Abstract
Background
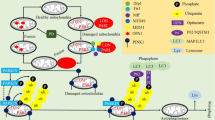
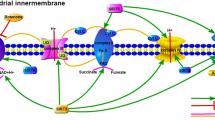
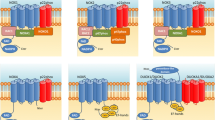
The prevalence of neurodegenerative disorders such as Parkinson’s disease (PD) is increased by age. Alleviation of their symptoms and protection of normal neurons against degeneration are the main aspects of the researches to establish novel therapeutic strategies. Many studies have shown that mitochondria as the most important organelles in the brain which show impairment in PD models. Succinate dehydrogenase (SDH) as a component of the oxidative phosphorylation system in mitochondria connects Krebs cycle to the electron transport chain. Dysfunction or inhibition of the SDH can trigger mitochondrial impairment and disruption in ATP generation. Excessive in lipid synthesis and induction of the excitotoxicity as inducers in PD are controlled by SDH activity directly and indirectly. On the other hand, mutation in subunits of the SDH correlates with the onset of neurodegenerative disorders. Therefore, SDH could behave as one of the main regulators in neuroprotection.
Objective
In this review we will consider contribution of the SDH and its related mechanisms in PD.
Methods
Pubmed search engine was used to find published studies from 1977 to 2016. “Succinate dehydrogenase”, “lipid and brain”, “mitochondria and Parkinson’s disease” were the main keywords for searching in the engine.
Results
Wide ranges of studies (59 articles) in neurodegenerative disorders especially Parkinson’s disease like genetics of the Parkinson’s disease, effects of the mutant SDH on cell activity and physiology and lipid alteration in neurodegenerative disorders have been used in this review.
Conclusion
Mitochondria as key organelles in the energy generation plays crucial roles in PD. ETC complex in this organelle consists four complexes which alteration in their activities cause ROS generation and ATP depletion. Most of complexes are encoded by mtDNA while complex II is the only part of the ETC which is encoded by nuclear genome. So, focusing on the SDH and related pathways which have important role in neuronal survival and SDH has a potential to further studies as a novel neuroprotective agent.
Similar content being viewed by others
References
Ali S F, David S N, Newport G D, Cadet J L, Slikker W Jr (1994). MPTPinduced oxidative stress and neurotoxicity are age-dependent: evidence from measures of reactive oxygen species and striatal dopamine levels. Synapse, 18(1): 27–34
Beal M F, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman B T (1993). Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem, 61(3): 1147–1150
Berliocchi L, Bano D, Nicotera P (2005). Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond B Biol Sci, 360(1464): 2255–2258
Bonifati V (2007). Genetics of parkinsonism. Parkinsonism Relat Disord, 13(Suppl 3): S233–S241
Cecchini G (2003). Function and structure of complex II of the respiratory chain. Annu Rev Biochem, 72(1): 77–109
Chen H, Zhang S M, Hernán M A, Willett W C, Ascherio A (2003). Dietary intakes of fat and risk of Parkinson’s disease. Am J Epidemiol, 157(11): 1007–1014
Cole N B, Murphy D D, Grider T, Rueter S, Brasaemle D, Nussbaum R L (2002). Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem, 277(8): 6344–6352
Davis R E, Williams M (2012). Mitochondrial function and dysfunction: an update. J Pharmacol Exp Ther, 342(3): 598–607
de Lau L M, Breteler M M (2006). Epidemiology of Parkinson’s disease. Lancet Neurol, 5(6): 525–535
de Rijk MC, Breteler MM, Graveland G A, Ott A, Grobbee D E, van der Meché F G, Hofman A (1995). Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology, 45(12): 2143–2146
Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F (2004). SREBP transcription factors: master regulators of lipid homeostasis. Biochimie, 86(11): 839–848
Etschmaier K, Becker T, Eichmann T O, Schweinzer C, Scholler M, Tam-Amersdorfer C, Poeckl M, Schuligoi R, Kober A, Chirackal Manavalan A P, Rechberger G N, Streith I E, Zechner R, Zimmermann R, Panzenboeck U (2011). Adipose triglyceride lipase affects triacylglycerol metabolism at brain barriers. J Neurochem, 119(5): 1016–1028
Exner N, Lutz A K, Haass C, Winklhofer K F (2012). Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J, 31(14): 3038–3062
Fahy E, Subramaniam S, Brown H A, Glass C K, Merrill A H Jr, Murphy R C, Raetz C R, Russell D W, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze M S, White S H, Witztum J L, Dennis E A (2005). A comprehensive classification system for lipids. J Lipid Res, 46(5): 839–861
Fernández A, Llacuna L, Fernández-Checa J C, Colell A (2009). Mitochondrial cholesterol loading exacerbates amyloid beta peptideinduced inflammation and neurotoxicity. J Neurosci, 29(20): 6394–6405
Fernandez-Gomez F J, Galindo M F, Gómez-Lázaro M, Yuste V J, Comella J X, Aguirre N, Jordán J (2005). Malonate induces cell death via mitochondrial potential collapse and delayed swelling through an ROS-dependent pathway. Br J Pharmacol, 144(4): 528–537
Fujimoto T, Parton R G (2011). Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol, 3(3): 3
Gitler A D, Bevis B J, Shorter J, Strathearn K E, Hamamichi S, Su L J, Caldwell K A, Caldwell G A, Rochet J C, McCaffery J M, Barlowe C, Lindquist S (2008). The Parkinson’s disease protein alphasynuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA, 105(1): 145–150
Guo L, Shestov A A, Worth A J, Nath K, Nelson D S, Leeper D B, Glickson J D, Blair I A (2016). Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J Biol Chem, 291(1): 42–57
Gutman M, Kearney E B, Singer T P (1971). Control of succinate dehydrogenase in mitochondria. Biochemistry, 10(25): 4763–4770
Hallett P J, Standaert D G (2004). Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther, 102(2): 155–174
Hanagasi H A, Ayribas D, Baysal K, Emre M (2005). Mitochondrial complex I, II/III, and IV activities in familial and sporadic Parkinson’s disease. Int J Neurosci, 115(4): 479–493
Hattori N, Tanaka M, Ozawa T, Mizuno Y (1991). Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Ann Neurol, 30(4): 563–571
Horton J D, Goldstein J L, Brown MS (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest, 109(9): 1125–1131
Ishii T, Miyazawa M, Onodera A, Yasuda K, Kawabe N, Kirinashizawa M, Yoshimura S, Maruyama N, Hartman P S, Ishii N (2011). Mitochondrial reactive oxygen species generation by the SDHC V69E mutation causes low birth weight and neonatal growth retardation. Mitochondrion. 11(1): 155–165
Ivatt R M, Whitworth A J (2014). SREBF1 links lipogenesis to mitophagy and sporadic Parkinson disease. Autophagy, 10(8): 1476–1477
Jenner P (2003). Oxidative stress in Parkinson’s disease. Ann Neurol, 53(Suppl 3): S26–36; discussion S36–28
Jodeiri Farshbaf M, Ghaedi K, Megraw T L, Curtiss J, Shirani Faradonbeh M, Vaziri P, Nasr-Esfahani M H (2016). Does PGC1/FNDC5/BDNF elicit the beneficial effects of exercise on neurodegenerative Disorders? Neuromolecular Med, 18(1): 1–15
Jung K H, Chu K, Lee S T, Park H K, Kim J H, Kang K M, Kim M, Lee S K, Roh J K (2009). Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochem Biophys Res Commun, 378(3): 507–512
Khatchadourian A, Bourque S D, Richard V R, Titorenko V I, Maysinger D (2012). Dynamics and regulation of lipid droplet formation in lipopolysaccharide (LPS)-stimulated microglia. Biochim Biophys Acta, 1821(4): 607–617
Kühlbrandt W (2015). Structure and function of mitochondrial membrane protein complexes. BMC Biol, 13(1): 89
Langston J W, Ballard P, Tetrud J W, Irwin I (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science, 219(4587): 979–980
Legros F, Malka F, Frachon P, Lombès A, Rojo M (2004). Organization and dynamics of human mitochondrial DNA. J Cell Sci, 117(Pt 13): 2653–2662
Linderholm H, Essén-Gustavsson B, Thornell L E (1990). Low succinate dehydrogenase (SDH) activity in a patient with a hereditary myopathy with paroxysmal myoglobinuria. J Intern Med, 228(1): 43–52
Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E (2009). Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROSdependent pathway. Cell Death Differ, 16(6): 899–909
Lipton J O, Sahin M (2014). The neurology of mTOR. Neuron, 84(2): 275–291
Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham B H, Quintana A, Bellen H J (2015). Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell, 160(1-2): 177–190
Lodge D (2009). The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology, 56(1): 6–21
Martin L J (2010). Mitochondrial and Cell Death Mechanisms in Neurodegenerative Diseases. Pharmaceuticals (Basel), 3(4): 839–915
Meijer A J (2003). Amino acids as regulators and components of nonproteinogenic pathways. J Nutr, 133(6 Suppl 1): 2057S–2062S
Okamoto K, Kimura A, Donishi T, Imbe H, Goda K, Kawanishi K, Tamai Y, Senba E (2006). Persistent monoarthritis of the temporomandibular joint region enhances nocifensive behavior and lumbar spinal Fos expression after noxious stimulation to the hindpaw in rats. Exp Brain Res, 170(3): 358–367
Owen O E, Kalhan S C, Hanson RW (2002). The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem, 277(34): 30409–30412
Perier C, Vila M (2012). Mitochondrial biology and Parkinson’s disease. Cold Spring Harb Perspect Med, 2(2): a009332
Porstmann T, Santos C R, Griffiths B, Cully M, Wu M, Leevers S, Griffiths J R, Chung Y L, Schulze A (2008). SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab, 8(3): 224–236
Przedborski S (2005). Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat Disord, 11(Suppl 1): S3–S7
Ralph S J, Moreno-Sánchez R, Neuzil J, Rodríguez-Enríquez S (2011). Inhibitors of succinate: quinone reductase/Complex II regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm Res, 28(11): 2695–2730
Recchia A, Debetto P, Negro A, Guidolin D, Skaper S D, Giusti P (2004). Alpha-synuclein and Parkinson’s disease. FASEB J, 18(6): 617–626
Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, Richard-Bulteau H, Vignaud A, Baas D, Defour A, Freyssenet D, Tanti J F, Le-Marchand-Brustel Y, Ferrier B, Conjard-Duplany A, Romanino K, Bauché S, Hantaï D, Mueller M, Kozma S C, Thomas G, Rüegg MA, Ferry A, Pende M, Bigard X, Koulmann N, Schaeffer L, Gangloff Y G (2009). Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol, 187(6): 859–874
Rothstein J D (1996). Excitotoxicity hypothesis. Neurology, 47: S19–25; discussion S26
Rottenberg H, Gutman M (1977). Control of the rate of reverse electron transport in submitochondrial particles by the free energy. Biochemistry, 16(14): 3220–3227
Schapira A H, Cooper J M, Dexter D, Clark J B, Jenner P, Marsden C D (1990). Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem, 54(3): 823–827
Schmitt M, Dehay B, Bezard E, Garcia-Ladona F J (2016). Harnessing the trophic and modulatory potential of statins in a dopaminergic cell line. Synapse, 70(3): 71–86
Schulz J B (2005). Neuronal pathology in Parkinson’s disease. Cell Tissue Res, 320(1): 211
Schulz J B, Falkenburger B H (2004). Neuronal pathology in Parkinson’s disease. Cell Tissue Res, 318(1): 135–147
Schwall C T, Greenwood V L, Alder N N (2012). The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochim Biophys Acta, 1817(9): 1588–1596
Selman C, Tullet J M, Wieser D, Irvine E, Lingard S J, Choudhury A I, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson I C, Schuster E, Batterham R L, Kozma S C, Thomas G, Carling D, Okkenhaug K, Thornton J M, Partridge L, Gems D, Withers D J (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science, 326(5949): 140–144
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z (2005). Crystal structure of mitochondrial respiratory membrane protein complex II. Cell, 121(7): 1043–1057
Van Vranken J G, Bricker D K, Dephoure N, Gygi S P, Cox J E, Thummel C S, Rutter J (2014). SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell Metab, 20(2): 241–252
Villa-Cuesta E, Holmbeck MA, Rand DM (2014). Rapamycin increases mitochondrial efficiency by mtDNA-dependent reprogramming of mitochondrial metabolism in Drosophila. J Cell Sci, 127(Pt 10): 2282–2290
Wübbeler J H, Hiessl S, Meinert C, Poehlein A, Schuldes J, Daniel R, Steinbüchel A (2015). The genome of Variovorax paradoxus strain TBEA6 provides new understandings for the catabolism of 3,3′-thiodipropionic acid and hence the production of polythioesters. J Biotechnol, 209: 85–95
Yasuda T, Nakata Y, Mochizuki H (2013). α-Synuclein and neuronal cell death. Mol Neurobiol, 47(2): 466–483
Younce C, Kolattukudy P (2012). MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem, 30(2): 307–320
Zhou Q, Sheng M (2013). NMDA receptors in nervous system diseases. Neuropharmacology, 74: 69–75
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farshbaf, M.J. Succinate dehydrogenase in Parkinson’s disease. Front. Biol. 12, 175–182 (2017). https://doi.org/10.1007/s11515-017-1450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-017-1450-6