Abstract
Background
The antibody–drug conjugate sacituzumab govitecan is approved for metastatic triple-negative breast cancer and has shown promising results in various other types of cancer. Its costs may limit patient access to this novel effective treatment modality.
Objective
The purpose of this study was to develop an evidence-based rational dosing regimen that results in targeted drug exposure within the therapeutic range while minimizing financial toxicity, to improve treatment access.
Patients and Methods
Exposure equivalent dosing strategies were developed based on pharmacokinetic modeling and simulation by using the published pharmacokinetic model developed by the license holder. The alternative dose was based on the principle of using complete vials to prevent spillage and on the established non-linear relationship between body weight and systemic exposure. Equivalent exposure compared to the approved dosing regimen of 10 mg/kg was aimed for. Equivalent exposure was conservatively defined as calculated geometric mean ratios within the 0.9–1.11 boundaries for area under the concentration–time curve (AUC), trough concentration (Ctrough) and maximum concentration (Cmax) of the alternative dosing regimen compared to the approved dosing regimen. Since different vial sizes are available for the European Union (EU) and United States (US) market, because body weight distributions differ between these populations, we performed our analysis for both scenarios.
Results
Dosing regimens of sacituzumab govitecan for the EU (< 50 kg: 400 mg, 50–80 kg: 600 mg, and > 80 kg: 800 mg) and US population (< 40 kg: 360 mg, 40–65 kg: 540 mg, 65–90 kg: 720 mg, and > 90 kg: 900 mg) were developed, based on weight bands. The geometric mean ratios for all pharmacokinetic outcomes were within the predefined equivalence boundaries, while the quantity of drug used was 21.5% and 19.0% lower for the EU and US scenarios, respectively.
Conclusions
With the alternative dosing proposal, an approximately 20% reduction in drug expenses for sacituzumab govitecan can be realized while maintaining an equivalent and more evenly distributed exposure throughout the body weight range, without notable increases in pharmacokinetic variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
By using pharmacokinetic modeling, an evidence-based rational dosing regimen was developed that resulted in targeted drug exposure within the therapeutic range while minimizing financial toxicity, to improve treatment access. |
With the developed alternative dosing regimen, an approximately 20% reduction in drug expenses for sacituzumab govitecan can be realized. |
1 Introduction
Patients with metastatic triple-negative breast cancer (mTNBC) have poor survival outcomes, since they lack druggable targets like estrogen receptor, progesterone receptor, and human epidermal growth factor receptor. Although addition of immunotherapy to chemotherapy has shown promising first-line clinical activity in a subset of triple-negative breast cancer (TNBC) patients, single-agent chemotherapy remains the standard treatment for previously treated (beyond first line) mTNBC. TNBC is intrinsically chemo-sensitive in an early stage but shows low response rates and short progression-free survival. Sacituzumab govitecan is a novel antibody–drug conjugate that has shown promising results in the treatment of various types of cancer, including mTNBC, metastatic urothelial carcinoma (mUC), and non-small cell lung cancer (NSCLC) (combined with pembrolizumab) [1, 2]. Sacituzumab govitecan is composed of an anti-Trop-2 monoclonal antibody conjugated to the topoisomerase I inhibitor SN-38, via a cleavable linker. Trop-2 is a transmembrane glycoprotein that is overexpressed in many epithelial cancers, making it an attractive target for therapeutic intervention [2,3,4].
Progression-free survival and overall survival of mTNBC patients were significantly longer with sacituzumab govitecan than with single-agent chemotherapy in the phase III ASCENT trial. The median overall survival was 12.1 months (95% confidence interval [CI] 10.7–14.0) with sacituzumab govitecan and 6.7 months (95% CI 5.8–7.7) with chemotherapy (hazard ratio for death 0.48; 95% CI 0.38–0.59; P < 0.001) [3]. Although sacituzumab govitecan is proven effective in this specific patient population, its substantial price tag has raised a lot of debate. In the United Kingdom, the National Institute of Health and Care Excellence (NICE) initially did not approve this drug due to an unfavorable pharmacoeconomic profile with an incremental cost-effectiveness ratio (ICER) of £196,929 per quality-adjusted life-year (QALY) gained [5]. Also, in the Netherlands, sacituzumab govitecan is currently not reimbursed due to the high costs [6], thus limiting patients access to an effective treatment. The current registered dose is 10 mg/kg on day 1 and 8 of a 21-day cycle. In the European Union (EU) and United States (US) setting, different vial sizes (200 mg in the EU and 180 mg in the United States of America [USA]) are available.
In clinical practice, there is often unnecessary drug spillage due to discarding partially used vials [7]. Furthermore, dosing of monoclonal antibodies according to the linear milligram per kilogram body weight (mg/kg) paradigm causes unnecessary pharmacokinetic variability due to the non-linear relationship between body weight and systemic exposure [8, 9]. By accounting for this non-linearity the dose may be further individualized while maintaining effective exposure.
At the approved dosage of sacituzumab govitecan, no significant relationship between systemic exposure and efficacy was found. However, a clear relationship between exposure and toxicity could be identified [10]. Since the linear mg/kg dosing paradigm results in relative overexposure in the high body weight range, a more even distribution of exposure across all body weights has the potential to reduce toxicity, without compromising efficacy.
The purpose of our investigation was, therefore, to develop a rational weight-based and cost-saving dosing regimen for sacituzumab govitecan, resulting in pharmacokinetically equivalent exposure to the approved 10 mg/kg dosing regimen.
2 Methods
Our general approach was based on the principle used in the recent US Food and Drug Administration (FDA) guidance for in silico dose development of programmed cell death (ligand) receptor-1 (PD [L]-1) blocking antibodies for treatment of patients with cancer [11]. This guidance allows the adoption of alternative doses of monoclonal antibodies in the label of approved drugs without the necessity of a clinical study, when it can be shown by using pharmacokinetic modeling that equivalent exposure can be achieved with an alternative dose compared to the approved dose. Since there is a general rationale for such a strategy for all monoclonal antibodies based on pharmacokinetic and pharmacodynamic behavior, the principle of this guideline can be used as guidance for monoclonal bodies targeting other receptors as well [9].
2.1 Pharmacokinetic Model Simulation
In short, in this guideline, equivalent exposure is defined as follows: the geometric means of the area under the concentration–time curve (AUC) over the least common time interval and trough concentration (Ctrough) following the alternative dosing regimen at steady state and/or in the first least common time interval are no more than 20% lower. For our in silico evaluation of alternative dosing regimens, we used the population pharmacokinetic model for sacituzumab govitecan, as published in the European Medicines Agency (EMA) public assessment report [10]. This linear two-compartment model described the pharmacokinetics of the intact antibody–drug conjugate. Using this model, a Monte Carlo simulation was performed using the non-linear mixed effects modeling software package NONMEM V7.5 (Icon, Dublin, Ireland). The NONMEM model code is supplied in the supplemental material of this manuscript.
2.2 Acceptance Criteria
Furthermore, according to this guideline the geometric mean of steady-state maximum concentration (Cmax) should not increase more than 25%. These criteria are well-aligned with bioequivalence boundaries for the geometric mean ratios for the AUC and Cmax of 0.8–1.25 for drugs without a narrow therapeutic window [12]. Since we consider antibody–drug conjugate drugs to have a narrow therapeutic index, we used the more conservative equivalence criteria of 0.9–1.11 [13] for the predicted geometric mean ratios, as previously proposed for these kinds of drugs for the current analysis.
2.3 Virtual Patient Population
We investigated alternative dosing regimens for the EU and US setting, as the vial sizes (200 mg in EU and 180 mg in the USA) and weight-banded dose recommendation will be different. For this purpose, we created two virtual populations of 500 participants: an EU population, with demographic data from the International Cancer Research Partnership, and a US population from the National Health and Nutrition Examination Survey (NHANES) database, both obtained from the PopGen virtual human population generator [14]. Since serum albumin was a covariate for the peripheral distribution compartment of sacituzumab govitecan, we assumed a serum albumin of 40 g/L [15], with an inter-individual geometric coefficient of variation of 5% during all our simulations.
2.4 General Approach
First, we predicted the pharmacokinetics of sacituzumab govitecan associated with the approved dose for both populations. For these simulations, we assumed exact dosing based on the body weight, without rounding. Thereafter, we explored alternative dosing regimens using the whole vials for the US and EU population separately. As the pharmacokinetics of the cytotoxic payload of sacituzumab govitecan (SN-38) are directly proportional to the pharmacokinetics of the total antibody–drug conjugate, the obtained predicted pharmacokinetics of the intact antibody–drug conjugate were used for our analysis of pharmacokinetic equivalence.
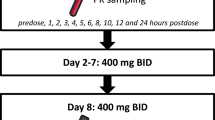
The simulated reference dosing regimen was 10 mg/kg on day 1 and 8 of a 21-day cycle, in line with the drug label. The alternative dosing regimen was based on the same administration days and 21-day cycle, with individual doses based on weight bands with the use of complete vials, while accounting for the established allometric (non-linear) relationship between body size and pharmacokinetics. For all dosing regimens, the average quantity of drug used per dose was calculated, under the assumption of complete wastage of a partially used vial.
3 Results
For the EU setting, the selected alternative weight-band dosing regimen was as follows: < 50 kg 400 mg, 50–80 kg 600 mg, and > 80 kg 800 mg on day 1 and 8 of a 21-day cycle. For the US setting, the selected dosing regimen was < 40 kg 360 mg, 40–65 kg 540 mg, 65–90 kg 720 mg, and > 90 kg 900 mg on day 1 and 8 of a 21-day cycle.
The results of our analysis are presented in Table 1a (EU setting) and Table 1b (US setting) and are visually depicted in Figs. 1, 2, respectively. As observed, all predicted Ctrough and AUC values for the alternative dosing regimens were slightly lower, yet within the equivalence criteria. As no AUC or dose in the population was higher than associated with the reference dosing regimen, none of the Cmax of the alternative dosing regimens deviated > 11% from the Cmax from the reference dosing regimens for the population (data not shown). Furthermore, as presented in Figs. 1C, D and 2C, D, the alternative dosing approach reduces variability in exposure (Ctrough) over the body weight range. Furthermore, a more even distribution in exposure metrics across the body weight ranges can be observed for the alternative dose. Additional visual representations can be found in the electronic supplementary material (Figs. S1A–C and S2A–C, where the different exposure metric distributions per population are presented as histograms). For more in-depth comparison, the proportion of patients receiving the alternative dose resulting in exposure that falls outside the 20th and 80th quantiles of the AUCs of the registered dose are presented in Table S1 (see the electronic supplementary material).
A Distribution body weight of studied population (EU). B Comparison of exposure metrics (Ctrough 1st cycle) between registered dose and alternative dose. C Ctrough at steady state distribution per body weight group registered dose. D Ctrough at steady state distribution per body weight group alternative dose. E Comparison of exposure metrics (Ctrough steady state) between registered dose and alternative dose. F Comparison of exposure metrics (AUC first cycle) between registered dose and alternative dose. AUC area under the concentration–time curve, Cmin minimum blood plasma concentration, Ctrough trough concentration, EU European Union
A Distribution body weight of studied population (USA). B Comparison of exposure metrics (Ctrough 1st cycle) between registered dose and alternative dose. C Ctrough at steady state distribution per body weight group registered dose. D Ctrough at steady state distribution per body weight group alternative dose. E Comparison of exposure metrics (Ctrough steady state) between registered dose and alternative dose. F Comparison of exposure metrics (AUC 1st cycle) between registered dose and alternative dose. AUC area under the concentration–time curve, Ctrough trough concentration, USA United States of America
As observed in these tables, approximately 20% savings in drug expenses can be realized while maintaining effective exposure, with predicted exposure to be equivalent to the approved dose with < 10% deviation. Furthermore, it can be observed that the developed allometric weight-band dosing algorithm with the use of complete vials was not at the expense of pharmacokinetic variability, since the differences in coefficients of variation (CV) in AUC and Ctrough between regimens were negligible.
4 Discussion
In the current study, we show that further optimization of the sacituzumab govitecan dosing regimen was cost saving as well as leads to a more even distribution in systemic exposure to active drug across the full weight range. Approximately 20% of drug expenses can be saved while maintaining equivalent exposure for each patient. The use of complete vials did not come with more pharmacokinetic variability, showing that our proposed dosing regimen is as individualized as the approved mg/kg dosing regimen.
The predicted geometric mean ratios for exposure of the alternative dosing regimens of approximately 0.91 are within the bioequivalence boundaries for drugs with a narrow therapeutic index [13], as well as within the boundaries for pharmacokinetic equivalency as proposed by the FDA in their recent guidance for in silico dose optimization of PD(L)-1 inhibitors [11], and the bioequivalence limits as proposed by the EMA [16] and FDA [12]. These latter boundaries are often applied for generic and biosimilar drugs. The marginally lower predicted exposure associated with the alternative dose as developed by us can therefore be considered as equivalent to the registered dosing regimen. In addition, a thorough exposure-response analysis of sacituzumab govitecan as documented in the EMA public assessment report [10] did not reveal a significant relationship between systemic exposure and efficacy within the exposure range of the approved dose of 10 mg/kg, but did report a significant and positive relationship between systemic exposure and toxicity. Notably, in an exposure-response analysis where lower doses were also included [17], a relationship was found. This indicates that maintaining an equivalent exposure, as proposed in this study, is pivotal to ensuring adequate efficacy. Furthermore, since the proposed alternative dosing regimen results in less relative over-exposure for high body weights, it may be postulated that toxicity may be even reduced. Since the methodology applied by us has already been used to change the posology in the label of approved drugs without performing a clinical study [18, 19] and since the analysis was based on the population pharmacokinetic model as reported by the manufacturer and because we used conservative criteria for equivalence, we consider the level of evidence sufficiently high to implement this dosing strategy in the clinic.
Although sacituzumab govitecan is considered an effective drug, it is not without adverse effects. In the case of severe toxicity in clinical practice, it is recommended by the label of sacituzumab govitecan to perform a 25% dose reduction and use colony stimulating factor in case of neutropenia. If there is a recurrence of toxicity, a further dose reduction of 50% is advised. In addition, it is known that UGT1A1 metabolizes the SN-38 payload of sacituzumab govitecan, and polymorphisms of the gene can lead to decreased enzyme activity and increased toxicity [20]. Early UGT1A1 testing as standard practice may identify patients with UGT1A1 *28/*28 genotype at risk for excess toxicity, who may undergo early dose reductions to prevent discontinuation of this important advanced breast cancer treatment [21]. However, consensus has not yet been established on a specific dose reduction or mandatory UGT1A1 testing. In our analysis, we assumed a 100% dose at all times for all patients. In the ASCENT trial, dose reductions due to adverse events occurred with similar frequency in the two groups (22% of the patients who received sacituzumab govitecan) [3]. It may be argued that savings in drug expenses in practice may deviate. Since the number of vials administered per dose in the alternative dosing regimens were approximately three to five, one may consider reducing the dose by one vial for the occurrence of dose-limiting toxicity. Subsequently, further reduction is possible by another vial at a second occurrence, as this closely resembles the 25% and 50% dose reductions in the label and will also prevent drug spillage by prevention of partially used vials.
5 Conclusion
In conclusion, we show that drug expenses for treatment with sacituzumab govitecan can be reduced by optimizing the dose while maintaining equivalent exposure. However, the true cause of drugs being expensive and therefore not reimbursed by the healthcare system is not the dose, but the price as chosen by the license holder. Ideally, the root cause for high drug costs is tackled, but this requires a worldwide fundamental system change. Until then, the alternative dose as proposed by us can be used to reduce drug expenses associated with sacituzumab govitecan treatment.
References
Early Activity With Frontline Sacituzumab Govitecan/Pembro in Metastatic NSCLC [press release]. 2023, September 10. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2023/9/gileads-phase-2-evoke02-study-of-trodelvy-sacituzumab-govitecanhziy-in-combination-with-keytruda-pembrolizumab-demonstrates-promising-clinica.
Rugo HS, Bardia A, Marme F, Cortes J, Schmid P, Loirat D, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol. 2022;40(29):3365–76. https://doi.org/10.1200/JCO.22.01002.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–41. https://doi.org/10.1056/NEJMoa2028485.
Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80(10):1019–25. https://doi.org/10.1007/s40265-020-01337-5.
NICE says new triple negative advanced breast cancer drug too expensive for NHS use. 2022. Available from: https://www.nice.org.uk/news/article/nice-says-new-triple-negative-advanced-breast-cancer-drug-too-expensive-for-nhs-use.
Government D. Negatief vergoedingsbesluit Trodelvy [updated 29-03-2023. Available from: https://www.rijksoverheid.nl/actueel/nieuws/2023/03/28/geneesmiddel-trodelvy-niet-in-basispakket.
Liran O, Prus J, Gordon N, Almog V, Gruenewald T, Goldstein DA. A real-world analysis of cancer drug wastage due to oversized vials. J Am Pharm Assoc (2003). 2018;58(6):643–6. https://doi.org/10.1016/j.japh.2018.06.004.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32. https://doi.org/10.1146/annurev.pharmtox.48.113006.094708.
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51(2):119–35. https://doi.org/10.2165/11596370-000000000-00000.
European Medicines Agency. Assessment report Trodelvy, International non-proprietary name: sacituzumab govitecan. 2021. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
FDA. Pharmacokinetic-Based Criteria for Supporting Alternative Dosing Regimens of Programmed Cell Death Receptor-1 (PD-1) or Programmed Cell Death-Ligand 1 (PD-L1) Blocking Antibodies for Treatment of Patients with Cancer 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetic-based-criteria-supporting-alternative-dosing-regimens-programmed-cell-death-receptor.
FDA. Statistical approaches to establishing bioequivalence. 2001. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-approaches-establishing-bioequivalence.
Yu LX, Jiang W, Zhang X, Lionberger R, Makhlouf F, Schuirmann DJ, et al. Novel bioequivalence approach for narrow therapeutic index drugs. Clin Pharmacol Ther. 2015;97(3):286–91. https://doi.org/10.1002/cpt.28.
McNally K, Cotton R, Hogg A, Loizou G. PopGen: A virtual human population generator. Toxicology. 2014;315:70–85. https://doi.org/10.1016/j.tox.2013.07.009.
Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. https://doi.org/10.1016/j.addr.2018.07.011.
European Medicines Agency. Guideline on the investigation of bioequivalence. 2010. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf.
Singh I, Sathe AG, Singh P, Diderichsen PM, Fauchet F, Maringwa J, et al. Exposure-response analyses of sacituzumab govitecan (SG) efficacy and safety in patients (pts) with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2022;40(16_suppl):1076.
Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68–75. https://doi.org/10.1016/j.ejca.2020.02.016.
Bi Y, Liu J, Furmanski B, Zhao H, Yu J, Osgood C, et al. Model-informed drug development approach supporting approval of the 4-week (Q4W) dosing schedule for nivolumab (Opdivo) across multiple indications: a regulatory perspective. Ann Oncol. 2019;30(4):644–51. https://doi.org/10.1093/annonc/mdz037.
Wong M, Behrendt CE, Yu W, Bosserman LD, Lavasani SM, Patel N, et al. UGT1A1 *28/*28 genotype and risk of toxicity and disease progression in breast cancer patients treated with sacituzumab govitecan-hziy. J Clin Oncol. 2023;41(16_suppl):1033.
Rugo HS, Tolaney SM, Loirat D, Punie K, Bardia A, Hurvitz SA, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. https://doi.org/10.1038/s41523-022-00467-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Competing Interest Related to this Work
D.J.A.R. Moes, J.J.M.A. Hendrikx, H.J. Guchelaar, R.H.J. Mathijssen, J.L. Bakker, V.O. Dezentjé, N. de Rouw, N.P. van Erp, E.F. Smit, M.M. van den Heuvel, T.H. Oude Munnink, M. van Kats, S. Croes, J. R. Kroep, J. Zwaveling, and R. ter Heine declare no competing interest related to this work.
Institutional Review Board Statement
Not applicable since this is an in silico study.
Informed Consent
Not applicable since this is an in silico study.
Data Availability
Data are available on reasonable request from the authors.
Code Availability
Code available in the supplemental material.
Author Contributions
Conception and design: D.J.A.R. Moes and R. Ter Heine. Acquisition of data (acquired and managed virtual patients): D.J.A.R. Moes and R. Ter Heine. Analysis and interpretation of data: All authors. Writing, review, and/or revision of the manuscript: All authors. Final approval of manuscript: All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Moes, D.J.A.R., Hendrikx, J.J.M.A., Guchelaar, HJ. et al. Model-Informed Development of a Cost-Saving Dosing Regimen for Sacituzumab Govitecan. Targ Oncol (2024). https://doi.org/10.1007/s11523-024-01075-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11523-024-01075-8