations to establish a cost-effective treatment for lung cancer. Another factor that should be weighed is that the pharmaceutical sector is responsible for a significant proportion of all carbon emissions [44]. By development of more sustainable dosing strategies with less drug wastage as presented here, one can directly contribute to the reduction of the carbon footprint of pharmaceuticals.
The criteria for a clinically equivalent exposure used in our analysis were derived from the FDA guideline for in silico dose development for programmed cell death-1 and programmed cell ligand-1 inhibitors [10], but some drugs (amivantamab, bevacizumab, ipilimumab, ramucirumab, and trastuzumab deruxtecan) in our study do not belong to these classes of drugs. However, we postulate that this FDA guidance may be applicable to other classes of therapeutic antibodies as well. We propose that if it is known that additional variability in exposure may cause relevant changes in the benefit-to-risk ratio of a drug (e.g., as with ipilimumab) [14, 15], stricter criteria should be used, for example: a maximum deviation of 10% in Ctrough, Cmax, and AUC and no increase in the variability of these pharmacokinetic endpoints. Our alternative dosing regimen for ipilimumab complies with these stricter criteria, while resulting in less drug wastage.
Although our alternative dosing regimens contribute to less drug expenses in the short term, we argue that high drug costs are symptoms of an underlying drug pricing system that should be fixed, for example by implementing pricing transparency [45]. Our proposal should be considered a “free-market solution” for a “free-market problem,” which can be used to limit drug expenses until we fundamentally change how the development of innovative drugs is funded and reimbursed.
5 Conclusions
We have developed alternative dosing regimens that will result in a reduction in drug wastage while maintaining clinically equivalent drug exposures, based on solid clinical pharmacological data from the license holders of the drugs. These dosing regimens may aid the reduction in costs and drug wastage without impacting drug efficacy and safety and may be implemented in routine practice without the necessity of performing an additional clinical study.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–74.
Leighl NB, Nirmalakumar S, Ezeife DA, Gyawali B. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:1–12.
Liran O, Prus J, Gordon N, Almog V, Gruenewald T, Goldstein DA. A real-world analysis of cancer drug wastage due to oversized vials. J Am Pharm Assoc. 2018;58:643–6.
Bai S, Jorga K, Xin Y, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51:119–35.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Hendrikx J, Haanen J, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212–21.
Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–8.
Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43.
US FDA. Pharmacokinetic-based criteria for supporting alternative dosing regimens of programmed cell death receptor-1 (PD-1) or programmed cell death-ligand 1 (PD-L1) blocking antibodies for treatment of patients with cancer: guidance for industry. 05-01-2023. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetic-based-criteria-supporting-alternative-dosing-regimens-programmed-cell-death-receptor. Accessed 4 Apr 2023.
Baverel PG, Dubois VFS, Jin CY, et al. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther. 2018;103:631–42.
Lala M, Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68–75.
McNally K, Cotton R, Hogg A, Loizou G. PopGen: a virtual human population generator. Toxicology. 2014;315:70–85.
Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res. 2013;19:3977–86.
Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64.
EMA. Opdivo CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/opdivo-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211.
EMA. Rybrevant CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/rybrevant-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
FDA. Drug approval package: RYBREVANT. 02-11-2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761210Orig1s000TOC.cfm. Accessed 4 Apr 2023.
EMA. Tecentriq CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/tecentriq-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
Morrissey KM, Marchand M, Patel H, et al. Alternative dosing regimens for atezolizumab: an example of model-informed drug development in the postmarketing setting. Cancer Chemother Pharmacol. 2019;84:1257–67.
US FDA. Drug approval package: TECENTRIQ. 02-11-2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000TOC.cfm. Accessed 4 Apr 2023.
EMA. Avastin scientific discussion. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/scientific-discussion/avastin-epar-scientific-discussion_en.pdf. Accessed 4 Apr 2023.
Han K, Peyret T, Marchand M, et al. Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother Pharmacol. 2016;78:341–51.
EMA. Imfinzi CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/imfinzi-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
EMA. Yervoy summary of product characteristics. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/product-information/yervoy-epar-product-information_en.pdf. Accessed 4 Apr 2023.
Sanghavi K, Zhang J, Zhao X, et al. Population pharmacokinetics of ipilimumab in combination with nivolumab in patients with advanced solid tumors. CPT Pharmacometrics Syst Pharmacol. 2020;9:29–39.
Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit–risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–8.
Long G, Tykodi S, Schneider J, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29:2208–13.
Zhang J, Sanghavi K, Shen J, et al. Population pharmacokinetics of nivolumab in combination with ipilimumab in patients with advanced malignancies. CPT Pharmacometrics Syst Pharmacol. 2019;8:962–70.
EMA. Keytruda CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/keytruda-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
Ahamadi M, Freshwater T, Prohn ME, et al. Model-based characterization of the pharmacokinetics of pembrolizumab: a humanized anti–PD-1 monoclonal antibody in advanced solid tumors. CPT Pharmacometr Syst Pharmacol. 2017;6:49–57.
EMA. Cyramza CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/cyramza-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
O’Brien L, Westwood P, Gao L, Heathman M. Population pharmacokinetic meta-analysis of ramucirumab in cancer patients. Br J Clin Pharmacol. 2017;83:2741–51.
EMA. Enhertu CHMP assessment report. 02-11-2022. Available from: https://www.ema.europa.eu/en/documents/assessment-report/enhertu-epar-public-assessment-report_en.pdf. Accessed 4 Apr 2023.
Yin O, Xiong Y, Endo S, et al. Population pharmacokinetics of trastuzumab deruxtecan in patients with HER2-positive breast cancer and other solid tumors. Clin Pharmacol Ther. 2021;109:1314–25.
Sundaramurthi P, Chadwick S, Narasimhan C. Physicochemical stability of pembrolizumab admixture solution in normal saline intravenous infusion bag. J Oncol Pharm Pract. 2020;26:641–6.
Paccaly AJ, Migden MR, Papadopoulos KP, et al. Fixed dose of cemiplimab in patients with advanced malignancies based on population pharmacokinetic analysis. Adv Ther. 2021;38:2365–78.
Yoo SH, Keam B, Kim M, et al. Low-dose nivolumab can be effective in non-small cell lung cancer: alternative option for financial toxicity. ESMO Open. 2018;3: e000332.
Patil VM, Noronha V, Menon N, et al. Results of phase III randomized trial for use of docetaxel as a radiosensitizer in patients with head and neck cancer, unsuitable for cisplatin-based chemoradiation. J Clin Oncol. 2023 Jan 27;JCO2200980. https://doi.org/10.1200/JCO.22.00980. Online ahead of print.
Agrawal S, Feng Y, Roy A, Kollia G, Lestini B. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer. 2016;4:1–11.
Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95.
Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31–41.
Ray A, Sharma S, Sadasivam B. Carbovigilance: curtailing the global pharmaceutical carbon footprint. Future Healthc J. 2021;8: e683.
Uyl-de Groot CA, Löwenberg B. Sustainability and affordability of cancer drugs: a novel pricing model. Nat Rev Clin Oncol. 2018;15:405–6.
Author information
Authors and Affiliations
Contributions
Rob ter Heine designed the study, performed the analysis and wrote the manuscript. Dirk Jan A.R. Moes and Nikki de Rouw performed the analysis, checked the model code and wrote the manuscript. Michel M. van den Heuvel, Berber Piet, Maarten J. Deenen, Anthonie J. van der Wekken, Lizza E.L. Hendriks, Sander Croes, Robin M.J.M. van Geel, Frank G.A. Jansman, Rogier C. Boshuizen, Eric J. Franssen, Arthur J. Smit, Daphne W. Dumoulin, Thijs H. Oude Munnink, Egbert F. Smit, Hieronymus J. Derijks, Cor H. van der Leest and Jeroen J.M.A. Hendrikx interpreted the data and results and wrote the manuscript.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Rob ter Heine has received fees or funding from Stichting Treatmeds and Amgen. Anthonie van der Wekken has received fees or funding from AstraZeneca, Boehringer Ingelheim, Pfizer Roche, Takeda, Janssen Cilag, Lilly, and Merck. Michel van den Heuvel has received fees or funding from Amgen, Astrazeneca, BMS, Janssen Pharmaceutica, Stichting Treatmeds, Merck, MSD, Novartis, Pamgene, Pfizer, Roche, Roche diagnostics, Abbvie, Astrazeneca, BMS, Lilly, MSD, Novartis, Pfizer, and Roche. Daphne Dumoulin has received funding from Roche, BMS, MSD, Astra Zeneca, Amgen, and Pfizer. Cor van der Leest has received fees or funding from BMS and MSD en Janssen. Lizza Hendriks has received fees or funding from Roche Genentech, AstraZeneca, Boehringer Ingelheim, Takeda, Merck, Pfizer, Benecke, Medtalks, VJOncology, high5oncology, BMS, Eli Lilly, Takeda, MSD, Merck, Novartis, Amgen, and Janssen. Sander Croes, Robin van Geel, Frank Jansman, Rogier Boshuizen, Egbert Smit, Arthur Smit, Thijs Oude Munnink, Hieronymus Derijks, Jeroen Hendrikx, Dirk Jan Moes, and Nikki de Rouw have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All model files to generate the presented data are provided in the Electronic Supplementary Material.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
ter Heine, R., van den Heuvel, M.M., Piet, B. et al. A Systematic Evaluation of Cost-Saving Dosing Regimens for Therapeutic Antibodies and Antibody-Drug Conjugates for the Treatment of Lung Cancer. Targ Oncol 18, 441–450 (2023). https://doi.org/10.1007/s11523-023-00958-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00958-6