Abstract
Sotorasib (LUMAKRAS™ in the USA and LUMYKRAS™ in the EU) is an orally active, first-in-class G12C-mutant KRAS (KRASG12C) inhibitor. By binding irreversibly to KRASG12C, sotorasib inhibits downstream signalling pathways which are associated with cell growth and differentiation. Sotorasib is indicated for the treatment of adults with advanced, previously treated, KRAS G12C mutation-positive non-small cell lung cancer (NSCLC) in multiple countries, including the countries of the EU and the USA. A clinically relevant objective response rate was observed in patients with KRAS G12C mutation-positive NSCLC during the primary analysis and in an updated analysis of the phase I/II CodeBreaK 100 trial. Furthermore, a clinically relevant response duration was reported in updated analyses of the trial. Sotorasib has a manageable tolerability profile, with permitted dose modifications to manage toxicity. In summary, sotorasib is a promising KRASG12C inhibitor that increases the available treatment options for patients with KRAS G12C mutation-positive NSCLC who were previously treated with platinum-based chemotherapy and/or immunotherapy.
Plain Language Summary
KRAS is a protein that is involved in cell signalling pathways, including those that are associated with cell growth and differentiation. KRAS mutations are detected in 23% of patients with non-small cell lung cancer (NSCLC), with the G12C mutation being the most common. G12C-mutant KRAS (KRASG12C) is kept in an activated state, which is associated with cancer. Sotorasib (LUMAKRAS™ in the USA and LUMYKRAS™ in the EU), which is taken orally once daily, is the first approved drug that inhibits KRASG12C; it permanently binds to KRASG12C and locks it in an inactivated state. Sotorasib is approved for adults who have advanced, previously treated, KRAS G12C mutation-positive NSCLC. In a clinical trial in patients with KRAS G12C mutation-positive NSCLC, a clinically relevant proportion of patients responded to sotorasib treatment. Furthermore, the duration of effectiveness with sotorasib was considered to be clinically relevant. Adverse reactions with sotorasib treatment were manageable; the dose may be decreased and/or sotorasib treatment may be temporarily stopped to manage adverse reactions. Overall, sotorasib is a promising treatment option for patients with KRAS G12C mutation-positive NSCLC who have received at least one prior systemic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.21086737. |
First approved drug that inhibits KRASG12C; irreversibly binds to the unique cysteine residue at codon 12 |
Once daily oral treatment |
Clinically relevant objective response rate observed in patients with KRAS G12C mutation-positive NSCLC |
Manageable tolerability profile |
1 Introduction
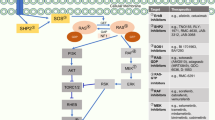
The rat sarcoma virus (RAS) family of GTPases are molecular switches that are involved in various cell signalling pathways, such as epidermal growth factor receptor (EGFR)-related signalling [1]. The downstream signalling pathways of RAS are complex; however, the canonical pathway is the RAF-MEK-ERK pathway, which subsequently results in the transcription of genes related to cell proliferation and differentiation [1]. Mutant variants of the Kirsten RAS (KRAS) isotype are frequently implicated in the oncogenesis of several types of cancer, including non-small cell lung cancer (NSCLC) [1, 2]. Mutations in the KRAS gene are detected in 23% of patients with NSCLC [3], with the most common being a single point missense mutation of glycine to cysteine at codon 12 (G12C) [1]; G12C mutations were observed in 46% of patients who had KRAS mutation-positive NSCLC [3, 4].
KRAS cycles between an active GTP-bound form and an inactive GDP-bound form during its function as a molecular switch [1, 2]. KRAS constitutively hydrolyses GTP to GDP, and the rate of hydrolysis or GTP exchange may be modulated by upstream factors [1, 2]. The G12C mutation prevents stabilization of the hydrolysis transition state, thereby keeping KRAS in the active GTP-bound state and perpetuating downstream signalling [2]. Competitive inhibitors of G12C-mutant KRAS (KRASG12C) or agents targeting downstream signalling were not successful due to low efficacy or tolerability [2]. Platinum-based chemotherapy and/or anti-programmed death 1 (PD-1) or anti-programmed death ligand 1 (PD-L1) immunotherapy are typically recommended for the treatment of KRAS mutation-positive NSCLC (Sect. 7); however, these therapies do not target KRAS G12C mutant-positive disease.
Sotorasib (LUMAKRAS™ in the USA and LUMYKRAS™ in the EU) is an orally active, first-in-class, small molecule, KRASG12C inhibitor [5], which is approved for the treatment of advanced, previously treated, KRAS G12C mutation-positive NSCLC in multiple countries including the USA [6], those of the EU [7], Australia, Brazil, Canada and the UK [8]. This article summarises the pharmacologic properties and clinical data for sotorasib in the management of NSCLC from a US and EU perspective.
2 Pharmacodynamic Properties of Sotorasib
Sotorasib irreversibly binds to the unique cysteine of KRASG12C, locking it in the inactive GDP-bound state, thereby inhibiting downstream signalling [6]. In two KRASG12C cell lines, sotorasib strongly inhibited the downstream phosphorylation of ERK1/2 [half-maximal inhibitory concentration (IC50) ≈ 0.03 µM] and subsequently inhibited the viability of these cell lines (IC50 ≈ 0.006 and 0.009 µM) [9]. Furthermore, sotorasib inhibited the growth of the majority of assessed KRASG12C cell lines (IC50 0.004–0.032 µM), but had no effect on the growth of non-KRAS mutant cell lines (IC50 > 7.5 µM). In animal models, sotorasib inhibited the growth of three patient-derived KRASG12C xenografts, but had no effect on the growth of a KRASG12V xenograft; in mice treated with sotorasib 200 mg/kg, the near-complete inhibition of ERK phosphorylation yielded durable anti-tumour responses in 8 of 10 mice [9].
Resistance to sotorasib may occur because of acquired mutations during treatment. The acquired mutations were heterogenous in nature [10], and the most common were alterations in receptor tyrosine kinases (RTKs), which were detected in 24% of 67 patients with NSCLC in the CodeBreaK 100 trial (Sect. 4); other acquired alterations were mutations in EGFR (9% of patients) and secondary RAS alterations (3%), with 12% of patients harbouring more than one alteration [11]. RTKs function upstream of KRAS and they may increase KRAS activity via the guanine nucleotide exchange factor Son of Sevenless (SOS) 1 or 2. Subsequently, KRAS shifts towards the active GTP-bound state, which is insensitive to KRAS inhibitors [2]. RTKs may also bypass KRAS inhibition via alternative signalling pathways (e.g. KRAS-independent activation of the PI3K-AKT-mTOR pathway) [2]. Secondary RAS alterations are mutations or the amplification of genes associated with KRAS (e.g. other single point mutations including G12D, G12V, G12F and V8L) or in homologous RAS GTPases (e.g. NRAS) [10]. MET amplification was observed in a KRASG12C cell line exposed to sotorasib. MET amplification stabilises the active, GTP-bound form of KRAS via SOS [12].
3 Pharmacokinetic Properties of Sotorasib
The pharmacokinetics of orally administered sotorasib are non-linear and time-dependent over the dosage range of 180–960 mg once daily, with similar systemic exposures between doses at steady state [6]. The median time to peak plasma concentration is 1 h. The 24-h area under the plasma-time curve (AUC24) is increased by 25% when sotorasib 960 mg is administered with a high-fat, high calorie meal, in comparison with administration under fasting state. Steady state is reached within 22 days, with a mean accumulation ratio of 0.56. The mean volume of distribution at steady state is 211 L and in vitro plasma protein binding is 89%. The mean terminal elimination half-life is 5 h, and in patients receiving sotorasib 960 mg once daily, the apparent clearance at steady state is 26.2 L/h. Sotorasib is primarily metabolized by non-enzymatic conjugation and oxidative metabolism via CYP3A enzymes. From a single radiolabelled dose of sotorasib, 74% and 6% of the dose was recovered from the faeces and urine, respectively; 53% and 1% of the dose was excreted unchanged into the faeces and urine, respectively [6].
No clinically significant differences were observed in the pharmacokinetics of sotorasib with respect to age, sex, race, body weight, line of therapy, Eastern Cooperative Oncology Group (ECOG) performance-status, kidney disease [in patients with an estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2] or mild hepatic impairment [6]. The pharmacokinetics of sotorasib has not been studied in patients with eGFR < 30 mL/min/1.73 m2 or moderate to severe hepatic impairment [6].
Concomitant use of acid-reducing agents decreases the systemic exposure of sotorasib [6]. Repeat doses of omeprazole (a proton pump inhibitor) decreased the maximum plasma concentration (Cmax) and AUC of a single sotorasib dose by 65% and 57%, respectively, under fed conditions; a decrease of 57% and 42%, respectively, was reported under fasted conditions [6, 13]. A single dose of famotidine (a histamine-H2 receptor antagonist) taken 10 h prior to and 2 h after a single dose of sotorasib decreased the fed-state Cmax and AUC of sotorasib by 35% and 38%, respectively [6]. Repeat doses of rifampicin (a strong CYP3A4 inhibitor) also decreased the single-dose Cmax and AUC of sotorasib by 35% and 51%, respectively [6, 14].
Sotorasib decreased the Cmax and AUC of midazolam (a sensitive CYP3A4 substrate) by 48% and 53%, respectively [6]. The coadministration of digoxin (a P-gp substrate) with sotorasib increased digoxin Cmax and AUC by 91% and 21%, respectively. In vitro, sotorasib may induce CYP2C8, 2C9 and 2B6, and may inhibit BCRP [6].
4 Therapeutic Efficacy of Sotorasib
The efficacy of sotorasib in the treatment of KRAS G12C mutation-positive NSCLC was investigated in the open-label, multicentre phase I/II CodeBreaK 100 trial in patients with advanced, previously treated, KRAS G12C mutation-positive solid tumours (Fig. 1). Data from the phase I portion of the trial indicated potential efficacy with sotorasib 180–960 mg once daily (the primary endpoint was safety), with 19 patients (32.2%) with NSCLC achieving a confirmed partial response (PR) [15]. Patients in the phase I portion of CodeBreaK 100 were excluded from the primary efficacy analyses of the phase II portion of the trial [16].
Trial design of the open-label phase I/II CodeBreaK 100 trial in adults with advanced, previously-treated, KRAS G12C mutation-positive solid tumours, including NSCLC [16, 17]. Efficacy results are reported in the animated figure (available online). KRAS Kirsten rat sarcoma virus, NSCLC non-small cell lung cancer. a123 patients with evaluable disease during the primary analysis; 124 patients during the updated analysis
Supplementary file2 (MP4 6715 KB)
Eligible patients (aged ≥ 18 years) had locally advanced or metastatic KRAS G12C mutation-positive NSCLC [centrally confirmed via polymerase chain reaction (PCR)], progressive disease following anti-PD-1 or anti-PD-L1 immunotherapy and/or platinum-based chemotherapy, and an ECOG performance-status of 0 or 1 (0–5 scale; higher scores indicate greater disability) [16, 17]. Key exclusion criteria included the presence of active, untreated brain metastases, prior treatment with > 3 lines of therapy, treatment with a systemic anticancer therapy within 28 days, radiation therapy within 2 weeks or treatment with a direct KRASG12C inhibitor. Enrolled patients were treated orally with sotorasib 960 mg once daily until disease progression, unacceptable toxicity or withdrawal of consent [16, 17].
Patients’ median age at baseline was 64 years, and half of the patients were female [16, 17]. The majority (82%) of patients were white, 15% were Asian and 2% were black. Almost all patients (97%) had metastatic disease and were previously treated with a median of two lines of treatment; 43%, 35% and 22% of patients received one, two or three lines of prior therapy, respectively. Platinum-based chemotherapy was previously administered in 90% of patients and anti-PD-1 or anti-PD-L1 immunotherapy in 91% of patients; most patients (81%) received both treatment modalities [16, 17].
The primary endpoint was objective response rate (ORR), which was planned to be assessed when the trial had 105 patients with evaluable NSCLC [16]. The primary analysis was performed in 123 patients with a data cutoff date of 1 Sep 2020 [17]. Tumour response was assessed by blinded independent central review using RECIST version 1.1 criteria and contrast-enhanced computed tomography or magnetic resonance imaging [16, 17]. A prespecified ORR benchmark of 23% was selected based on the ORR reported with ramucirumab plus docetaxel as a second-line treatment in patients with advanced NSCLC during the REVEL trial [16, 17]; a minimum ORR of 32% was required to exclude the benchmark from the range of the 95% confidence interval (CI) [17, 18].
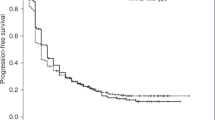
The ORR with sotorasib treatment during the primary analysis was 37.4% (Table 1), including 1.6% of patients who achieved a complete response (CR) [17]. The lower bound of the 95% CI of the ORR was higher than the prespecified benchmark of 23%, and the ORR was higher than 32%. The disease control rate (DCR) was 80.5% and the median duration of response (DOR) was 8.4 months; however, the median follow-up for DOR was 6.9 months during this analysis. Median progression-free survival (PFS) and overall survival (OS) with sotorasib treatment were 6.7 and 12.0 months, respectively (Table 1) [17].
The efficacy results of the primary analysis were supported by an updated analysis [data cutoff date 15 Mar 2021; median treatment and follow-up durations 5.5 and 15.3 months] (Table 1) [16]. The ORR and DCR were 37.1% and 80.6%, respectively, which included 3.2% of patients who achieved a CR. The 95% CI range of the ORR with sotorasib excluded the prespecified benchmark of 23%. Kaplan-Meier estimates of ORR at 3, 6 and 9 months were 90.5% (95% CI 76.7–96.3%), 70.8% (95% CI 54.3–82.2%) and 57.3% (95% CI 40.4–71.0%), respectively. The median DOR, PFS and OS were 11.1, 6.8 and 12.5 months, respectively [16]. In a later analysis (data cutoff date 20 Jun 2021), the median DOR was 11.1 months (Table 1) [7].
Response to sotorasib treatment was observed in patients with co-mutations in genes which frequently occur with NSCLC (e.g. STK11 and KEAP1) and in patients with higher PD-L1 expression [16]. In exploratory subgroup analyses (data cutoff date 15 Mar 2021), the ORR was 40% (8/20 patients) versus 39% (33/84) in patients with wild-type versus mutant TP53; 39% (27/69) versus 40% (14/35) in patients with wild-type versus mutant STK11 and 44% (37/84) versus 20% (4/20) in patients with wild-type versus mutant KEAP1. The ORR in patients with < 1%, 1–49% and ≥ 50% PD-L1 expression were 48% (21/44), 39% (13/33) and 22% (2/9), respectively [16].
In a pooled analysis of 174 patients with NSCLC enrolled in the phase I and II portions of CodeBreaK 100, the ORR was 40.7% (Table 1) [19]. The median DOR, PFS and OS were 12.3, 6.3 and 12.5 months, respectively. One-year and two-year OS rates were 50.8% and 30.3%, respectively. The data cutoff date was not reported for this analysis [19].
Patient-reported quality of life (QoL) during sotorasib treatment did not appear to be impacted after up to 231 days of treatment (n > 20) [20]. QoL was assessed using the European Organization for Research and Treatment of Cancer QoL Questionnaire Core 30 (EORTC QLQ-C30) questionnaire in addition to its lung cancer module (EORTC QLQ-CL13). EORTC QLQ-C30 global health status/QoL and physical functioning scores were generally consistent with baseline. Scores associated with symptoms in EORTC QLQ-C30 (e.g. fatigue, nausea, vomiting, pain) and lung-related symptoms in EORTC QLQ-CL13 were stable or improved versus baseline [20].
The efficacy of sotorasib was demonstrated in the open-label, comparator-controlled, phase III CodeBreaK 200 trial [21]. Patients with KRAS G12C mutation-positive NSCLC who had progressive disease following treatment with a platinum-based chemotherapy and a checkpoint inhibitor received sotorasib 960 mg orally once daily (n = 169) or docetaxel 75 mg/m2 intravenously once every 3 weeks (n = 151). The PFS was significantly (p = 0.002) extended with sotorasib than with docetaxel treatment [hazard ratio 0.66; 95% CI 0.51–0.86] (primary endpoint). Additionally, a significant (p < 0.001) difference in the ORR was reported (28.1% with sotorasib vs 13.2% with docetaxel). The median follow-up duration was 17.7 months [21].
4.1 Companion Diagnostic
The detection of KRAS G12C mutations from a liquid biopsy via a peripheral blood draw (Guardant360® CDx) was generally consistent with PCR [22]. The performance of the liquid biopsy was evaluated against PCR using archival tissue samples and plasma samples obtained from 112 patients enrolled in the CodeBreaK 100 trial. The overall percent agreement between the liquid biopsy and PCR was 0.82 (95% CI 0.76–0.87); the positive percent agreement was 0.71 (95% CI 0.62–0.79) and the negative percent agreement was 1.00 (95% CI 0.95–1.00) [22].
5 Tolerability of Sotorasib
Sotorasib had a manageable tolerability profile in 359 patients with solid tumours who were treated with sotorasib 960 mg once daily during the CodeBreaK 100 trial (Sect. 4) [7]; a similar tolerability profile was reported in patients during the CodeBreaK 200 trial [21]. The most common adverse drug reactions (ADRs) were diarrhoea (34% of patients), nausea (25%) and fatigue (21%); the most common grade ≥ 3 ADRs were increased alanine aminotransferase [ALT] (5%), increased aspartate aminotransferase [AST] (4%) and diarrhoea (4%). The most common ADRs leading to dose modification were increased ALT (6%), diarrhoea (6%), increased AST (6%), nausea (3%), increased blood alkaline phosphatase (3%) and vomiting (2%). Increased ALT, increased AST and drug-induced liver injury (all 1%) were the most common ADRs leading to discontinuation of sotorasib. Cases of ALT and AST elevation were transient in nature, with a median time to onset of 8 weeks. In patients who had hepatotoxic reactions, 26% were treated with corticosteroids [7].
Cases of interstitial lung disease (ILD) and pneumonitis were reported with sotorasib treatment [6, 7]. In 359 patients who received sotorasib, ILD or pneumonitis was reported in 0.8% of patients with all reactions being grade 3 or 4 in severity at onset; the median time to first onset was 2 weeks. Sotorasib was discontinued in 0.6% of patients due to ILD or pneumonitis [7].
In 126 patients with locally advanced or metastatic KRAS G12C mutation-positive NSCLC from the phase II portion of the CodeBreaK 100 trial, any-grade treatment-related adverse events (TRAEs) were reported in 69.8% of patients (Fig. 2) [16]. Grade ≥ 3 TRAEs were reported in 20.6% of patients and events occurring in more than one patient were mostly liver-related, including ALT increase (6.3%), AST increase (5.6%), diarrhoea (4.0%), gamma-glutamyl transferase increase (2.4%), drug-induced liver injury (1.6%) and pneumonitis (1.6%). Dose modification was required in 22.2% and 15.9% of patients due to all-grade or grade ≥ 3 TRAEs, respectively. Treatment discontinuation due to all-grade or grade ≥ 3 TRAEs occurred in 7.1% and 4.0% of patients, respectively. Fatal adverse events occurred in 15.9% of patients; however, none were considered treatment-related [16].
Treatment-related AEs with an incidence ≥ 5% of patients with locally advanced or metastatic non-small cell lung cancer enrolled in the phase II portion of CodeBreaK 100 (n = 126) [16]. AEs adverse events
6 Dosage and Administration of Sotorasib
Sotorasib is indicated in the USA for the treatment of adults with KRAS G12C mutant-positive locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one systemic therapy [6]. In the EU, sotorasib is indicated as a monotherapy for the treatment of adults with KRAS G12C mutant-positive locally advanced or metastatic NSCLC, who have progressed on, or are intolerant to, platinum-based chemotherapy and/or anti-PD-1/PD-L1 immunotherapy [7]. The recommended dosage of sotorasib is 960 mg taken orally once daily in both the USA and the EU [6, 7]; two dose reductions, with each reduction halving the dose, and temporary discontinuation of sotorasib are permitted for the management of adverse reactions [6, 7].
Monitoring patients for hepatotoxicity or new or worsening pulmonary symptoms is recommended (Sect. 5), including liver function tests conducted every three weeks for the first three months of treatment, and monthly or as clinically indicated thereafter [6, 7]. Consult local prescribing information for specific recommendations regarding treatment modification or discontinuation, detailed dosage instructions, drug interactions, contraindications and additional warnings and precautions.
7 Current Status of Sotorasib in the Management of Non-small Cell Lung Cancer
Sotorasib is a first-in-class KRASG12C inhibitor [5], which is generally recommended as a second- or subsequent-line treatment in NSCLC guidelines [23,24,25,26]. The US National Comprehensive Cancer Network recommends the use of sotorasib in patients with metastatic, KRAS G12C mutant-positive NSCLC who have progressive disease following treatment with platinum-based chemotherapy with or without immunotherapy [23]. Similar recommendations were made by the UK National Institute for Health and Care Excellence, which recommends sotorasib for adults who have locally advanced or metastatic KRAS G12C mutant-positive NSCLC whose disease has progressed on, or are intolerant to platinum-based chemotherapy or anti-PD-1/PD-L1 immunotherapy [24]. The European Society for Medical Oncology guidelines were published prior to the approval of sotorasib, and recommend recruitment into open trials for patients with KRAS G12C mutant-positive NSCLC; platinum-based chemotherapy and/or immunotherapy are recommended for patients without actionable oncogenic drivers [25, 26].
Sotorasib inhibited KRASG12C in preclinical models (Sect. 2) and demonstrated clinically relevant efficacy against advanced, previously treated, KRAS G12C mutant-positive NSCLC (Sect. 4). The ORR met prespecified benchmarks in the primary and updated analyses and was considered clinically relevant during the phase II portion of CodeBreaK 100 in patients with KRAS G12C mutant-positive NSCLC [16, 17]. Responses to sotorasib treatment were also reported in patients who had co-mutations in other genes (e.g. STK11 and KEAP1) typically associated with worse outcomes in NSCLC [16]. Findings in an updated analysis of patients from the phase II portion of CodeBreaK 100 support a clinically relevant response duration with sotorasib treatment [18]. Efficacy results were supported by a pooled and updated analysis of patients from the phase I and II portions of CodeBreaK 100 [19]. Additionally, the phase III CodeBreaK 200 trial demonstrated a significant extension in PFS with sotorasib versus docetaxel [21]. Further study on the efficacy of sotorasib based on coexisting mutations, PD-1/PD-L1 and/or resistance markers is warranted.
Sotorasib had a manageable tolerability profile during the CodeBreaK 100 trial (Sect. 5). Treatment discontinuation due to TRAEs occurred in < 8% of patients with KRAS G12C mutant-positive NSCLC receiving sotorasib during the phase II portion of the trial [16]. Although elevated AST or ALT were among the most commonly reported grade ≥ 3 ADRs in patients with solid tumours and in those with KRAS G12C mutant-positive NSCLC, these were transient in nature (Sect. 5) [7, 16]. Hepatotoxicity, ILD and pneumonitis are adverse reactions of special interest with sotorasib treatment [6, 7]; dose modification is permitted for the management of adverse reactions (Sect. 6), and corticosteroids were administered for the management of hepatotoxicity in approximately one-quarter of affected patients (Sect. 5) [7].
In summary, sotorasib is an orally active, first-in-class KRASG12C inhibitor, which demonstrated clinically relevant efficacy and a manageable tolerability profile in a clinical trial. Sotorasib is a promising treatment option for patients with KRAS G12C mutant-positive NSCLC who have received at least one prior systemic therapy.
Data Selection sotorasib: 422 records identified
Duplicates removed | 192 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 182 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 22 |
Cited efficacy/tolerability articles | 10 |
Cited articles not efficacy/tolerability | 16 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were sotorasib, LUMAKRAS, LUMYKRAS, AMG 510 and AMG510. Records were limited to those in English language. Searches last updated 5 Oct 22 | |
Change history
03 December 2022
ESM Update.
02 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11523-022-00939-1
References
Huang L, Guo Z, Wang F, et al. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6(1):386.
Kim D, Xue JY, Lito P. Targeting KRAS(G12C): from inhibitory mechanism to modulation of antitumor effects in patients. Cell. 2020;183(4):850–9.
Finn SP, Addeo A, Dafni U, et al. Prognostic impact of KRAS G12C mutation in patients with NSCLC: results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2021;16(6):990–1002.
Arbour KC, Rizvi H, Plodkowski AJ, et al. Treatment outcomes and clinical characteristics of patients with KRAS-G12C–mutant non-small cell lung cancer. Clin Cancer Res. 2021;27(8):2209–15.
Blair HA. Sotorasib: first approval. Drugs. 2021;81(13):1573–9.
Amgen Inc. LUMAKRAS™ (sotorasib): US prescribing information. 2021. https://dailymed.nlm.nih.gov/. Accessed 5 Oct 2022.
European Medicines Agency. LUMYKRAS™ (sotorasib): EU summary of product characteristics. 2022. https://www.ema.europa.eu/. Accessed 5 Oct 2022.
US Food and Drug Administration. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. 2021. https://www.fda.gov/. Accessed 5 Oct 2022.
Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23.
Zhao Y, Murciano-Goroff YR, Xue JY, et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature. 2021;599(7886):679–83.
Li BT, Velcheti V, Price TJ, et al. Largest evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated non–small cell lung cancer (NSCLC) and colorectal cancer (CRC): plasma biomarker analysis of CodeBreaK100. J Clin Oncol. 2022;40(16 Suppl):102.
Suzuki S, Yonesaka K, Teramura T, et al. KRAS inhibitor resistance in MET-amplified KRAS (G12C) non-small cell lung cancer induced by RAS- and non-RAS-mediated cell signaling mechanisms. Clin Cancer Res. 2021;27(20):5697–707.
Cardona P, Simiens M, Purkis J, et al. An open-label study to evaluate the effect of omeprazole on the pharmacokinetics of sotorasib (AMG 510) in healthy subjects [abstract no. PII-009]. Clin Pharm Ther. 2021;109(Suppl S1):S31.
Cardona P, Spring M, Purkis J, et al. An open-label study to evaluate the effect of single and multiple doses of rifampin on the pharmacokinetics of sotorasib (AMG 510) in healthy subjects [abstract no. PII-1010]. Clin Pharm Therap. 2021;109(Suppl S1):S31.
Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17.
Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–81.
US Center for Drug Evaluation and Research. 214665Orig1s000 (sotorasib) multi-discipline review. 2020. https://www.fda.gov/. Accessed 5 Oct 2022.
European Medicines Agency. LUMYKRAS™ (sotorasib): assessment report. 2022. https://www.ema.europa.eu/. Accessed 5 Oct 2022.
Dy G, Govindan R, Velcheti V, et al. Long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100 [abstract no. CT008]. Cancer Res. 2022;82(12 Suppl):CT008.
Spira A, Wilson FH, Shapiro G, et al. Patient-reported outcomes (PRO) from the phase 2 CodeBreaK 100 trial evaluating sotorasib in KRAS p.G12C mutated non-small cell lung cancer (NSCLC) [abstract no. 9057]. J Clin Oncol. 2021;39(15 Suppl):9057.
Johnson ML, de Langen AJ, Waterhouse DM, et al. Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study [abstract no. LBA10]. Ann Oncol. 2022;33(Suppl 7):S1417–8.
Bauml JM, Li BT, Velcheti V, et al. Clinical validation of Guardant360 CDx as a blood-based companion diagnostic for sotorasib. Lung Cancer. 2021;166:270–8.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): non-small cell lung cancer. 2022. https://www.nccn.org/. Accessed 5 Oct 2022.
National Institute for Health and Care Excellence. Sotorasib for previously treated KRAS G12C mutation-positive advanced non-small-cell lung cancer. 2022. https://www.nice.org.uk/. Accessed 5 Oct 2022.
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192-237.
Planchard D, Popat S, Kerr K, et al. Clinical practice living guidelines: metastatic non-small-cell lung cancer. 2020. https://www.esmo.org/. Accessed 5 Oct 2022.
Acknowledgements
During the peer review process, the manufacturer of sotorasib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Arnold Lee is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: D. Lang, Department of Pulmonology, Johannes Kepler University Hospital Linz, Linz, Austria; A. Russo, Universita degli Studi di Palermo, Palermo, Italy; P.J. Souquet, Centre Hospitalier Lyon Sud, Hospices Civils de Lyon, Lyon, France.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, A. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. Targ Oncol 17, 727–733 (2022). https://doi.org/10.1007/s11523-022-00922-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00922-w