Abstract
Background
In pancreatic cancer, systemic treatment options in addition to chemotherapy remain scarce, and so far only a small proportion of patients benefit from targeted therapies.
Objective
The patients with pancreatic cancer discussed in the CCCMunichLMU Molecular Tumor Board were reviewed to gain a better real-world understanding of the challenges and chances of precision oncology in this hard-to-treat cancer.
Methods
Patients with pancreatic cancer who received comprehensive genomic profiling and were discussed in the interdisciplinary Molecular Tumor Board between May 2017 and July 2022 were included. These patients’ medical charts, comprehensive genomic profiling results, and Molecular Tumor Board recommendations were analyzed in this retrospective cohort study.
Results
Molecular profiles of 165 patients with pancreatic cancer were discussed in the Molecular Tumor Board. In the 149 cases where comprehensive genomic profiling was successful, KRAS mutations were detected in 87.9%, TP53 in 53.0%, and CDKN2A in 14.1%. 33.3% of KRAS wild-type patients harbored targetable mutations, while these were only found in 19.1% of patients with the KRAS mutation; however, this difference was not statistically significant. 63.8% of patients with successful testing received a targeted treatment recommendation by the Molecular Tumor Board; however, only 3.2% of these were put into practice. Compared to a historic cohort of patients with pancreatic cancer with synchronous metastatic disease diagnosed between 2010 and 2017, the patients from the pancreatic cancer cohort with synchronous metastatic disease had a longer survival.
Conclusions
This single-center experience emphasizes the challenges of targeted treatment in pancreatic cancer. Very few patients ultimately received the recommended therapies, highlighting the need for more and better targeted treatment options in pancreatic cancer, early comprehensive genomic profiling to allow sufficient time to put Molecular Tumor Board recommendations into practice, and close cooperation with clinical trial units to give patients access to otherwise not available targeted treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While 63.8% of patients with pancreatic cancer received a recommendation for a targeted therapy from the Molecular Tumor Board based on their molecular profiles, only 3.2% of therapeutic recommendations were put into practice. |
KRAS wild-type patients seemed to harbor targetable mutations more often than the patients with the KRAS mutation; however, this difference was not statistically significant. |
This study highlights the need for better targeted treatment options and clinical trials in pancreatic cancer. |
1 Introduction
The increasing availability of molecularly targeted therapies has transformed the treatment landscape in solid cancers such as biliary tract cancer, non-small cell lung cancer, and prostate cancer, which is why extended molecular testing is recommended routinely in these entities [1]. In pancreatic cancer, however, treatment options in addition to chemotherapy are scarce and 5-year overall survival has remained consistently low at approximately 10% [2, 3]. As per national guidelines, patients with metastatic pancreatic cancer have access to gemcitabine- and 5-FU-based therapies in the first-line setting. 5-FU/nanoliposomal irinotecan is approved after pretreatment with gemcitabine. Targeted therapies for patients with pancreatic cancer are currently limited to PARP inhibitors in the setting of platinum sensitivity and germline BRCA1/2 mutation, TRK inhibitors, and erlotinib [4]. The European Medicines Agency has not followed the US Food and Drug Administration approval for pembrolizumab in mismatch repair-deficiency/microsatellite instability (MSI) pancreatic cancer. Accordingly, this therapeutic option is considered off-label in Europe including Germany. As of now, the American Society of Clinical Oncology clinical guidelines recommend early testing for MSI-high (MSI-h) or mismatch repair deficiency, BRCA mutations, and NTRK gene fusions in patients with pancreatic cancer who would likely be able to receive targeted therapies in case actionable alterations are found [5, 6]. Unfortunately, only a small subgroup of patients with pancreatic cancer can benefit from these therapies so far, making further advances necessary to broaden the target population and overcome the challenges presented to physicians and patients in this disease.
With KRAS being the most frequent somatic mutation and a major oncogenic driver in pancreatic cancer [7], recent studies have been focusing on targeting it. In part, this has been accomplished by KRAS G12C inhibitors; although not yet approved in pancreatic cancer, patients with KRAS G12C alterations may be treated within clinical trials investigating substances such as adagrasib and sotorasib (NCT03600883, NCT03785249). The by far more frequent KRAS G12D mutation has been successfully used as a target of T-cell receptor engineered T cells in a 71-year-old patient, leading to a 72% size reduction in visceral metastases ongoing at 6 months [8]. While larger clinical trials are necessary to better understand the tolerability and effectiveness of such a treatment, reports like this foster hope for significant advances in a hard-to-treat entity such as pancreatic cancer.
In addition to the dominant subgroup of patients with KRAS-mutated pancreatic cancer, the KRAS wild-type population has gained attention especially in regard to molecularly guided therapies. It has been shown that this subgroup is enriched with targetable alterations and harbors a higher proportion of MSI-h and tumor mutational burden-high patients [9], which emphasizes the importance of knowing the KRAS mutation status of patients with pancreatic cancer in order to identify individuals with a higher likelihood to benefit from comprehensive genomic profiling (CGP).
Multiple attempts to define molecular subtypes of pancreatic cancer have led to different classifications. While Puleo et al. identified five subtypes based on next-generation sequencing [10], immunohistochemistry, and gene expression analysis of tumor cells and the microenvironment, Waddell et al. analyzed structural variations of the tumor to define a stable, locally arranged, scattered, and unstable subtype [11]. In a larger analysis with 456 tumors, Bailey et al. defined four subtypes by a genomic analysis that correlated with histopathological characteristics; squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine [12]. So far, it remains unclear whether the knowledge of these molecular subtypes can be leveraged in a clinical routine; however, a trial stratifying first-line chemotherapy depending on a classic or basal molecular subtype according to Moffit et al. [13] (NCT04683315), and a trial evaluating the efficacy of chemotherapy with regard to a molecular subtype (NCT03977233) are ongoing [14].
To gain a better understanding of the current status and challenges of precision oncology in pancreatic cancer at our comprehensive cancer center, we have gathered real-world data from our Molecular Tumor Board (MTB) from 2017 to 2022. Here, we report the retrospective analysis of CGP results and medical charts of 165 patients with pancreatic cancer.
2 Material and Methods
2.1 MTB
Starting in 2016, patients’ CGP results have been discussed in the University Hospital Munich’s interdisciplinary MTB. The LMU Munich University hospital is a high-volume tertiary care clinic with a certified interdisciplinary pancreas center. In the MTB, clinicians, pathologists, tumor geneticists, and experts on precision oncology evaluate CGP results while considering the clinical situation and medical history of the patient. Based on the interdisciplinary discussion and literature research, treatment recommendations are made for approved targeted therapies, off-label therapies, and available clinical trials if possible. Some patients are referred to the MTB by external physicians to discuss already available CGP results. In case a therapeutic recommendation is made, the evidence level is indicated according to the European Society of Medical Oncology Scale for Clinical Actionability of Molecular Targets and the National Center for Tumor Diseases and the decision whether to follow it is ultimately made by the treating physician. As of December 2022, more than 2700 patients have been discussed in the MTB.
2.2 Patient Population
This is a retrospective cohort study. All patients with pancreatic cancer who received CGP and were discussed in the University Hospital Munich’s MTB between 29 May, 2017 and 11 July, 2022 were included in the analysis. Patients with neuroendocrine pancreatic tumors or ultimately a diagnosis of a different malignancy than pancreatic cancer were excluded, as well as patients who were discussed in the MTB but did not receive CGP (Fig. 1). Median follow-up time was 13.1 months (range 0–181.8 months). Molecular testing had been recommended beforehand either by the entity-specific tumor board or by the coordinator of the clinic’s precision oncology program, or patients had been referred from external physicians for discussion of their available CGP results. The study has been approved by the local Ethics Committee of the Ludwig Maximilian University of Munich (21-0869). Additionally, we included a comparative cohort of patients with synchronous metastatic pancreatic cancer who had been diagnosed between December 2010 and August 2017 at our comprehensive cancer center in the survival analysis, this study has also been approved by the local ethics committee (284-10).
2.3 Sequencing Assays
The number of assays for molecular testing at the accredited pathology of the University Hospital Munich has increased over recent years through the addition of broader panels and the possibility to analyze the tumor mutational burden. The various assays performed at our center have been described in a previous report [15]. The allele frequency threshold for inclusion in the analysis was set at 5%; however, in cases where inclusion of low-frequency mutations was more sensible, especially in otherwise all wild-type tumors, this threshold was disregarded.
2.4 Follow-Up
Medical charts were retrospectively reviewed and analyzed to follow up on the included patients. Baseline characteristics were collected from physician’s reports closest to the CGP. Results of molecular testing and therapy recommendations were taken from the pathologist’s report and the MTB statement.
2.5 Statistical Analysis
Descriptive and statistical analysis, as well as the generation of graphs were performed with IBM SPSS Statistics Version 28.0 and Microsoft 365 Version 2206. Correlations between the presence of targetable alterations and different variables were measured by either Phi and Cramer’s V or Eta, as appropriate. The comparison of the mean age of two groups was performed using the Student’s t test. Survival time was calculated from the initial diagnosis to either death or the date of last contact. Survival curves were estimated by the Kaplan–Meier method and compared statistically using the Log-rank test. Four patients were not included in the survival analysis because the date of initial diagnosis was unknown. Statistical significance was determined as a p value <0.05.
3 Results
3.1 Patient Characteristics
Comprehensive genomic profiling results of 165 patients with pancreatic cancer were discussed in the MTB during the study period. The median age at initial diagnosis was 62 years (range 29–83 years) and the population consisted of 63% men and 37% women. The median time interval between initial diagnosis and MTB was 5.5 months, in this time span, the patients had progressed on or had shown intolerance towards a median of one line of systemic therapy (7.3% missing). At initial diagnosis, 60% of patients already had metastatic disease, while 37% presented with resectable or locally advanced disease (3% missing). The proportion of patients with metastatic pancreatic cancer increased to 87% until the timepoint of the MTB. 96.4% of the patients in this study had pancreatic ductal adenocarcinoma, but also rare histological subtypes such as (adeno)squamous, sarcomatoid, and acinar cell carcinoma were included and comprised the remaining 3.6%. Most patients had been referred to the MTB from our medical oncology department (58.2%), 19.4% from our department for gastroenterology, and 18.8% had been referred from external physicians. In the vast majority of patients, tumor tissue was used for CGP. Testing by liquid biopsy was only done in five cases, in two of them, no mutations could be detected, potentially owing to low circulating tumor DNA levels in the blood. Comprehensive genomic profiling was successful in 90.3% of cases, in the remaining cases, insufficient quality of tumor material was the most common reason for unsuccessful testing. Among the samples where testing failed, no common characteristic regarding collection method or source was found. In ten patients, testing was repeated with a different assay and/or tumor tissue. Fifteen patients were discussed in the MTB twice, either because new CGP results were available after initially unsuccessful testing, or not all information regarding the case had been available at the timepoint of the first discussion. Baseline characteristics of the 165 included patients can be found in Table 1.
3.2 Molecular Alterations
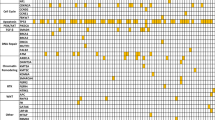
In patients where CGP was technically successful, a median of two pathogenic alterations were detected. Figure 2 shows the frequency of pathogenic alterations, the most commonly mutated genes being KRAS (87.9%), TP53 (53.0%), and CDKN2A (14.1%). Three of the patients with the KRAS mutation harbored G12C alterations (2.3%). In three cases, BRCA1/2 mutations were detected. Additionally, five patients with pancreatic cancer with BRCA1/2 germline mutations were discussed in our MTB between May 2017 and July 2022; however, as they did not undergo CGP they were not included in this analysis. Eigtheen patients were KRAS wild-type (12.1%), including four all wild-type cases in which even after manual curation no pathogenic alterations were found.
In the group of KRAS wild-type patients, 33.3% harbored targetable pathogenic alterations, while in the group of patients with the KRAS mutation, only 19.1% had targetable alterations; however, no statistically significant correlation could be drawn (Phi and Cramer’s V correlation coefficient 0.114; p = 0.163). An overview of the detected mutations in KRAS wild-type patients is depicted in Table 2. Among the patients with KRAS wild-type tumors, one had a sarcomatoid histological subtype, and two had acinar cell carcinomas. Fifteen KRAS wild-type patients were microsatellite stable, microsatellite status was unknown in the remaining three patients.
The proportion of patients with targetable alterations was similar between male and female patients (21.9% and 18.9%). Furthermore, there was a weak correlation between age and targetable mutations (Eta coefficient 0.104).
3.3 Targeted Treatment Recommendations
There were 63.8% of patients who received a recommendation for a targeted therapy from the MTB based on their CGP results. This number includes the combination of off-label treatment with trametinib and hydroxychloroquine based on case reports [16, 17] and the inclusion criteria of the THREAD trial that was recommended in most patients with the KRAS mutation discussed between June 2019 and March 2022 (evidence level NCT1C, European Society of Medical Oncology Scale for Clinical Actionability of Molecular Targets IIIA). Excluding this treatment option, only 23.5% of patients received a recommendation for a targeted therapy. An overview of the recommended treatments can be found in Fig. 3, the most common being trametinib and hydroxychloroquine (suggested in 62 cases), MEK inhibition and CDK4/6 inhibition (suggested in 14 cases), and olaparib (suggested in 9 cases). The olaparib recommendations were given conditionally upon sensitivity towards platin-containing regimens and the detection of BRCA germline mutations. To our knowledge, only three patients ultimately received the recommended targeted therapy, therefore, only 3.2% of therapeutic recommendations were put into practice.
Of the three patients who received targeted therapies, one patient with RET/PTC1 fusion was treated with a RET inhibitor for 2 months and died shortly after upon progression of the pancreatic cancer. Another patient with the KRAS mutation received trametinib and hydroxychloroquine for 2 months, afterwards the treatment was ended because of the progression of the disease and the patient died 3 months later. The third patient had KRAS and CDKN2A mutations and started treatment with a MEK inhibitor and a CDK4/6 inhibitor (palbociclib/trametinib) but died 1 month later because of the progression of the disease. One more patient had been recommended trametinib and hydroxychloroquine in April 2020, and although this MTB recommendation was not followed, the patient started treatment with palbociclib and trametinib in February 2022. The patient initially had stable disease during this treatment combination except for a progressive metastasis in the abdominal wall, which was treated with stereotactic radiation. Unfortunately, in August 2022, the patient had to be switched to chemotherapy again because of systemic progression of the disease.
3.4 Outcome
Median overall survival (mOS) in patients with synchronous metastatic disease was 14.1 months (95% confidence interval 10.4–17.8), and 24.6 months (95% confidence interval 20.4–28.8) in patients without metastases at the initial diagnosis (p = 0.002). Although mOS seemed longer in female patients (16.3 months vs 13.0 months in synchronous metastatic disease and 28.9 months vs 22.0 months in patients without metastases at initial diagnosis), the difference was not statistically significant (p = 0.844 and p = 0.308, respectively). There were also no significant differences in the survival of patients with or without druggable mutations. Although KRAS wild-type patients seemed to have a longer mOS (23.4 months vs 13.1 months in synchronous metastatic disease (Fig. 4a) and 44.6 months vs 23.6 months in locally limited disease at the initial diagnosis), this difference was also not statistically significant (p = 0.339 and p = 0.474, respectively).
Furthermore, we compared the group of 88 patients with synchronous metastatic disease to a historic cohort of 90 patients with pancreatic cancer with synchronous metastatic disease who had been diagnosed at our comprehensive cancer center between December 2010 and August 2017. The baseline characteristics of this group including also some patients with metachronous metastatic disease have been reported previously [18]. The proportion of male and female patients was similar in both cohorts (MTB cohort 63.3% male vs comparative cohort 65.9% male). Patients in the MTB cohort were younger than patients in the comparative cohort (mean age at initial diagnosis 59.8 years and 62.6 years, respectively; p = 0.049) and also had a longer mOS than patients in the comparative cohort (Fig. 4b).
4 Discussion
In this retrospective cohort study, we took a closer look at consecutive patients with pancreatic cancer who received CGP and were discussed in our local MTB. Patients analyzed here had a lower median age at initial diagnosis than what has been reported for pancreatic cancer in Germany in 2018 [19], possibly explained by a selection bias, as treating physicians might rather opt for CGP in younger patients with a good performance status. The distribution of male versus female patients was unbalanced with approximately twice as many male than female patients in the evaluated cohort. The age standardized incidence rate in Germany was 10.8 for female patients, and 15.1 for male patients in 2018. However, the incidence of pancreatic cancer in Germany in 2018 was similar among male and female patients (9860 and 9160, respectively) [19]. Therefore, the question arises why twice as many male patients with pancreatic cancer have been discussed in our MTB than female patients. In Germany in 2018, the median age at diagnosis of pancreatic cancer was 72 years in male patients and 76 years in female patients [19]. Considering the lower median age of our MTB cohort, fewer female patients might have been included because of the lower sex-specific incidence in the respective age group. With MTBs serving as an important referring system especially for early clinical trials, efforts to ensure a more balanced distribution of sex are critical. It has been reported multiple times that female patients are under-represented in clinical studies, especially phase I trials [20,21,22,23]. Standardized local regulations as to which patients should receive CGP can be helpful to minimize a potential selection bias by the treating physician.
Philip and colleagues have analyzed 2483 patients with pancreatic cancer, of whom 266 were KRAS wild-type (10.7%) and found that patients wtih KRAS wild-type were more likely to harbor targetable alterations or to be MSI-h [9]. In our cohort, the prevalence of KRAS wild-type tumors was comparable to 12.1% and targetable alterations appeared to be enriched in this population as well. However, most likely because of a too small sample size, statistical significance was not reached. Additionally, we could not observe a higher likelihood of MSI-h in KRAS wild-type patients. In the cohort reported by Philip and colleagues, pseudopapillary, acinar, sarcomatoid, and mucinous tumor histology stood out with a higher KRAS wild-type prevalence (100.0%, 81.8%, 14.3%, and 13.3%, respectively) [9]. These observations were in line with our MTB cohort that included one sarcomatoid pancreatic cancer and two acinar cell carcinomas, which were all KRAS wild-type. In the outcome analysis performed by Philip and colleagues, 5324 patients were included, and KRAS wild-type patients had a longer overall survival than patients with KRAS-mutated pancreatic cancer [9]. Although in our cohort there was a tendency towards a longer mOS in KRAS wild-type patients, the sample size was too small to draw a definitive conclusion from our data.
Only three patients with BRCA1/2 mutated pancreatic cancer were identified in our MTB cohort, which is lower than the prevalence of germline and somatic mutations reported in a large meta-analysis (BRCA1 0.9%, BRCA2 3.5%). A reason for this may be that patients eligible for treatment with platin-based chemotherapy received early germline testing for BRCA1/2 mutations as recommended by guidelines [4, 6], and while five patients with BRCA1/2 germline mutations were discussed in our MTB between May 2017 and July 2022, additional CGP was performed in only two of these cases.
What stands out when looking at our analysis is that only a small number of patients received the suggested targeted therapy—three patients out of 95 who had been recommended a treatment option. In the evaluation of the 1000 first cases discussed in our MTB, 17% of the therapeutic recommendations were realized [15]. The substantially lower proportion in the pancreatic cancer cohort lies in the nature of the disease and limited promising treatment options. Therapeutic recommendations made by the MTB have been put into practice more often in breast cancer (16%) [24] and central nervous system malignancies (15%) [25]. Ding and colleagues have reported a comparable rate of patients with pancreatic cancer receiving targeted treatment based on CGP (3%) [26]. A reason for this number being especially low in pancreatic cancer may be owing to the short survival and fast deterioration typical for this disease, not allowing for targeted treatments to be applied after standard-of-care treatments. Additionally, off-label treatments can be delayed by the need to apply for cost coverage by insurance companies beforehand. Low evidence levels also influence the decisions of treating physicians, stressing even more the need for further clinical trials to identify targeted treatment options that present realistic chances of improved survival and quality of life. Strategies to increase the number of patients with pancreatic cancer receiving targeted therapies in the future include the close cooperation of MTBs with early clinical trial units, performing CGP as early as possible, as well as identifying the right patients who are willing and able to follow targeted treatment recommendations [15, 27]. Another important aspect is the improvement of follow-ups, especially of patients referred to the MTB from external physicians, and their support in gaining access to the suggested therapies, either in clinical trials or as off-label use.
A large proportion of the therapeutic recommendations from our MTB is made up by trametinib combined with hydroxychloroquine put forward in case reports and prospectively investigated in the THREAD trial (NCT03825289). MEK inhibition has been found to elicit autophagy in pancreatic cancer, therefore the MEK inhibitor trametinib was combined with the autophagy inhibitor hydroxychloroquine, case reports of this combination have described partial responses or disease stabilization in a few patients [16, 17]. However, based on sobering experiences from our center and other institutions (personal communication), the combination has not been recommended any further since April 2022.
It became obvious that the patients in our MTB cohort had a longer mOS than the comparative cohort. This may be explained by a selection bias as patients with a very short survival are more likely to not receive CGP and discussion in the MTB between diagnosis and death. Furthermore, because CGP should be performed in patients fit enough and willing to undergo experimental treatment, patients with a lower performance status would not have been included in the MTB cohort. Finally, the comparative cohort consists of patients diagnosed between 2010 and 2017, while the MTB cohort consists of patients who were discussed in our MTB between 2017 and 2022. Therefore, although there might be some overlap between the patient groups, the improved outcome in the MTB cohort could also be related to the establishment of improved chemotherapeutic treatment options over the years [28, 29].
There are some limitations to this retrospective analysis: as a single-center study, it only reflects the experiences from our MTB, and a selection bias towards younger and motivated patients with a good performance status is to be assumed. Furthermore, because an increasing number of patients is referred to our MTB for the discussion of CGP results, follow-up with regard to treatment adherence and survival is challenging and not always possible. The implementation of a structured follow-up program within the MTB is a step that has been taken to improve this in the future [15]. Additionally, owing to the small sample size especially of rare subgroups, the significance of statistical analyses is limited.
5 Conclusions
In summary, even in the setting of a dedicated pancreatic cancer center and a precision oncology program, pancreatic cancer remains a hard-to-treat malignancy not only with conventional chemotherapy, but also with targeted treatments. The main oncogenic driver in pancreatic cancer, KRAS, is not yet broadly targetable besides KRAS G12C mutations. Looking at how rarely patients received a recommended targeted treatment in this cohort and how all three patients who did deteriorated too fast to adequately evaluate the efficacy of the received therapies, the question might arise whether CGP in pancreatic cancer should be done at all. Nonetheless, there are reasons to support CGP in pancreatic cancer. Treatment options are constantly developing, and in recent years, the number of Food and Drug Administration-approved targeted therapies has increased substantially [30]. Now, many patients need to undergo CGP in order for very few patients to benefit from this; however, the difference for these few patients could become enormous in the future. Especially in a malignancy with a high unmet clinical need for new therapies, such as pancreatic cancer, clinical trials investigating novel treatment options are essential, and their recruitment can be fueled by MTBs [31]. Although challenging, we believe broad CGP, discussion in MTBs, and striving for higher patient numbers to receive and benefit from targeted treatments are important goals that especially comprehensive cancer centers and university hospitals should pursue.
References
Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–505. https://doi.org/10.1016/j.annonc.2020.07.014.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Dorman K, Heinemann V, Kobold S, von Bergwelt-Baildon M, Boeck S. Novel systemic treatment approaches for metastatic pancreatic cancer. Expert Opin Investig Drugs. 2022;31(3):249–62. https://doi.org/10.1080/13543784.2022.2037552.
Seufferlein T, Mayerle J, Böck S, Brunner T, Ettrich TJ, Grenacher L, et al. S3-leitlinie zum exokrinen pankreaskarzinom—Kurzversion 2.0—Dezember 2021, AWMF-Registernummer: 032/010O. Z Gastroenterol. 2022;60(6):991–1037. https://doi.org/10.1055/a-1771-6811.
Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(24):2545–56. https://doi.org/10.1200/jco.2018.78.9636.
Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38(27):3217–30. https://doi.org/10.1200/jco.20.01364.
Singh RR, O’Reilly EM. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs. 2020;80(7):647–69. https://doi.org/10.1007/s40265-020-01304-0.
Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP, et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med. 2022;386(22):2112–19. https://doi.org/10.1056/NEJMoa2119662.
Philip PA, Azar I, Xiu J, Hall MJ, Hendifar AE, Lou E, et al. Molecular characterization of KRAS tild-type yumors in patients with pancreatic adenocarcinoma. Clin Cancer Res. 2022;28(12):2704–14. https://doi.org/10.1158/1078-0432.Ccr-21-3581.
Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155(6):1999–2013.e3. https://doi.org/10.1053/j.gastro.2018.08.033.
Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. https://doi.org/10.1038/nature14169.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. https://doi.org/10.1038/nature16965.
Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. https://doi.org/10.1093/annonc/mdw443.
Rodríguez Gil Y, Jiménez Sánchez P, Muñoz Velasco R, García García A, Sánchez-Arévalo Lobo VJ. Molecular alterations in pancreatic cancer: transfer to the clinic. Int J Mol Sci. 2021;22(4):2077. https://doi.org/10.3390/ijms22042077.
Heinrich K, Miller-Phillips L, Ziemann F, Hasselmann K, Rühlmann K, Flach M, et al. Lessons learned: the first consecutive 1000 patients of the CCCMunichLMU Molecular Tumor Board. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04165-0.
Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25(4):620–7. https://doi.org/10.1038/s41591-019-0367-9.
Xavier CB, Marchetti KR, Castria TB, Jardim DLF, Fernandes GS. Trametinib and hydroxychloroquine (HCQ) combination treatment in KRAS-mutated advanced pancreatic adenocarcinoma: detailed description of two cases. J Gastrointest Cancer. 2021;52(1):374–80. https://doi.org/10.1007/s12029-020-00556-z.
Dorman K, Gerckens M, Kruger S, Krueger K, Mayer Z, Rupp A, et al. Serum biomarker panel diagnostics in pancreatic ductal adenocarcinoma: the clinical utility of soluble interleukins, IFN-γ, TNF-α and PD-1/PD-L1 in comparison to established serum tumor markers. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04112-z.
Krebs—Bauchspeicheldrüsenkrebs. https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Bauchspeicheldruesenkrebs/bauchspeicheldruesenkrebs_node.html. Accessed 26 Aug 2022.
Pinnow E, Sharma P, Parekh A, Gevorkian N, Uhl K. Increasing participation of women in early phase clinical trials approved by the FDA. Womens Health Issues. 2009;19(2):89–93. https://doi.org/10.1016/j.whi.2008.09.009.
Labots G, Jones A, de Visser SJ, Rissmann R, Burggraaf J. Gender differences in clinical registration trials: is there a real problem? Br J Clin Pharmacol. 2018;84(4):700–7. https://doi.org/10.1111/bcp.13497.
Ayuso E, Geller RJ, Wang J, Whyte J, Jenkins M. Evaluation of worldwide clinical trials by gender: an FDA perspective. Contemp Clin Trials. 2019;80:16–21. https://doi.org/10.1016/j.cct.2019.03.007.
Sosinsky AZ, Rich-Edwards JW, Wiley A, Wright K, Spagnolo PA, Joffe H. Enrollment of female participants in United States drug and device phase 1–3 clinical trials between 2016 and 2019. Contemp Clin Trials. 2022;115:106718. https://doi.org/10.1016/j.cct.2022.106718.
Sultova E, Westphalen CB, Jung A, Kumbrink J, Kirchner T, Mayr D, et al. Implementation of precision oncology for patients with metastatic breast cancer in an interdisciplinary MTB setting. Diagnostics (Basel). 2021;11(4):733. https://doi.org/10.3390/diagnostics11040733.
von Baumgarten L, Kumbrink J, Jung A, Reischer A, Flach M, Liebmann S, et al. Therapeutic management of neuro-oncologic patients: potential relevance of CSF liquid biopsy. Theranostics. 2020;10(2):856–66. https://doi.org/10.7150/thno.36884.
Ding D, Javed AA, Cunningham D, Teinor J, Wright M, Javed ZN, et al. Challenges of the current precision medicine approach for pancreatic cancer: a single institution experience between 2013 and 2017. Cancer Lett. 2021;497:221–8. https://doi.org/10.1016/j.canlet.2020.10.039.
Subbiah V, Kurzrock R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer. 2018;4(2):101–9. https://doi.org/10.1016/j.trecan.2017.12.004.
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. https://doi.org/10.1056/NEJMoa1011923.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. https://doi.org/10.1056/NEJMoa1304369.
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6(1):201. https://doi.org/10.1038/s41392-021-00572-w.
Dienstmann R, Garralda E, Aguilar S, Sala G, Viaplana V, Ruiz-Pace F, et al. Evolving landscape of molecular prescreening strategies for oncology early clinical trials. JCO Precis Oncol. 2020;4:PO.19.00398. https://doi.org/10.1200/po.19.00398.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no specific funding and no funding was provided for the publication of this article.
Conflict of interest
KD has received travel support from Servier, GSK, and BMS. DZ has received travel support from AstraZeneca, Amgen, and BMS, as well as honoraria from AstraZeneca. LW has received honoraria from Roche, Servier, and Amgen. GB has received honoraria from Falk Foundation and Akcea. WGK has received honoraria from BMS. MS has received research grants from Bayer Healthcare and SIRTEX Medical, as well as lecture honoraria from Bayer Healthcare, SIRTEX Medical, Cook, Boston Scientific, LIAM, Siemens, Balt, Astra Zeneca, and Falk. SC has received research grants from Elekta, Viewray, and Brainlab and speaker fees/travel support from Elekta, Viewray, C-RAD, Roche, and Brainlab. JK received honoraria and reimbursement for travel and accommodation for participation in advisory boards and in the speakers’ bureau from AstraZeneca, Novartis, and Roche Pharma. MvB has received research support from and serves on the speakers’ bureau at Gilead, Miltenyi Biotec, MSD Sharpe & Dohme, Roche, Mologen, Novartis, Astellas, and BMS. SB reports personal fees from Celgene/BMS, AstraZeneca, Servier, Janssen-Cilag, and MSD (honoraria for scientific presentations and a paid consultant), as well as research support from Celgene outside the submitted work. VH reports grants, honoraria, and non-financial support from Amgen, Astra-Zeneca, Baxalta, Boehringer-Ingelheim, Celgene/BMS, Halozyme, Lilly, Merck, MSD, Novartis, OncoSil, Pierre-Fabre, Roche, Sanofi, Servier, Shire, Sirtex, Taiho, and Terumo. CBW has received honoraria from Amgen, Bayer, BMS, Chugai, Celgene, Falk, GSK, MSD, Merck, Janssen, Ipsen, Roche, Servier, SIRTeX, and Taiho; served on advisory boards for Bayer, BMS, Celgene, Janssen, MSD, Servier, Shire/Baxalta, Rafael Pharmaceuticals, RedHill, and Roche; has received travel support by Bayer, Celgene, Janssen, RedHill, Roche, Servier, and Taiho and research grants (institutional) by Roche. CBW serves as an officer for the European Society of Medical Oncology, Deutsche Krebshilfe, and Arbeitsgemeinschaft internistische Onkologie, and is a member of the European Union Commission expert group: Mission Board for cancer. The remaining authors have no conflicts of interest to declare.
Ethics approval
This retrospective chart review study involving human participants was in accordance with the principles of the Declaration of Helsinki and its later amendments. The study has been approved by the local Ethics Committee of the Ludwig Maximilian University of Munich (project number 21-0869), as has the study of the comparative group (284–10).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KD, DZ, LR, and CBW. The first draft of the manuscript was written by KD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and agree to be accountable for the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dorman, K., Zhang, D., Heinrich, K. et al. Precision Oncology in Pancreatic Cancer: Experiences and Challenges of the CCCMunichLMU Molecular Tumor Board. Targ Oncol 18, 257–267 (2023). https://doi.org/10.1007/s11523-023-00950-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00950-0