ABSTRACT
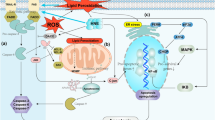
Succinate:quinone reductase (SQR) of Complex II, occupying a unique central point in the mitochondrial respiratory system as a major source of electrons driving reactive oxygen species (ROS) production, is an ideal pharmaceutical target for modulating ROS levels in normal cells to prevent oxidative stress-induced damage or increase ROS in cancer cells, inducing cell death. Value of drugs like diazoxide to prevent ROS production, protecting normal cells, while vit. E analogues promote ROS in cancer cells to kill them, is highlighted. As pharmaceuticals, agents may prevent degenerative disease; their modes of action are being fully explored. Evidence that SDH/Complex II is tightly coupled to NADH/NAD+ ratio in all cells, impacted by available supplies of Krebs cycle intermediates as essential NAD-linked substrates, and NAD+-dependent regulation of SDH/Complex II are reviewed, as are links to NAD+-dependent dehydrogenases, Complex I and E3 dihiydrolipoamide dehydrogenase to produce ROS. We collate and discuss diverse sources of information relating to ROS production in different biological systems, focussing on evidence for SQR as main source of ROS production in mitochondria, particularly its relevance to protection from oxidative stress and to mitochondrial-targeted anticancer drugs (mitocans) as novel cancer therapies.






Similar content being viewed by others
Abbreviations
- α-TOS:
-
alpha-tocopheryl succinate
- ∆μH + :
-
electrochemical H+ gradient across the inner mitocondrial membrane
- 2-OG:
-
2-oxoglutarate
- 2-OGDH:
-
2-oxoglutarate dehydrogenase
- 3-BrPyr:
-
3-bromopyruvate
- ANT:
-
adenine nucleotide translocator
- DCA:
-
dichloroacetate
- DCPIP:
-
dichlorophenolindophenol
- DLD:
-
dihydrolipoamide dehydrogenase
- FFAs:
-
free fatty acids
- FH:
-
fumarate hydratase
- MCA:
-
metabolic control analysis
- MPTP:
-
mitochondrial permeability transition pore
- O2 −•:
-
superoxide
- OAA:
-
oxaloacetate
- OXPHOS:
-
oxidative phosphorylation
- PDH:
-
pyruvate dehydrogenase
- SDH:
-
succinate dehydrogenase
- SMPs:
-
submitochondrial particles
- SOD:
-
superoxide dismutase
- SQR:
-
succinate quinone reductase
- TTFA:
-
thenoyltrifluoroacetone
- UQ:
-
ubiquinone
- UQH:
-
semiquinone
- UQH2 :
-
ubiquinol
REFERENCES
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–57.
Maklashina E, Cecchini G. The quinone-binding and catalytic site of complex II. Biochim Biophys Acta. 2010;1797:1877–82.
Xiong Y, Petersen PL, Lee C-P. Polarographic assays of mitochondrial functions. In: Celis JE, editor. Cell biology: a laboratory handbook. Oxford: Elsevier Academic Press; 2006. p. 259–64.
Brand MD. Measurement of the intramitochondrial P/O ratio. Biochem Biophys Res Commun. 1979;91:592–8.
E.Gnaiger. Mitochondrial Pathways through Complexes I and II: Convergent Electron Transfer at the Q-Junction and Additive Effects of Substrate Combinations. In E.Gnaiger (ed.), Mitochondrial Pathways and Respiratory Control, OROBOROS MiPNet publications, Innsbruck, 2007, pp. 1–13.
Garcia-Palmer FJ. Lack of functional assembly in mitochondrial supercomplexes: a new insight into impaired mitochondrial function? Cardiovasc Res. 2008;80:3–4.
Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del SM, et al. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta. 2010;1797:633–40.
Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008.
Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–83.
Lenaz G, Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–73.
Dudkina NV, Kouril R, Bultema JB, Boekema EJ. Imaging of organelles by electron microscopy reveals protein-protein interactions in mitochondria and chloroplasts. FEBS Lett. 2010;584:2510–5.
Schagger H, Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem. 2001;276:37861–7.
Schafer E, Dencher NA, Vonck J, Parcej DN. Three-dimensional structure of the respiratory chain supercomplex I1III2IV1 from bovine heart mitochondria. Biochemistry. 2007;46:12579–85.
Rich PR, Marechal A. The mitochondrial respiratory chain. Essays Biochem. 2010;47:1–23.
D.G.Nicholls and S.J.Ferguson. Bioenergetics 3, Academic Press, 2002.
Bianchi C, Genova ML, Parenti CG, Lenaz G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem. 2004;279:36562–9.
Moreno-Sanchez R, Bravo C, Westerhoff HV. Determining and understanding the control of flux. An illustration in submitochondrial particles of how to validate schemes of metabolic control. Eur J Biochem. 1999;264:427–33.
Kroger A, Klingenberg M. Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem. 1973;39:313–23.
Kroger A, Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem. 1973;34:358–68.
M.Gutman. Kinetic analysis of electron flux through the quinones in the mitochondrial system. In G.Ed.Lenaz (ed.), Coenzyme Q, John Wiley, Chichester, UK, 1985, pp. 215–234.
Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti CG, Lenaz G. Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett. 1992;311:107–9.
Stoner CD. Steady-state kinetics of the overall oxidative phosphorylation reaction in heart mitochondria. Determination of the coupling relationships between the respiratory reactions and miscellaneous observations concerning rate-limiting steps. J Bioenerg Biomembr. 1984;16:115–41.
Hatefi Y. Introduction–preparation and properties of the enzymes and enzymes complexes of the mitochondrial oxidative phosphorylation system. Methods Enzymol. 1978;53:3–4.
Yu CA, Yu L, King TE. Soluble cytochrome b-c1 complex and the reconstitution of succinate-cytochrome c reductase. J Biol Chem. 1974;249:4905–10.
Benard G, Faustin B, Galinier A, Rocher C, Bellance N, Smolkova K, et al. Functional dynamic compartmentalization of respiratory chain intermediate substrates: implications for the control of energy production and mitochondrial diseases. Int J Biochem Cell Biol. 2008;40:1543–54.
Lee C, Johansson B, King TE. Reconstitution of respiratory control of succinate oxidation in submitochondrial particles. Biochem Biophys Res Commun. 1969;35:243–8.
Tushurashvili PR, Gavrikova EV, Ledenev AN, Vinogradov AD. Studies on the succinate dehydrogenating system. Isolation and properties of the mitochondrial succinate-ubiquinone reductase. Biochim Biophys Acta. 1985;809:145–59.
Choudhry ZM, Kotlyar AB, Vinogradov AD. Studies on the succinate dehydrogenating system. Interaction of the mitochondrial succinate-ubiquinone reductase with pyridoxal phosphate. Biochim Biophys Acta. 1986;850:131–8.
Yu L, Yu CA. Interaction between succinate dehydrogenase and ubiquinone-binding protein from succinate-ubiquinone reductase. Biochim Biophys Acta. 1980;593:24–38.
Kalina M, Weavers B, Pearse AG. Ultrastructural localization of succinate dehydrogenase in mouse liver mitochondria; a cytochemical study. J Histochem Cytochem. 1971;19:124–30.
Barnes SJ, Weitzman PD. Organization of citric acid cycle enzymes into a multienzyme cluster. FEBS Lett. 1986;201:267–70.
Beeckmans S, Kanarek L. Enzyme-enzyme interactions as modulators of the metabolic flux through the citric acid cycle. Biochem Soc Symp. 1987;54:163–72.
Lyubarev AE, Kurganov BI. Supramolecular organization of tricarboxylic acid cycle enzymes. Biosystems. 1989;22:91–102.
Robinson Jr JB, Inman L, Sumegi B, Srere PA. Further characterization of the Krebs tricarboxylic acid cycle metabolon. J Biol Chem. 1987;262:1786–90.
Robinson Jr JB, Srere PA. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985;260:10800–5.
Moore GE, Gadol SM, Robinson Jr JB, Srere PA. Binding of citrate synthase and malate dehydrogenase to mitochondrial inner membranes: tissue distribution and metabolite effects. Biochem Biophys Res Commun. 1984;121:612–8.
Beeckmans S, Van DE, Kanarek L. Immobilized enzymes as tools for the demonstration of metabolon formation. A short overview. J Mol Recognit. 1993;6:195–204.
Beeckmans S, Van DE, Kanarek L. Clustering of sequential enzymes in the glycolytic pathway and the citric acid cycle. J Cell Biochem. 1990;43:297–306.
van der Laarse WJ, Diegenbach PC, Elzinga G. Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil. 1989;10:221–8.
Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–52.
Kacser H, Burns JA. The control of flux. Biochem Soc Trans. 1995;23:341–66.
Kacser H, Burns JA. The control of flux. Symp Soc Exp Biol. 1973;27:65–104.
Heinrich R, Rapoport SM, Rapoport TA. Metabolic regulation and mathematical models. Prog Biophys Mol Biol. 1977;32:1–82.
Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974;42:89–95.
Moreno-Sanchez R, Torres-Marquez ME. Control of oxidative phosphorylation in mitochondria, cells and tissues. Int J Biochem. 1991;23:1163–74.
Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992;284(Pt 1):1–13.
Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, et al. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol Cancer. 2009;8:41.
Fan TW, Kucia M, Jankowski K, Higashi RM, Ratajczak J, Ratajczak MZ, et al. Rhabdomyosarcoma cells show an energy producing anabolic metabolic phenotype compared with primary myocytes. Mol Cancer. 2008;7:79.
Kwong LK, Sohal RS. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch Biochem Biophys. 1998;350:118–26.
Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52.
Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–80.
Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–58.
Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46:1283–97.
Gutman M. In: Lenaz G, editor. Kinetic analysis of electron flux through the quinones in the mitochondrial system. Chichester: Coenzyme Q, John Wiley; 1985. p. 215–34.
Gutman M, Silman N. Mutual inhibition between NADH oxidase and succinoxidase activities in respiring submitochondrial particles. FEBS Lett. 1972;26:207–10.
Pryde KR, Hirst J. Superoxide Is Produced by the Reduced Flavin in Mitochondrial Complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem. 2011;286:18056–65.
Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–12.
Bortolami S, Comelato E, Zoccarato F, Alexandre A, Cavallini L. Long chain fatty acyl-CoA modulation of H(2)O (2) release at mitochondrial complex I. J Bioenerg Biomembr. 2008;40:9–18.
Zoccarato F, Cavallini L, Bortolami S, Alexandre A. Succinate modulation of H2O2 release at NADH:ubiquinone oxidoreductase (Complex I) in brain mitochondria. Biochem J. 2007;406:125–9.
Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–77.
Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–44.
Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95.
Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–8.
Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382:511–7.
Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem. 2004;279:39414–20.
Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–7.
Zoccarato F, Cavallini L, Alexandre A. Succinate is the controller of O2-/H2O2 release at mitochondrial complex I: negative modulation by malate, positive by cyanide. J Bioenerg Biomembr. 2009;41:387–93.
Lambert AJ, Buckingham JA, Brand MD. Dissociation of superoxide production by mitochondrial complex I from NAD(P)H redox state. FEBS Lett. 2008;582:1711–4.
Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–67.
Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–61.
Grivennikova VG, Kareyeva AV, Vinogradov AD. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim Biophys Acta. 2010;1797:939–44.
Tomitsuka E, Kita K, Esumi H. The NADH-fumarate reductase system, a novel mitochondrial energy metabolism, is a new target for anticancer therapy in tumor microenvironments. Ann N Y Acad Sci. 2010;1201:44–9.
Kareyeva AV, Grivennikova VG, Cecchini G, Vinogradov AD. Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production. FEBS Lett. 2011;585:385–9.
Papa S, Lofrumento NE, Paradies G, Quagliariello E. Mechanism of inhibition by uncouples of succinate oxidation in isolated mitochondria. Biochim Biophys Acta. 1969;180:35–44.
Wojtczak L, Wojtczak AB, Ernster L. The inhibition of succinate dehydrogenase by oxalacetate. Biochim Biophys Acta. 1969;191:10–21.
Wojtczak AB. Inhibitory action of oxaloacetate on succinate oxidation in rat-liver mitochondria and the mechanism of its reversal. Biochim Biophys Acta. 1969;172:52–65.
Moser MD, Matsuzaki S, Humphries KM. Inhibition of succinate-linked respiration and complex II activity by hydrogen peroxide. Arch Biochem Biophys. 2009;488:69–75.
Dohm GL, Tapscott EB. Oxaloacetate inhibition of succinate oxidation in tightly coupled liver mitochondria with ferricyanide as an electron acceptor. Biochem Biophys Res Commun. 1973;52:246–53.
Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, et al. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J. 2008;409:491–9.
Pisarenko OI, Khlopkov VN, Ruuge EK. A 1H NMR study of succinate synthesis from exogenous precursors in oxygen-deprived rat heart mitochondria. Biochem Int. 1986;12:145–53.
Oestreicher AB, Van den Bergh SG, Slater EC. The inhibition by 2,4-dinitrophenol of the removal of oxaloacetate formed by the oxidation of succinate by rat-liver and -heart mitochondria. Biochim Biophys Acta. 1969;180:45–55.
Piccoli C, Scrima R, Boffoli D, Capitanio N. Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem J. 2006;396:573–83.
Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science. 2006;311:1430–6.
Hunte C, Zickermann V, Brandt U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science. 2010;329:448–51.
Zoccarato F, Cappellotto M, Alexandre A. Clorgyline and other propargylamine derivatives as inhibitors of succinate-dependent H(2)O(2) release at NADH:UBIQUINONE oxidoreductase (Complex I) in brain mitochondria. J Bioenerg Biomembr. 2008;40:289–96.
Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P) + oxidation-reduction state. Biochem J. 2002;368:545–53.
Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–8.
Vinogradov AD. Respiratory complex I: structure, redox components, and possible mechanisms of energy transduction. Biochemistry (Mosc). 2001;66:1086–97.
Tuena M, Gomez-Puyou A, Pena A, Chavez E, Sandoval F. Effect of ATP on the oxidation of succinate in rat brain mitochondria. Eur J Biochem. 1969;11:283–90.
Ezawa I, Ogata E. Ca2+ −induced activation of succinate dehydrogenase and the regulation of mitochondrial oxidative reactions. J Biochem. 1979;85:65–74.
Ezawa I, Ogata E. Ca2+ requirement in ATP-induced activation of uncoupled oxidation of succinate in isolated rat-liver mitochondria. Eur J Biochem. 1977;77:427–35.
Rustin P, Lance C. Succinate-driven reverse electron transport in the respiratory chain of plant mitochondria. The effects of rotenone and adenylates in relation to malate and oxaloacetate metabolism. Biochem J. 1991;274(Pt 1):249–55.
Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–405.
Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972–9.
Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–31.
Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K + channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–5.
Ardehali H, O’Rourke B. Mitochondrial K(ATP) channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16.
Facundo HT. J.G.de Paula, and A.J. Kowaltowski. Mitochondrial ATP-sensitive K + channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039–48.
Schafer G, Wegener C, Portenhauser R, Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem Pharmacol. 1969;18:2678–81.
Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K + channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–9.
Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–9.
Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, et al. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K + channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–82.
Szewczyk A, Marban E. Mitochondria: a new target for K channel openers? Trends Pharmacol Sci. 1999;20:157–61.
Brustovetsky T, Shalbuyeva N, Brustovetsky N. Lack of manifestations of diazoxide/5-hydroxydecanoate-sensitive KATP channel in rat brain nonsynaptosomal mitochondria. J Physiol. 2005;568:47–59.
Minners J, Lacerda L, Yellon DM, Opie LH, McLeod CJ, Sack MN. Diazoxide-induced respiratory inhibition - a putative mitochondrial K(ATP) channel independent mechanism of pharmacological preconditioning. Mol Cell Biochem. 2007;294:11–8.
Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial K(ATP) channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–5.
Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–41.
Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893–902.
Kopustinskiene DM, Toleikis A, Saris NE. Adenine nucleotide translocase mediates the K(ATP)-channel-openers-induced proton and potassium flux to the mitochondrial matrix. J Bioenerg Biomembr. 2003;35:141–8.
Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–70.
Grimmsmann T, Rustenbeck I. Direct effects of diazoxide on mitochondria in pancreatic B-cells and on isolated liver mitochondria. Br J Pharmacol. 1998;123:781–8.
Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–74.
Paddenberg R, Goldenberg A, Faulhammer P, Braun-Dullaeus RC, Kummer W. Mitochondrial complex II is essential for hypoxia-induced ROS generation and vasoconstriction in the pulmonary vasculature. Adv Exp Med Biol. 2003;536:163–9.
Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, et al. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2003;284:L710–9.
Paddenberg R, Faulhammer P, Goldenberg A, Gries B, Heinl J, Kummer W. Impact of modulators of mitochondrial ATP-sensitive potassium channel (mitoK(ATP)) on hypoxic pulmonary vasoconstriction. Adv Exp Med Biol. 2009;648:361–8.
B.B.Queliconi, A.P.Wojtovich, S.M.Nadtochiy, A.J.Kowaltowski, and P.S.Brookes. Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta (2010).
Drose S, Hanley PJ, Brandt U. Ambivalent effects of diazoxide on mitochondrial ROS production at respiratory chain complexes I and III. Biochim Biophys Acta. 2009;1790:558–65.
Junemann S, Heathcote P, Rich PR. On the mechanism of quinol oxidation in the bc1 complex. J Biol Chem. 1998;273:21603–7.
Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–54.
Liu B, Zhu X, Chen CL, Hu K, Swartz HM, Chen YR, et al. Opening of the mitoKATP channel and decoupling of mitochondrial complex II and III contribute to the suppression of myocardial reperfusion hyperoxygenation. Mol Cell Biochem. 2010;337:25–38.
Miyadera H, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Tomoda H, et al. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase). Proc Natl Acad Sci U S A. 2003;100:473–7.
Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104:121–9.
S.Drose, L.Bleier, and U.Brandt. A common mechanism links differently acting complex II inhibitors to cardioprotection: modulation of mitochondrial reactive oxygen species production. Mol Pharmacol (2011).
Schafer G, Portenhauser R, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide. I. Action on succinate dehydrogenase and TCA-cycle oxidations. Biochem Pharmacol. 1971;20:1271–80.
Drose S, Brandt U, Hanley PJ. K + −independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281:23733–9.
Sarewicz M, Borek A, Cieluch E, Swierczek M, Osyczka A. Discrimination between two possible reaction sequences that create potential risk of generation of deleterious radicals by cytochrome bc. Implications for the mechanism of superoxide production. Biochim Biophys Acta. 2010;1797:1820–7.
Borek A, Sarewicz M, Osyczka A. Movement of the iron-sulfur head domain of cytochrome bc(1) transiently opens the catalytic Q(o) site for reaction with oxygen. Biochemistry. 2008;47:12365–70.
Folbergrova J, Ljunggren B, Norberg K, Siesjo BK. Influence of complete ischemia on glycolytic metabolites, citric acid cycle intermediates, and associated amino acids in the rat cerebral cortex. Brain Res. 1974;80:265–79.
Benzi G, Arrigoni E, Marzatico F, Villa RF. Influence of some biological pyrimidines on the succinate cycle during and after cerebral ischemia. Biochem Pharmacol. 1979;28:2545–50.
Benzi G, Pastoris O, Dossena M. Relationships between gamma-aminobutyrate and succinate cycles during and after cerebral ischemia. J Neurosci Res. 1982;7:193–201.
Khazanov VA, Poborsky AN, Kondrashova MN. Air saturation of the medium reduces the rate of phosphorylating oxidation of succinate in isolated mitochondria. FEBS Lett. 1992;314:264–6.
A.N.Poborskii. [Effect of research conditions on succinate oxidation in brain mitochondria in circulatory hypoxia]. Patol Fiziol Eksp Ter:10–12 (1997).
Konig T, Nicholls DG, Garland PB. The inhibition of pyruvate and Ls(+)-isocitrate oxidation by succinate oxidation in rat liver mitochondria. Biochem J. 1969;114:589–96.
Hohl C, Oestreich R, Rosen P, Wiesner R, Grieshaber M. Evidence for succinate production by reduction of fumarate during hypoxia in isolated adult rat heart cells. Arch Biochem Biophys. 1987;259:527–35.
Grivennikova VG, Gavrikova EV, Timoshin AA, Vinogradov AD. Fumarate reductase activity of bovine heart succinate-ubiquinone reductase. New assay system and overall properties of the reaction. Biochim Biophys Acta. 1993;1140:282–92.
Ackrell BA. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 2000;466:1–5.
Pisarenko OI. Mechanisms of myocardial protection by amino acids: facts and hypotheses. Clin Exp Pharmacol Physiol. 1996;23:627–33.
Pisarenko OI, Khlopkov VN, Ruuge EK. A 1H NMR study of succinate synthesis from exogenous precursors in oxygen-deprived rat heart mitochondria. Biochem Int. 1986;12:145–53.
Penney DG, Cascarano J. Anaerobic rat heart. Effects of glucose and tricarboxylic acid-cycle metabolites on metabolism and physiological performance. Biochem J. 1970;118:221–7.
Taegtmeyer H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ Res. 1978;43:808–15.
Taegtmeyer H, Lesch M. Mechanisms of de novo alanine synthesis in hypoxic heart muscle. Verh Dtsch Ges Kreislaufforsch. 1977;43:269.
Sanborn T, Gavin W, Berkowitz S, Perille T, Lesch M. Augmented conversion of aspartate and glutamate to succinate during anoxia in rabbit heart. Am J Physiol. 1979;237:H535–41.
Freminet A, Leclerc L, Poyart C, Huel C, Gentil M. Alanine and succinate accumulation in the perfused rat heart during hypoxia. J Physiol (Paris). 1980;76:113–7.
Peuhkurinen KJ, Takala TE, Nuutinen EM, Hassinen IE. Tricarboxylic acid cycle metabolites during ischemia in isolated perfused rat heart. Am J Physiol. 1983;244:H281–8.
Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000;279:F927–43.
Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–31.
Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+−dependent malic enzyme. J Biol Chem. 1984;259:6215–21.
Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Moreno-Sanchez R. Bioenergetic pathways in tumor mitochondria as targets for cancer therapy and the importance of the ROS-induced apoptotic trigger. Mol Aspects Med. 2010;31:29–59.
Mates JM, Segura JA, Campos-Sandoval JA, Lobo C, Alonso L, Alonso FJ, et al. Glutamine homeostasis and mitochondrial dynamics. Int J Biochem Cell Biol. 2009;41:2051–61.
Marino G, Kroemer G. Ammonia: a diffusible factor released by proliferating cells that induces autophagy. Sci Signal. 2010;3:e19.
Kovacevic Z, McGivan JD. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983;63:547–605.
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–93.
Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–19.
Erickson JW, Cerione RA. Glutaminase: A hot spot for regulation of cancer cell metabolism? Oncotarget. 2010;1:734–40.
Ishii N, Ishii T, Hartman PS. The role of the electron transport SDHC gene on lifespan and cancer. Mitochondrion. 2007;7:24–8.
Ishii N, Ishii T, Hartman PS. The role of the electron transport gene SDHC on lifespan and cancer. Exp Gerontol. 2006;41:952–6.
Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16.
Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004.
Kaelin Jr WG. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–73.
Brahimi-Horn MC, Pouyssegur J. Harnessing the hypoxia-inducible factor in cancer and ischemic disease. Biochem Pharmacol. 2007;73:450–7.
Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–101.
Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–66.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85.
Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–39.
Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–9.
King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–82.
Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–32.
MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–9.
Blank A, Schmitt AM, Korpershoek E, Van NF, Rudolph T, Weber N, et al. SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr Relat Cancer. 2010;17:919–28.
Brieger J, Bedavanija A, Gosepath J, Maurer J, Mann WJ. Vascular endothelial growth factor expression, vascularization and proliferation in paragangliomas. ORL J Otorhinolaryngol Relat Spec. 2005;67:119–24.
Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, Munoz I, Schiavi F, Montero-Conde C, et al. Research resource: transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24:2382–91.
Ni Y, Zbuk KM, Sadler T, Patocs A, Lobo G, Edelman E, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–8.
Briere JJ, Favier J, Benit P, El Ghouzzi V, Lorenzato A, Rabier D, et al. Mitochondrial succinate is instrumental for HIF1alpha nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Hum Mol Genet. 2005;14:3263–9.
Briere JJ, Favier J, El Ghouzzi V, Djouadi F, Benit P, Gimenez AP, et al. Succinate dehydrogenase deficiency in human. Cell Mol Life Sci. 2005;62:2317–24.
Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE. Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–20.
Grivennikova VG, Cecchini G, Vinogradov AD. Ammonium-dependent hydrogen peroxide production by mitochondria. FEBS Lett. 2008;582:2719–24.
Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RN, Kristal BS, Brown AM. Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem. 2002;277:10064–72.
Okamura-Ikeda K, Hosaka H, Maita N, Fujiwara K, Yoshizawa AC, Nakagawa A, et al. Crystal structure of aminomethyltransferase in complex with dihydrolipoyl-H-protein of the glycine cleavage system: implications for recognition of lipoyl protein substrate, disease-related mutations, and reaction mechanism. J Biol Chem. 2010;285:18684–92.
Link TA, von Jagow G. Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group. J Biol Chem. 1995;270:25001–6.
Raffaello A, Rizzuto R. Mitochondrial longevity pathways. Biochim Biophys Acta. 2011;1813:260–8.
M.H.Vendelbo and K.S.Nair. Mitochondrial longevity pathways. Biochim Biophys Acta (2011).
Tahara EB, Barros MH, Oliveira GA, Netto LE, Kowaltowski AJ. Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J. 2007;21:274–83.
Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95.
L.Gil del Valle. Oxidative stress in aging: Theoretical outcomes and clinical evidences in humans. Biomed Pharmacother (2010).
Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–88.
Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24:7771–8.
Huennekens F, Basford RE, Gabrio BW. An oxidase for reduced diphosphopyridine nucleotide. J Biol Chem. 1955;213:951–67.
Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269:22459–62.
Bunik VI, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur J Biochem. 2002;269:5004–15.
Cooney GJ, Taegtmeyer H, Newsholme EA. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J. 1981;200:701–3.
McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425.
Moreno-Sanchez R, Hogue BA, Hansford RG. Influence of NAD-linked dehydrogenase activity on flux through oxidative phosphorylation. Biochem J. 1990;268:421–8.
Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–20.
Bunik VI. 2-Oxo acid dehydrogenase complexes in redox regulation. Eur J Biochem. 2003;270:1036–42.
Muhling J, Tiefenbach M, Lopez-Barneo J, Piruat JI, Garcia-Flores P, Pfeil U, et al. Mitochondrial complex II participates in normoxic and hypoxic regulation of alpha-keto acids in the murine heart. J Mol Cell Cardiol. 2010;49:950–61.
Olsson JM, Xia L, Eriksson LC, Bjornstedt M. Ubiquinone is reduced by lipoamide dehydrogenase and this reaction is potently stimulated by zinc. FEBS Lett. 1999;448:190–2.
Xia L, Bjornstedt M, Nordman T, Eriksson LC, Olsson JM. Reduction of ubiquinone by lipoamide dehydrogenase. An antioxidant regenerating pathway. Eur J Biochem. 2001;268:1486–90.
Ventura FV, Ruiter JP, Ijlst L, de Almeida IT, Wanders RJ. Inhibitory effect of 3-hydroxyacyl-CoAs and other long-chain fatty acid beta-oxidation intermediates on mitochondrial oxidative phosphorylation. J Inherit Metab Dis. 1996;19:161–4.
Ventura FV, Ruiter J, Ijlst L, de Almeida IT, Wanders RJ. Differential inhibitory effect of long-chain acyl-CoA esters on succinate and glutamate transport into rat liver mitochondria and its possible implications for long-chain fatty acid oxidation defects. Mol Genet Metab. 2005;86:344–52.
Ventura FV, Ruiter JP, Ijlst L, Almeida IT, Wanders RJ. Inhibition of oxidative phosphorylation by palmitoyl-CoA in digitonin permeabilized fibroblasts: implications for long-chain fatty acid beta-oxidation disorders. Biochim Biophys Acta. 1995;1272:14–20.
Beatrice MC, Pfeiffer DR. The mechanism of palmitoyl-CoA inhibition of Ca2+ uptake in liver and heart mitochondria. Biochem J. 1981;194:71–7.
Garland PB, Randle PJ. Control of pyruvate dehydrogenase in the perfused rat heart by the intracellular concentration of acetyl-coenzyme A. Biochem J. 1964;91:6C–7C.
Wieland O, Von Jagow-Westermann B, Stukowski B. Kinetic and regulatory properties of heart muscle pyruvate dehydrogenase. Hoppe Seylers Z Physiol Chem. 1969;350:329–34.
Bremer J. Pyruvate dehydrogenase, substrate specificity and product inhibition. Eur J Biochem. 1969;8:535–40.
Tsai CS, Burgett MW, Reed LJ. Alpha-keto acid dehydrogenase complexes. XX. A kinetic study of the pyruvate dehydrogenase complex from bovine kidney. J Biol Chem. 1973;248:8348–52.
Cooper RH, Randle PJ, Denton RM. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974;143:625–41.
Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976;154:327–48.
Hansford RG, Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978;191:65–81.
Garland PB, Randle PJ. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964;93:678–87.
Randle PJ, England PJ, Denton RM. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970;117:677–95.
Moore KH, Dandurand DM, Kiechle FL. Fasting induced alterations in mitochondrial palmitoyl-CoA metabolism may inhibit adipocyte pyruvate dehydrogenase activity. Int J Biochem. 1992;24:809–14.
Lai JC, Cooper AJ. Neurotoxicity of ammonia and fatty acids: differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme A derivatives. Neurochem Res. 1991;16:795–803.
Sauer SW, Okun JG, Hoffmann GF, Koelker S, Morath MA. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim Biophys Acta. 2008;1777:1276–82.
Luis PB, Ruiter JP, Aires CC, Soveral G, de Almeida IT, Duran M, et al. Valproic acid metabolites inhibit dihydrolipoyl dehydrogenase activity leading to impaired 2-oxoglutarate-driven oxidative phosphorylation. Biochim Biophys Acta. 2007;1767:1126–33.
Krebs HA, Johnson WA. Metabolism of ketonic acids in animal tissues. Biochem J. 1937;31:645–60.
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49:304–11.
Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–75.
James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004;423:47–56.
O’Malley Y, Fink BD, Ross NC, Prisinzano TE, Sivitz WI. Reactive oxygen and targeted antioxidant administration in endothelial cell mitochondria. J Biol Chem. 2006;281:39766–75.
Fink BD, O’Malley Y, Dake BL, Ross NC, Prisinzano TE, Sivitz WI. Mitochondrial targeted coenzyme Q, superoxide, and fuel selectivity in endothelial cells. PLoS One. 2009;4:e4250.
James AM, Sharpley MS, Manas AR, Frerman FE, Hirst J, Smith RA, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–18.
Plecita-Hlavata L, Jezek J, Jezek P. Pro-oxidant mitochondrial matrix-targeted ubiquinone MitoQ10 acts as anti-oxidant at retarded electron transport or proton pumping within Complex I. Int J Biochem Cell Biol. 2009;41:1697–707.
Mowery PC, Steenkamp DJ, Ackrell AC, Singer TP, White GA. Inhibition of mammalian succinate dehydrogenase by carboxins. Arch Biochem Biophys. 1977;178:495–506.
Trumpower BL, Simmons Z. Diminished inhibition of mitochondrial electron transfer from succinate to cytochrome c by thenoyltrifluoroacetone induced by antimycin. J Biol Chem. 1979;254:4608–16.
Don AS, Hogg PJ. Mitochondria as cancer drug targets. Trends Mol Med. 2004;10:372–8.
Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria as targets for cancer chemotherapy. Semin Cancer Biol. 2009;19:57–66.
Fantin VR, Leder P. Mitochondriotoxic compounds for cancer therapy. Oncogene. 2006;25:4787–97.
Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125–9.
Ralph SJ, Neuzil J. Mitochondria as targets for cancer therapy. Mol Nutr Food Res. 2009;53:9–28.
Ralph SJ, Neuzil J. Mitocans, a class of emerging anti-cancer drugs. Mol Nutr Food Res. 2009;53:7–8.
Ralph SJ, Low P, Dong L, Lawen A, Neuzil J. Mitocans: mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Pat Anticancer Drug Discov. 2006;1:327–46.
Neuzil J, Dong LF, Ramanathapuram L, Hahn T, Chladova M, Wang XF, et al. Vitamin E analogues as a novel group of mitocans: anti-cancer agents that act by targeting mitochondria. Mol Aspects Med. 2007;28:607–45.
Neuzil J, Dyason JC, Freeman R, Dong LF, Prochazka L, Wang XF, et al. Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II. J Bioenerg Biomembr. 2007;39:65–72.
Neuzil J, Tomasetti M, Zhao Y, Dong LF, Birringer M, Wang XF, et al. Vitamin E analogs, a novel group of ‘mitocans,’ as anticancer agents: the importance of being redox-silent. Mol Pharmacol. 2007;71:1185–99.
Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125–9.
Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer. 2001;84:87–9.
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–13.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51.
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12.
Moreno-Sanchez R, Saavedra E, Rodriguez-Enriquez S, Gallardo-Perez JC, Quezada H, Westerhoff HV. Metabolic control analysis indicates a change of strategy in the treatment of cancer. Mitochondrion. 2010;10:626–39.
Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, et al. alpha-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J Biol Chem. 2006;281:11819–25.
Stapelberg M, Gellert N, Swettenham E, Tomasetti M, Witting PK, Procopio A, et al. Alpha-tocopheryl succinate inhibits malignant mesothelioma by disrupting the fibroblast growth factor autocrine loop: mechanism and the role of oxidative stress. J Biol Chem. 2005;280:25369–76.
Weber T, Dalen H, Andera L, Negre-Salvayre A, Auge N, Sticha M, et al. Mitochondria play a central role in apoptosis induced by alpha-tocopheryl succinate, an agent with antineoplastic activity: comparison with receptor-mediated pro-apoptotic signaling. Biochemistry. 2003;42:4277–91.
Wang XF, Witting PK, Salvatore BA, Neuzil J. Vitamin E analogs trigger apoptosis in HER2/erbB2-overexpressing breast cancer cells by signaling via the mitochondrial pathway. Biochem Biophys Res Commun. 2005;326:282–9.
Swettenham E, Witting PK, Salvatore BA, Neuzil J. Alpha-tocopheryl succinate selectively induces apoptosis in neuroblastoma cells: potential therapy of malignancies of the nervous system? J Neurochem. 2005;94:1448–56.
Kang YH, Lee E, Choi MK, Ku JL, Kim SH, Park YG, et al. Role of reactive oxygen species in the induction of apoptosis by alpha-tocopheryl succinate. Int J Cancer. 2004;112:385–92.
Kogure K, Hama S, Manabe S, Tokumura A, Fukuzawa K. High cytotoxicity of alpha-tocopheryl hemisuccinate to cancer cells is due to failure of their antioxidative defense systems. Cancer Lett. 2002;186:151–6.
Allen RG, Balin AK. Effects of oxygen on the antioxidant responses of normal and transformed cells. Exp Cell Res. 2003;289:307–16.
Safford SE, Oberley TD, Urano M, St Clair DK. Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase. Cancer Res. 1994;54:4261–5.
Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS, et al. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993;90:3113–7.
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–5.
Neuzil J, Massa H. Hepatic processing determines dual activity of alpha-tocopheryl succinate: a novel paradigm for a shift in biological activity due to pro-vitamin-to-vitamin conversion. Biochem Biophys Res Commun. 2005;327:1024–7.
Wang XF, Dong L, Zhao Y, Tomasetti M, Wu K, Neuzil J. Vitamin E analogues as anticancer agents: lessons from studies with alpha-tocopheryl succinate. Mol Nutr Food Res. 2006;50:675–85.
Wang XF, Birringer M, Dong LF, Veprek P, Low P, Swettenham E, et al. A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res. 2007;67:3337–44.
Tomasetti M, Gellert N, Procopio A, Neuzil J. A vitamin E analogue suppresses malignant mesothelioma in a preclinical model: a future drug against a fatal neoplastic disease? Int J Cancer. 2004;109:641–2.
Dong LF, Low P, Dyason JC, Wang XF, Prochazka L, Witting PK, et al. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene. 2008;27:4324–35.
Oostveen FG, Au HC, Meijer PJ, Scheffler IE. A Chinese hamster mutant cell line with a defect in the integral membrane protein CII-3 of complex II of the mitochondrial electron transport chain. J Biol Chem. 1995;270:26104–8.
Albayrak T, Scherhammer V, Schoenfeld N, Braziulis E, Mund T, Bauer MK, et al. The tumor suppressor cybL, a component of the respiratory chain, mediates apoptosis induction. Mol Biol Cell. 2003;14:3082–96.
Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH. The quinone binding site in Escherichia coli succinate dehydrogenase is required for electron transfer to the heme b. J Biol Chem. 2006;281:32310–7.
Cheng VW, Ma E, Zhao Z, Rothery RA, Weiner JH. The iron-sulfur clusters in Escherichia coli succinate dehydrogenase direct electron flow. J Biol Chem. 2006;281:27662–8.
McLennan HR, Degli EM. The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J Bioenerg Biomembr. 2000;32:153–62.
Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–45.
Valis K, Prochazka L, Boura E, Chladova J, Obsil T, Rohlena J, et al. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 2011;71:946–54.
Prochazka L, Dong LF, Valis K, Freeman R, Ralph SJ, Turanek J, et al. alpha-Tocopheryl succinate causes mitochondrial permeabilization by preferential formation of Bak channels. Apoptosis. 2010;15:782–94.
Dong LF, Jameson VJ, Tilly D, Cerny J, Mahdavian E, Marin-Hernandez A, et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem. 2011;286:3717–28.
L.F.Dong, V.J.Jameson, D.Tilly, L.Prochazka, J.Rohlena, K.Valis, J.Truksa, R.Zobalova, E.Mahdavian, K.Kluckova, M.Stantic, J.Stursa, R.Freeman, P.K.Witting, E.Norberg, J.Goodwin, B.A.Salvatore, J.Novotna, J.Turanek, M.Ledvina, P.Hozak, B.Zhivotovsky, M.J.Coster, S.J.Ralph, R.A.Smith, and J.Neuzil. Mitochondrial targeting of alpha-tocopheryl succinate enhances its pro-apoptotic efficacy: A new paradigm of efficient cancer therapy. Free Radic Biol Med (2011).
D’Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–7.
D’Angelo G, Duplan E, Vigne P, Frelin C. Cyclosporin A prevents the hypoxic adaptation by activating hypoxia-inducible factor-1alpha Pro-564 hydroxylation. J Biol Chem. 2003;278:15406–11.
Holmuhamedov E, Lewis L, Bienengraeber M, Holmuhamedova M, Jahangir A, Terzic A. Suppression of human tumor cell proliferation through mitochondrial targeting. FASEB J. 2002;16:1010–6.
Ding J, Ge D, Guo W, Lu C. Diazoxide-mediated growth inhibition in human lung cancer cells via downregulation of beta-catenin-mediated cyclin D1 transcription. Lung. 2009;187:61–7.
van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens. 1996;14:1041–5.
Ozcan C, Holmuhamedov EL, Jahangir A, Terzic A. Diazoxide protects mitochondria from anoxic injury: implications for myopreservation. J Thorac Cardiovasc Surg. 2001;121:298–306.
Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H531–9.
Akao M, O’Rourke B, Kusuoka H, Teshima Y, Jones SP, Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res. 2003;92:195–202.
Ichinose M, Yonemochi H, Sato T, Saikawa T. Diazoxide triggers cardioprotection against apoptosis induced by oxidative stress. Am J Physiol Heart Circ Physiol. 2003;284:H2235–41.
Lenzen S, Panten U. Characterization of succinate dehydrogenase and alpha-glycerophosphate dehydrogenase in pancreatic islets. Biochem Med. 1983;30:349–56.
Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, et al. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;284:H1048–56.
C.Gleason, S.Huang, L.F.Thatcher, R.C.Foley, C.R.Anderson, A.J.Carroll, A.H.Millar, and K.B.Singh. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc Natl Acad Sci U S A (2011).
Hirst J. Towards the molecular mechanism of respiratory complex I. Biochem J. 2010;425:327–39.
J.R.Treberg, C.L.Quinlan, and M.D.Brand. Evidence for Two Sites of Superoxide Production by Mitochondrial NADH-Q Oxidoreductase (Complex I). J Biol Chem (2011).
Treberg JR, Brand MD. A model of the proton translocation mechanism of complex I. J Biol Chem. 2011;286:17579–84.
Ingledew WJ, Ohnishi T. An analysis of some thermodynamic properties of iron-sulphur centres in site I of mitochondria. Biochem J. 1980;186:111–7.
Lemos RS, Fernandes AS, Pereira MM, Gomes CM, Teixeira M. Quinol:fumarate oxidoreductases and succinate:quinone oxidoreductases: phylogenetic relationships, metal centres and membrane attachment. Biochim Biophys Acta. 2002;1553:158–70.
Covian R, Zwicker K, Rotsaert FA, Trumpower BL. Asymmetric and redox-specific binding of quinone and quinol at center N of the dimeric yeast cytochrome bc1 complex. Consequences for semiquinone stabilization. J Biol Chem. 2007;282:24198–208.
Snyder CH, Merbitz-Zahradnik T, Link TA, Trumpower BL. Role of the Rieske iron-sulfur protein midpoint potential in the protonmotive Q-cycle mechanism of the cytochrome bc1 complex. J Bioenerg Biomembr. 1999;31:235–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11095-011-0583-6
Rights and permissions
About this article
Cite this article
Ralph, S.J., Moreno-Sánchez, R., Neuzil, J. et al. Inhibitors of Succinate: Quinone Reductase/Complex II Regulate Production of Mitochondrial Reactive Oxygen Species and Protect Normal Cells from Ischemic Damage but Induce Specific Cancer Cell Death. Pharm Res 28, 2695–2730 (2011). https://doi.org/10.1007/s11095-011-0566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0566-7