Abstract
Background
Treatment options for patients with chemotherapy-refractory solid tumors are limited.
Objective
We conducted an open-label, single-arm, single-center phase II trial to evaluate the efficacy and safety of gefitinib in patients with chemotherapy-refractory solid tumors and EGFR amplification or sensitivity to an EGFR inhibitor identified through a drug-screening platform with patient-derived tumor cells (PDCs).
Patients and methods
EGFR amplification was detected by targeted sequencing. Sensitivity to an EGFR inhibitor was established in chemical screening using PDCs. Gefitinib (250 mg daily) was administered continuously in 28-day cycles until the occurrence of disease progression, unacceptable toxicity, or death due to any cause. The primary endpoint was the objective response rate (ORR).
Results
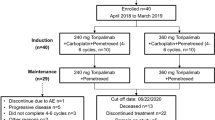
In total, 15 patients were assigned to the present study. The most common tumor type was glioblastoma multiforme (n = 9, 60%), followed by gastric cancer (n = 3, 20%), anal squamous cancer, rectal cancer, and sarcoma (each n = 1, 6.7%). Among 13 evaluable patients, one patient had a partial response and five had stable disease, with an ORR of 7.7% and a disease control rate of 46.1%. The median progression-free survival was 2.1 months (95% confidence interval [CI] 0.77–3.43). The most common adverse events were diarrhea (26.7%) and skin rash (26.7%).
Conclusion
Gefitinib demonstrated modest anti-tumor activity and a manageable safety profile in chemotherapy-refractory solid tumors with EGFR amplification or sensitivity to an EGFR inhibitor identified through a drug-screening platform with PDCs.
ClinicalTrials.gov identifier
NCT02447419.


Similar content being viewed by others
References
Mitchell RA, Luwor RB, Burgess AW. Epidermal growth factor receptor: Structure-function informing the design of anticancer therapeutics. Exp Cell Res. 2018;371(1):1–19.
Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7(10):2958–70.
Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGF-related peptides in tumor growth. Front Biosci. 2001;6:D685–707.
Sawyers CL. Opportunities and challenges in the development of kinase inhibitor therapy for cancer. Genes Dev. 2003;17(24):2998–3010.
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–63.
Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303.
Lassman AB, Aldape KD, Ansell PJ, Bain E, Curran WJ, Eoli M, et al. Epidermal growth factor receptor (EGFR) amplification rates observed in screening patients for randomized trials in glioblastoma. J Neurooncol. 2019;144(1):205–10.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46.
Cappuzzo F, Finocchiaro G, Grossi F, Bidoli P, Favaretto A, Marchetti A, et al. Phase II study of afatinib, an irreversible ErbB family blocker, in EGFR FISH-positive non-small-cell lung cancer. J Thorac Oncol. 2015;10(4):665–72.
Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23(28):6838–45.
Wang F, Fu S, Shao Q, Zhou YB, Zhang X, Zhang X, et al. High EGFR copy number predicts benefits from tyrosine kinase inhibitor treatment for non-small cell lung cancer patients with wild-type EGFR. J Transl Med. 2013;11:90.
Zhu CQ, da Cunha SG, Ding K, Sakurada A, Cutz JC, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26(26):4268–75.
Ruiz-Patino A, Castro CD, Ricaurte LM, Cardona AF, Rojas L, Zatarain-Barron ZL, et al. EGFR amplification and sensitizing mutations correlate with survival in lung adenocarcinoma patients treated with Erlotinib (MutP-CLICaP). Target Oncol. 2018;13(5):621–9.
Fiala O, Pesek M, Finek J, Minarik M, Benesova L, Sorejs O, et al. Epidermal growth factor receptor gene amplification in patients with advanced-stage NSCLC. Anticancer Res. 2016;36(1):455–60.
Sasaki H, Endo K, Okuda K, Kawano O, Kitahara N, Tanaka H, et al. Epidermal growth factor receptor gene amplification and gefitinib sensitivity in patients with recurrent lung cancer. J Cancer Res Clin Oncol. 2008;134(5):569–77.
Sone T, Kasahara K, Kimura H, Nishio K, Mizuguchi M, Nakatsumi Y, et al. Comparative analysis of epidermal growth factor receptor mutations and gene amplification as predictors of gefitinib efficacy in Japanese patients with nonsmall cell lung cancer. Cancer. 2007;109(9):1836–44.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, Brown University, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–75.
Kodack DP, Farago AF, Dastur A, Held MA, Dardaei L, Friboulet L, et al. Primary patient-derived cancer cells and their potential for personalized cancer patient care. Cell Rep. 2017;21(11):3298–309.
Ehrenberg KR, Gao J, Oppel F, Frank S, Kang N, Dieter SM, et al. Systematic generation of patient-derived tumor models in pancreatic cancer. Cells. 2019;8(2):142.
Lee JK, Liu Z, Sa JK, Shin S, Wang J, Bordyuh M, et al. Pharmacogenomic landscape of patient-derived tumor cells informs precision oncology therapy. Nat Genet. 2018;50(10):1399–411.
Franceschi E, Cavallo G, Lonardi S, Magrini E, Tosoni A, Grosso D, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96(7):1047–51.
Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133–42.
de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90(1):89–97.
Kreisl TN, Lassman AB, Mischel PS, Rosen N, Scher HI, Teruya-Feldstein J, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92(1):99–105.
Sepulveda-Sanchez JM, Vaz MA, Balana C, Gil-Gil M, Reynes G, Gallego O, et al. Phase II trial of dacomitinib, a pan-human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol. 2017;19(11):1522–31.
Maron SB, Alpert L, Kwak HA, Lomnicki S, Chase L, Xu D, et al. Targeted therapies for targeted populations: anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(6):696–713.
Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6(5):279–86.
Khan SA, Zeng Z, Shia J, Paty PB. EGFR Gene amplification and KRAS mutation predict response to combination targeted therapy in metastatic colorectal cancer. Pathol Oncol Res. 2017;23(3):673–7.
Shen WD, Chen HL, Liu PF. EGFR gene copy number as a predictive biomarker for resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer treatment: a meta-analysis. Chin J Cancer Res. 2014;26(1):59–71.
Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(1):37–48.
Ahn S, Kim HJ, Kang E, Kim EK, Kim SH, Kim JH, et al. Genomic profiling of multiple breast cancer reveals inter-lesional heterogeneity. Br J Cancer. 2020. https://doi.org/10.1038/s41416-019-0713-1.
Lin DC, Mayakonda A, Dinh HQ, Huang P, Lin L, Liu X, et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Res. 2017;77(9):2255–65.
Hiley C, de Bruin EC, McGranahan N, Swanton C. Deciphering intratumor heterogeneity and temporal acquisition of driver events to refine precision medicine. Genome Biol. 2014;15(8):453.
Kato S, Okamura R, Mareboina M, Lee S, Goodman A, Patel SP, et al. Revisiting epidermal growth factor receptor (EGFR) amplification as a target for anti-EGFR therapy: analysis of cell-free circulating tumor DNA in patients with advanced malignancies. JCO Precis. Oncol. 2019. https://doi.org/10.1200/PO.18.00180.
Jiang J, Greulich H, Janne PA, Sellers WR, Meyerson M, Griffin JD. Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res. 2005;65(19):8968–74.
Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405.
Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schutz M, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10(1):156–63.
Kwatra MM. A rational approach to target the epidermal growth factor receptor in glioblastoma. Curr Cancer Drug Targets. 2017;17(3):290–6.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43.
VanderLaan PA, Rangachari D, Mockus SM, Spotlow V, Reddi HV, Malcolm J, et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: correlation with clinical outcomes. Lung Cancer. 2017;106:17–211.
Bria E, Pilotto S, Amato E, Fassan M, Novello S, Peretti U, et al. Molecular heterogeneity assessment by next-generation sequencing and response to gefitinib of EGFR mutant advanced lung adenocarcinoma. Oncotarget. 2015;6(14):12783–95.
Hou H, Qin K, Liang Y, Zhang C, Liu D, Jiang H, et al. Concurrent TP53 mutations predict poor outcomes of EGFR-TKI treatments in Chinese patients with advanced NSCLC. Cancer Manag Res. 2019;11:5665–75.
Deng LL, Gao G, Deng HB, Wang F, Wang ZH, Yang Y. Co-occurring genetic alterations predict distant metastasis and poor efficacy of first-line EGFR-TKIs in EGFR-mutant NSCLC. J Cancer Res Clin Oncol. 2019;145(10):2613–24.
Algars A, Avoranta T, Osterlund P, Lintunen M, Sundstrom J, Jokilehto T, et al. Heterogeneous EGFR gene copy number increase is common in colorectal cancer and defines response to anti-EGFR therapy. PLoS ONE. 2014;9(6):e99590.
French PJ, Eoli M, Sepulveda JM, de Heer I, Kros JM, Walenkamp A, et al. Defining EGFR amplification status for clinical trial inclusion. Neuro Oncol. 2019;21(10):1263–72.
Yang ZY, Shen WX, Hu XF, Zheng DY, Wu XY, Huang YF, et al. EGFR gene copy number as a predictive biomarker for the treatment of metastatic colorectal cancer with anti-EGFR monoclonal antibodies: a meta-analysis. J Hematol Oncol. 2012;5:52.
Suzuki S, Dobashi Y, Sakurai H, Nishikawa K, Hanawa M, Ooi A. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas. An immunohistochemical and fluorescence in situ hybridization study. Cancer. 2005;103(6):1265–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of Interest
Seonggyu Byeon, Jung Yong Hong, Jeeyun Lee, Do-Hyun Nam, Se Hoon Park, Joon Oh Park, Young Suk Park, Ho Yeong Lim, Won Ki Kang, and Seung Tae Kim declare that they have no conflicts of interest that might be relevant to the contents of this article.
Rights and permissions
About this article
Cite this article
Byeon, S., Hong, J.Y., Lee, J. et al. Use of Gefitinib in EGFR-Amplified Refractory Solid Tumors: An Open-Label, Single-Arm, Single-Center Prospective Pilot Study. Targ Oncol 15, 185–192 (2020). https://doi.org/10.1007/s11523-020-00706-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00706-0