Abstract
Sotorasib (LUMAKRAS™) is a RAS GTPase family inhibitor being developed by Amgen for the treatment of solid tumours with KRAS mutations, including non-small cell lung cancer (NSCLC) and colorectal cancer. In May 2021, sotorasib was granted accelerated approval by the US FDA for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy. This article summarizes the milestones in the development of sotorasib leading to this first approval for KRAS G12C-mutated NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.15078999 |
RAS GTPase family inhibitor being developed by Amgen for the treatment of KRAS G12C-mutated NSCLC |
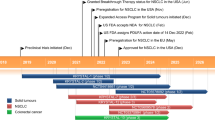
Received its first approval on 28 May 2021 in the USA |
Approved for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy |
1 Introduction
KRAS, a GTPase and member of the RAS family of proteins, is the most frequently mutated oncogene in cancer [1]. The KRAS G12C mutation is present in approximately 13% of patients with lung cancer, 3% of patients with colorectal cancer and 2% of patients with other solid tumours [1, 2]. Unlike other mutant KRAS proteins, KRAS G12C has been shown to cycle between its active GTP-bound and inactive GDP-bound states within cancer cells [2], thereby providing a basis for the development of targeted therapies [2, 3].
Sotorasib (LUMAKRAS™) is a RAS GTPase family inhibitor being developed by Amgen for the treatment of solid tumours with KRAS G12C mutations. Sotorasib was given orphan drug designation by the US FDA in June 2019 for KRAS G12C-positive non-small cell lung cancer (NSCLC) and colorectal cancer [4]. The drug was granted breakthrough therapy designation for advanced or metastatic KRAS G12C-mutated NSCLC in December 2020 [5], and a priority review was granted in February 2021 [6]. On 28 May 2021, sotorasib received its first approval in the USA for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy [7, 8]. This indication was approved under accelerated approval based on overall response rate (ORR) and duration of response (DOR), and its continued approval may be contingent upon verification and description of clinical benefit in confirmatory trial(s) [7]. The recommended dosage of sotorasib is 960 mg taken orally once daily (with or without food) until disease progression or unacceptable toxicity. Dosage modifications may be required because of adverse events (AEs). The recommended dose reduction levels are as follows: first reduction to 480 mg once daily; second reduction to 240 mg once daily. If patients are unable to tolerate a dosage of 240 mg once daily, treatment with sotorasib should be discontinued [7].

Key milestones in the development of sotorasib for the treatment of KRAS G12C-mutated non-small cell lung cancer. MAA Market Authorisation Application, NDA New Drug Application
Phase I/II clinical trials of sotorasib in KRAS G12C-mutated colorectal cancer and other solid tumours are currently underway in multiple countries.
1.1 Company Agreements
In January 2014, Amgen and Carmot Therapeutics entered into a research, development and license agreement [9]. The agreement was extended in February 2016 [10] and December 2017 [11]. Under the terms of the agreement, Carmot Therapeutics will apply its proprietary lead-identification technology, Chemotype Evolution, to discover and develop drug leads for therapeutic targets selected by Amgen [9,10,11].
1.2 Patent Information
Amgen has patent protection for sotorasib in the USA, with an estimated expiration date of 2038 [12].
2 Scientific Summary
2.1 Pharmacodynamics
Sotorasib is a first-in-class KRAS G12C inhibitor. It binds covalently and irreversibly to the cysteine residue of the KRAS G12C mutant [7]. Consequently, the KRAS protein is locked in an inactive state and its downstream signalling effects are blocked, without affecting wild-type KRAS [7].
Sotorasib inhibited SOS1-catalyzed nucleotide exchange of recombinant mutant KRAS G12C/C118A in vitro [13]. Cysteine proteome analysis of cells treated with sotorasib demonstrated that only the G12C-containing peptide of KRAS was covalently modified. Sotorasib inhibited KRAS signalling (as measured by ERK phosphorylation) in all KRAS G12C mutant cell lines, but not in cell lines without the KRAS G12C mutation. Sotorasib also selectively impaired the viability of KRAS G12C mutant lines. Coadministration of sotorasib with inhibitors of other cellular signalling pathways afforded evidence for synergistic effects on cell viability [13].

Chemical structure of sotorasib
In preclinical tumour models, sotorasib bound rapidly and irreversibly to KRAS G12C, thereby providing durable suppression of the mitogen-activated protein kinase (MAPK) signalling pathway [14]. Once daily administration of sotorasib led to tumour regression in mouse models of KRAS G12C cancer [14]. Sotorasib was also associated with prolonged survival and anti-tumour immunity in KRAS G12C models [7].
The recommended dosage of sotorasib (i.e. 960 mg once daily) was not associated with large mean increases (i.e. > 20 ms) in the corrected QT interval [7].
2.2 Pharmacokinetics
Sotorasib demonstrates non-linear, time-dependent pharmacokinetics over a dose range of 180–960 mg once daily, with similar systemic exposure [i.e. area under the concentration-time curve from zero to 24 h (AUC0–24h) and maximum plasma concentration (Cmax)] across doses at steady state [7]. Steady-state concentrations of sotorasib are achieved within 22 days, with no appreciable accumulation following repeated administration (mean accumulation ratio of 0.56). The median time to reach Cmax of sotorasib is 1 h. Compared with fasting conditions, administration of a single dose of sotorasib 960 mg with a high-fat, high-calorie meal increased sotorasib AUC0–24h by 25%. Sotorasib is 89% bound to plasma proteins and has a mean volume of distribution at steady state of 211 L [7].
Sotorasib is largely metabolized by non-enzymatic conjugation and oxidative metabolism with CYP3As [7]. Following administration of a single radiolabeled dose of sotorasib, 74% of the dose was recovered in faeces (53% as unchanged drug) and 6% in urine (1% as unchanged drug). The mean apparent clearance of sotorasib at steady state is 26.2 L/h and the mean terminal elimination half-life is 5 h [7].
Age (28–86 years), race (white, black or Asian), sex, body weight (36.8–157.9 kg), line of treatment, Eastern Cooperative Oncology Group performance status (ECOG PS; 0 or 1), mildly or moderately abnormal kidney function (estimated glomerular filtration rate ≥ 30 mL/min/1.73 m2) and mild hepatic impairment [alanine aminotransferase (ALT) or aspartate aminotransferase (AST) < 2.5 × upper limit of normal (ULN) or total bilirubin < 1.5 × ULN] did not affect the pharmacokinetics of sotorasib to a clinically significant extent [7]. The effect of severely abnormal kidney function or moderate to severe hepatic impairment on the pharmacokinetics of sotorasib is not known [7].
Coadministration of sotorasib with acid-reducing agents (e.g. omeprazole [15], famotidine [7]) or repeated doses of rifampicin (a strong CYP3A4 inducer) [16] decreased sotorasib Cmax and AUC. Coadministration of sotorasib with itraconazole [a combined strong CYP3A4 and P-glycoprotein (P-gp) inhibitor] [7], metformin (a MATE1/2K substrate) [17] or a single dose of rifampicin (an OATP1B1/1B3 inhibitor) [7, 16] did not affect the pharmacokinetics of sotorasib to a clinically significant extent. Sotorasib had no clinically relevant effect on the Cmax and AUC of metformin [17], but decreased the Cmax and AUC of the sensitive CYP3A4 substrate midazolam [7] and increased the Cmax and AUC of the P-gp substrate digoxin [18]. In vitro, sotorasib may induce CYP2B6, CYP2C8 and CYP2C9 and may inhibit BCRP, but it does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP2D6 [7].
Features and properties of sotorasib
Alternative names | AMG-510; LUMAKRAS™ |
Class | Anti-neoplastics, piperazines, pyridines, pyridones, pyrimidines, small molecules |
Mechanism of action | KRAS protein inhibitors |
Route of administration | Oral |
Pharmacodynamics | Forms an irreversible, covalent bond with the cysteine residue of the KRAS G12C mutant, thereby locking the protein in an inactive state and blocking its downstream signalling effects |
Pharmacokinetics | Non-linear, time-dependent pharmacokinetics over dose range of 180–960 mg once daily; median time to Cmax 1 h; mean Vd 211 L; mean apparent clearance 26.2 L/h; mean t½ 5 h |
Most frequent adverse events | |
Any grade | Decreased lymphocytes, decreased haemoglobin, diarrhoea, increased AST, increased ALT, musculoskeletal pain, decreased calcium, increased ALP, nausea, fatigue, hepatotoxicity, cough |
Grade 3 or 4 | Hepatotoxicity, increased ALT, increased AST, musculoskeletal pain, pneumonia, diarrhoea |
ATC codes | |
WHO ATC code | L01 (Anti-neoplastic agents) |
EphMRA ATC code | L1 (Anti-neoplastics) |
Chemical name | 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2-methyl-4-(prop-2enoyl)piperazin-1-yl]pyrido[2,3-d]pyrimidin-2(1H)-one |
2.3 Therapeutic Trials
2.3.1 Non-Small Cell Lung Cancer
2.3.1.1 Phase II (Monotherapy)
Sotorasib was associated with deep and durable responses in patients with locally advanced or metastatic KRAS G12C-mutated NSCLC in the registrational phase II portion of the ongoing, multicentre, phase I/II CodeBreaK 100 trial (NCT03600883) [19]. All patients had progressed on anti-programmed cell death protein 1 (PD-1) or anti-programmed death-ligand 1 (PD-L1) immunotherapy and/or platinum-based chemotherapy (and targeted therapy if EGFR, ALK and ROS1 alterations were identified) and had received ≤ 3 prior lines of therapy. A total of 126 patients (median age 63.5 years) received oral sotorasib 960 mg once daily until disease progression. The primary endpoint was confirmed objective response rate (ORR), assessed by blinded independent central review per RECIST 1.1 criteria [19].
At a median follow-up of 12.2 months (data cut-off 1 December 2020), the ORR was 37% [19]. The disease control rate (DCR; defined as objective response or stable disease) was 81%. The median time to response (TTR) was 1.4 months and the median DOR was 10.0 months. Median progression-free survival (PFS) was 6.8 months. At data cut-off, 43% of responders remained on treatment without disease progression [19]. Sotorasib was also associated with improvements in patient-reported outcomes, including global health status, quality of life, physical functioning and the severity of key lung cancer symptoms (e.g. cough, chest pain, dyspnoea) [20].
In exploratory analyses, the tumour response to sotorasib was seen across a range of biomarker subgroups, including patients with negative or low PD-L1 expression level and those with mutated STK11 [19]. The clinical benefit of sotorasib was also seen regardless of age (< 65 vs ≥ 65 years), ECOG PS (0 vs 1), metastatic disease (yes vs no), prior lines of therapy (1 vs ≥ 2), prior anti-PD-1/PD-L1 therapy (yes vs no), TP53 co-mutation (wild-type vs mutant), STK11 co-mutation (wild-type vs mutant), KEAP1 co-mutation (wild-type vs mutant) and tumour mutational burden (low vs high) [21].
2.3.1.2 Phase I (Dose Escalation and Expansion)
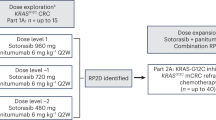
Sotorasib demonstrated anti-cancer activity in patients with KRAS G12C-mutated NSCLC participating in the earlier phase I portion of CodeBreaK 100 [22]. Eligible patients (aged ≥ 18 years) had histologically confirmed, locally advanced or metastatic NSCLC with the KRAS G12C mutation and had received previous platinum-based combination therapy, targeted therapies, or both. During the dose-escalation phase, patients received oral sotorasib 180, 360, 720 or 960 mg once daily in 21-day cycles until disease progression, unacceptable toxicity, withdrawal of consent or study end. A total of 59 patients with NSCLC were enrolled; of these, 90% had received previous anti-PD-1/PD-L1 therapies and 100% had received previous platinum-based chemotherapy [22].
At a median follow-up of 11.7 months (data cut-off 1 June 2020), the ORR was 32% (all partial responses) and the DCR was 88% [22]. Responses were seen across all dose levels. Among those in the 960 mg/day cohort (n = 34), the ORR was 35% and the DCR was 91%. Tumour shrinkage of any magnitude was seen in 71% of patients after 6 weeks. The median TTR was 1.4 months and the median DOR was 10.9 months. Median PFS was 6.3 months [22].
2.3.2 Colorectal Cancer
Sotorasib demonstrated clinical activity in patients with colorectal cancer participating in the phase I portion of CodeBreaK 100 (n = 42) [22]. All patients had received at least two previous lines of systemic therapy for metastatic colorectal cancer. At a median follow-up of 12.8 months (data cut-off 1 June 2020), 7% of patients had an ORR (all partial responses) and 74% of patients had disease control. The median duration of stable disease was 5.4 months. In the cohort receiving sotorasib 960 mg/day (n = 25), the ORR was 12% and the DCR was 80%. Median PFS was 4.0 months [22].
2.3.3 Other Solid Tumours
In patients with other solid tumours participating in the phase I portion of CodeBreaK 100 (n = 28), sotorasib was associated with an ORR of 14% and a DCR of 75% [22]. Partial responses were seen in patients with pancreatic cancer, endometrial cancer, appendiceal cancer and melanoma (all n = 1). Five patients remained on treatment at the time of data cut-off [22].
Key clinical trials of sotorasib (Amgen)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Sotorasib, docetaxel | NSCLC | III | Ongoing | Multinational | CodeBreaK 200; NCT04303780; EudraCT2019-003582-18 |
Sotorasib | NSCLC | N/Aa | Recruiting | Multinational | NCT04667234 |
Sotorasib | NSCLC | II | Not yet recruiting | Unknown | NCT04625647; NCI-2020-08103; S1900E, U10CA180888 |
Sotorasib, PD-1/PD-L1 inhibitors | NSCLC, colorectal cancer, solid tumours | I/II | Recruiting | Multinational | CodeBreaK 100; NCT03600883; EudraCT2018-001400-11 |
Sotorasib, anti-cancer therapies | NSCLC, colorectal cancer, solid tumours | Ib | Recruiting | Japan, USA | CodeBreaK 101; NCT04185883 |
Sotorasib | Solid tumours | I | Recruiting | Hong Kong, Taiwan | CodeBreaK 105; NCT04380753 |
Sotorasib | NSCLC, solid tumours | I | Recruiting | USA | NCT04887064 |
2.4 Adverse Events
Sotorasib 960 mg once daily had a manageable tolerability profile in the subset of 204 patients with KRAS G12C-mutated NSCLC enrolled in CodeBreaK 100 [7]. The most common (incidence ≥ 20%) AEs in patients receiving sotorasib were diarrhoea (42%), musculoskeletal pain (35%), nausea (26%), fatigue (26%), hepatotoxicity (25%) and cough (20%). The most common (incidence ≥ 30%) laboratory abnormalities were decreased lymphocytes (48%), decreased haemoglobin (43%), increased AST (39%), increased ALT (38%), decreased calcium (35%) and increased alkaline phosphatase (33%). The most common (incidence ≥ 5%) grade 3 or 4 AEs, including laboratory abnormalities, were hepatotoxicity (12%), increased ALT (11%), increased AST (9%), musculoskeletal pain (8%), pneumonia (7%) and diarrhoea (5%) [7].
Serious AEs occurred in 50% of patients receiving sotorasib, with pneumonia (8%), hepatotoxicity (3%) and diarrhoea (2%) reported most frequently (incidence ≥ 2%) [7]. Fatal AEs occurred in 3% of sotorasib recipients (respiratory failure, pneumonitis, cardiac arrest, cardiac failure, gastric ulcer and pneumonia). AEs led to permanent discontinuation of sotorasib in 9% of patients, with hepatotoxicity (5%) being the most common (incidence ≥ 2%) reason for discontinuing treatment. Sotorasib dose reductions because of AEs were required in 5% of patients and dosage interruptions because of AEs in 34% of patients. Dose reductions were most commonly (incidence > 2%) for increased ALT (3%) and increased AST (3%), and dosage interruptions were required most frequently (incidence ≥ 2%) for hepatotoxicity (11%), diarrhoea (8%), musculoskeletal pain (4%), nausea (3%) and pneumonia (3%) [7]. Most patients reported that they were ‘not at all’ (54–79%) or ‘a little bit’ (8–33%) bothered by sotorasib side effects on the GP5 item of the Functional Assessment of Cancer Therapy-General questionnaire [20].
The US prescribing information contains a warning stating that sotorasib may cause hepatotoxicity and potentially fatal interstitial lung disease (ILD)/pneumonitis [7]. In the pooled safety population of 357 patients with NSCLC and other solid tumours with KRAS G12C mutation enrolled in CodeBreaK 100, the incidence of grade 3 hepatotoxicity was 1.4%. Grade 3 and 4 ALT/AST elevations occurred in 6% and 0.6% of patients. The median time to first onset of increased ALT/AST was 9 weeks. Grade 3 or 4 ILD/pneumonitis occurred in 0.8% of patients; one case was fatal. The median time to first onset of ILD/pneumonitis was 2 weeks [7].
2.5 Companion Diagnostic
The US FDA has approved Guardant360® CDx, a blood-based liquid biopsy companion diagnostic developed by Guardant Health, for the identification of the KRAS G12C mutation in patients with NSCLC [23]. The therascreen® KRAS RGQ PCR Kit developed by QIAGEN has also been approved by the US FDA as a tissue-based companion diagnostic to identify the KRAS G12C mutation in NSCLC tumours [24].
2.6 Ongoing Clinical Trials
In addition to the ongoing phase I/II CodeBreaK 100 trial described above (NCT03600883), the randomized, open-label, multicentre, phase III CodeBreaK 200 trial (NCT04303780) is underway. CodeBreaK 200 will evaluate the efficacy and tolerability of sotorasib versus docetaxel in patients with previously treated advanced NSCLC harbouring the KRAS G12C mutation [25]. A phase II lung cancer master protocol (Lung MAP) trial (NCT04625647) plans to evaluate the efficacy and tolerability of sotorasib in patients with stage IV or recurrent KRAS G12C-mutated non-squamous NSCLC.
The open-label, multicentre, phase Ib CodeBreaK 101 trial (NCT04185883) is currently recruiting patients. The trial will evaluate the safety, tolerability and efficacy of sotorasib alone and in combination with other anti-cancer therapies in patients with advanced KRAS G12C-mutated solid tumours, including NSCLC and colorectal cancer [26]. Patients are also being recruited in CodeBreaK 105 (NCT04380753), an open-label, multicentre, phase I ethnic sensitivity study which will evaluate the safety, tolerability, pharmacokinetics and efficacy of sotorasib in patients of Chinese descent with KRAS G12C-mutated advanced/metastatic solid tumours.
Furthermore, an expanded access programme (NCT04667234) will provide treatment access to sotorasib and assess its safety in patients with previously treated locally advanced/unresectable/metastatic KRAS G12C-mutated NSCLC who are ineligible to participate in any ongoing sotorasib clinical trial.
3 Current Status
Sotorasib received its first approval on 28 May 2021 in the USA for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy [8].
Change history
23 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40265-021-01630-x
References
Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23.
Kim D, Xue JY, Lito P. Targeting KRAS(G12C): from inhibitory mechanism to modulation of antitumor effects in patients. Cell. 2020;183(4):850–9.
Singh RR, O’Reilly EM. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs. 2020;80(7):647–69.
Amgen. Amgen announces first clinical data evaluating novel investigational KRASG12C inhibitor AMG 510 at ASCO 2019 [media release]. 3 Jun 2019. https://www.amgen.com/newsroom/press-releases/2019/06/amgen-announces-first-clinical-data-evaluating-novel-investigational-krasg12c-inhibitor-amg-510-at-asco-2019.
Amgen. Amgen's sotorasib granted breakthrough therapy designation for advanced or metastatic non-small cell lung cancer patients with KRAS G12C mutation [media release]. 8 Dec 2020. https://www.amgen.com/newsroom/press-releases/2020/12/amgens-sotorasib-granted-breakthrough-therapy-designation-for-advanced-or-metastatic-nonsmall-cell-lung-cancer-patients-with-kras-g12c-mutation.
Amgen. FDA grants sotorasib priority review designation for the treatment of patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer [media release]. 16 Feb 2021. https://www.amgen.com/newsroom/press-releases/2021/02/fda-grants-sotorasib-priority-review-designation-for-the-treatment-of-patients-with-kras-g12c-mutated-locally-advanced-or-metastatic-non-small-cell-lung-cancer.
Amgen Inc. LUMAKRAS™ (sotorasib) tablets, for oral use: US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214665s000lbl.pdf. Accessed 5 July 2021.
US Food & Drug Administration. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC [media release]. 28 May 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc.
Carmot Therapeutics. Carmot Therapeutics enters into drug discovery collaboration with Amgen [media release]. 14 Jan 2014. https://www.businesswire.com/news/home/20140114005428/en/Carmot-Therapeutics-Enters-into-Drug-Discovery-Collaboration-with-Amgen.
Carmot Therapeutics. Carmot Therapeutics extends drug discovery collaboration with Amgen [media release]. 16 Feb 2016. https://carmot-therapeutics.us/carmot-therapeutics-extends-drug-discovery-collaboration-with-amgen/.
Carmot Therapeutics. Carmot enters a multi-year drug discovery collaboration with Amgen [media release]. 6 Dec 2017. https://carmot-therapeutics.us/carmot-enters-a-multi-year-drug-discovery-collaboration-with-amgen-collaboration-aims-to-identify-novel-molecules-intended-for-parkinsons-disease/.
Amgen. Amgen letter to shareholders. 2020. https://investors.amgen.com/static-files/c1b90d85-8945-4d11-8d1c-5ddc343fb699. Accessed 5 July 2021.
Saiki AY, Gaida K, Rex K, et al. Discovery and in vitro characterization of AMG 510—a potent and selective covalent small-molecule inhibitor of KRASG12C [abstract no. 4484]. Cancer Res. 2019;79(13 Suppl.).
Lanman BA, Chen JJ, Liu L, et al. Discovery of AMG 510, a first-in-human covalent inhibitor of KRASG12C for the treatment of solid tumors [abstract no. 4455]. Cancer Res. 2019;79(13 Suppl.).
Cardona P, Simiens M, Purkis J, et al. An open-label study to evaluate the effect of omeprazole on the pharmacokinetics of sotorasib (AMG 510) in healthy subjects [abstract no. PII-009]. Clin Pharmacol Ther. 2021;109(Suppl. 1):S31.
Cardona P, Spring M, Purkis J, et al. An open-label study to evaluate the effect of single and multiple doses of rifampin on the pharmacokinetics of sotorasib (AMG 510) in healthy subjects [abstract no. PII-010]. Clin Pharmacol Ther. 2021;109(Suppl. 1):S31.
Vuu I, Simiens M, Purkis J, et al. A phase I, open-label study to evaluate drug-drug interactions between metformin and sotorasib (AMG 510) in healthy subjects [abstract no. PIII-018]. Clin Pharmacol Ther. 2021;109(Suppl. 1):S50.
Cardona P, Spring M, Purkis J, et al. An open-label study to evaluate the effect of sotorasib (AMG 510) on digoxin pharmacokinetics in healthy subjects [abstract no. PII-011]. Clin Pharmacol Ther. 2021;109(Suppl. 1):S31.
Li B, Skoulidis F, Falchook G, et al. Registrational phase 2 trial of sotorasib in KRAS p.G12C mutant NSCLC: first disclosure of the Codebreak 100 primary analysis [abstract no. PS01.07 + oral presentation]. J Thorac Oncol. 2021;16(3 Suppl.):S61.
Spira A, Wilson FH, Shapiro G, et al. Patient-reported outcomes (PRO) from the phase 2 CodeBreaK 100 trial evaluating sotorasib in KRAS p.G12C mutated non-small cell lung cancer (NSCLC) [abstract no. 9057]. J Clin Oncol. 2021;39(15 Suppl.):474s.
Skoulidis F, Li BT, Govindan R, et al. Overall survival and exploratory subgroup analyses from the phase 2 CodeBreaK 100 trial evaluating sotorasib in pretreated KRAS p.G12C mutated non-small cell lung cancer [abstract no. 9003]. J Clin Oncol. 2021;39(15 Suppl.):460s.
Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17.
Guardant Health. Guardant360® CDx receives FDA approval as first and only liquid biopsy companion diagnostic for Amgen's LUMAKRAS™ (sotorasib) KRASG12C inhibitor for use in advanced non-small cell lung cancer [media release]. 28 May 2021. https://investors.guardanthealth.com/press-releases/press-releases/2021/Guardant360-CDx-Receives-FDA-Approval-as-First-and-Only-Liquid-Biopsy-Companion-Diagnostic-for-Amgens-LUMAKRAS-sotorasib-KRASG12C-Inhibitor-for-Use-in-Advanced-Non-Small-Cell-Lung-Cancer/default.aspx.
QIAGEN. QIAGEN launches first FDA-approved tissue companion diagnostic to identify the KRAS G12C mutation in NSCLC tumours and expand precision medicine options in lung cancer [media release]. 28 May 2021. https://corporate.qiagen.com/newsroom/press-releases/press-release-details/2021/QIAGEN-Launches-First-FDA-approved-Tissue-Companion-Diagnostic-to-Identify-the-KRAS-G12C-Mutation-in-NSCLC-Tumours-and-Expand-Precision-Medicine-Options-in-Lung-Cancer/default.aspx.
Reck M, Spira A, Besse B, et al. CodeBreak 200: a phase III multicenter study of sotorasib (AMG 510), a KRAS(G12C) inhibitor, versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC) harboring KRAS p.G12C mutation [abstract no. 1416TiP]. Ann Oncol. 2020;31(Suppl. 4):S894–5.
Hong DS, Strickler J, Fakih M, et al. Trial in progress: a phase 1b study of sotorasib, a specific and irreversible KRASG12C inhibitor, as monotherapy in non-small cell lung cancer (NSCLC) with brain metastasis and in combination with other anticancer therapies in advanced solid tumors (CodeBreaK 101) [abstract no. TPS2669]. J Clin Oncol. 2021;39(15 Suppl.):145s.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Hannah Blair is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Blair, H.A. Sotorasib: First Approval. Drugs 81, 1573–1579 (2021). https://doi.org/10.1007/s40265-021-01574-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01574-2