Abstract
Complex formation (leading to either coacervation or precipitation) offers a tool to generate plant-based novel food structures and textures. This study investigated the formation of complexes between soluble pea proteins and apple pectin upon varying the protein-to-pectin ratio (r = 2:1 to 10:1), pH (3–7), and temperature (25 and 85 °C) with a total biopolymer concentration set to 1% (w/w). The results showed that predominantly soluble biopolymer complexes were formed at pH 5, and at low ratio (r = 2:1), whereas lowering the pH to more acidic condition, and to higher ratios (r = 4:1–10:1) induced the formation of more insoluble biopolymer complexes. In general, the mean particle sizes of the biopolymer complexes ranged between approximately 20 and 100 μm. Upon heating to 85 °C, the amount of insoluble biopolymer complexes increased at pH 3–5 at all ratios, except at r = 2:1. In addition, the complex sizes became somewhat larger at r = 2:1 to 6:1 upon heat treatment, whereas only trivial size changes were observed at higher ratios (r = 8:1 to 10:1). Overall, electrostatic and hydrophobic interactions played a major role in the complex formation between the soluble pea proteins and apple pectin. These findings are important for designing solely plant-based food structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biopolymer complexes composed of different types of proteins and polysaccharides have been established as a useful tool for creating novel food structures, and functioning as encapsulation and delivery systems, gelling and film forming agents, and fat replacers [1,2,3,4,5,6,7]. So far, most of the past investigations have focused on animal-based proteins such as whey proteins, β-lactoglobulin and gelatin. However, increasing demand for vegan products has shifted the interest in complex formation with plant-based proteins. Peas (Pisum sativum L.) with high nutritional value and functional properties are emerging as an alternative for animal-derived proteins to provide texture to foods [8,9,10]. However, only limited number of studies on complex coacervation with pea proteins are available [11,12,13,14,15,16,17,18,19]. These studies demonstrated that pea proteins can be complexed with alginate [12], chitosan [11], gum arabic [14, 20], and pectins [13, 15,16,17,18,19].
In general, the complex coacervation describing the associative phase separation of biopolymers in diluted regime is induced predominantly by electrostatic interactions [3,4,5, 7] as was also demonstrated in the investigations on the pea protein – polysaccharide complexes [11,12,13,14,15,16,17,18,19]. This phenomenon occurs when biopolymers carry opposing net charges, thus facilitating formation of biopolymer complex and solvent-rich liquid phases. The physicochemical properties of the complex coacervates depend on intrinsic and extrinsic factors such as the biopolymer characteristics (e.g., type, molecular weight, charge density), system composition (concentration, mixing ratio), solvent conditions (e.g., solvent type, pH, ionic strength), and environmental factors (e.g., temperature) [7]. Changing intrinsic and extrinsic factors affected the complex coacervation behavior in mixtures composed of pea proteins and different types of polysaccharides [11,12,13,14,15,16,17,18,19]. Even within the same class of polysaccharides such as pectins, the differences in the intrinsic pectin characteristics changed the complex formation behavior [13, 15,16,17,18,19], demonstrating that the behavior is biopolymer specific. The previous complex coacervation studies on pea protein – pectin mixtures included the use of low- and high-methoxyl pectins derived mainly from citrus [13, 15, 16, 19, 21], whereas only a few studies with sugar beet [16, 18] or apple pectins [16] exist. Therefore, this study focused on using apple pectin to investigate the complex coacervation and precipitation with pea proteins to gain more insights into their behavior, especially to identify opportunities for improving the colloidal stability of pea proteins which are known to be prone to precipitation. This is especially important as the structural variations of pectins from different sources can improve or alter the complex formation and complex characteristics.
Briefly, the pectin structures typically vary between structures comprising linear homogalacturonan, branched rhamnogalacturonan (I, II) and/or xylogalacturonan backbones esterified with different types and number of carboxyl, methyl, and acetyl groups [22, 23]. For detailed descriptions of pectin structures, the reader is referred to literature [22, 23]. Apple pectin contains substantial segments of highly branched rhamnogalacturonans-I and II and xylogalacturonans as well as high amounts of neutral sugars (15–30%), making it a rather compact structure [22,23,24]. In comparison, citrus pectin is made of up to 90% linear homogalacturonans with some rhamnogalacturonans-I and minor segments of rhamnogalacturonans-II, but contains low amounts of neutral sugars (≤ 1%) [22, 25]. This less branched structure makes it less compact than apple pectin [24]. Sugar beet pectin, on the other hand, typically contains up to 45% highly branched rhamnogalacturonans-I [22] with a high content of neutral sugars (~80%) and ferulic acid [26]. The homogalacturonan regions rich in acetyl groups are typically unbranched, and the overall galacturonic acid content is lower (~55–58%) in sugar beet pectin than in apple and citrus pectins [27].
In this study, we used a commercially available apple pectin product. In general, one of the advantages of using pectins for complex coacervation is that they are widely used in food industry as gelling and thickening agents [28, 29], and different pectin types are available in commercial scale. In addition, we used a commercially available pea protein isolate product that was further processed to remove the insoluble protein fraction. The obtained water-soluble fraction entitled soluble pea proteins was investigated for its complex coacervation behavior with high-methoxyl apple pectin upon changing the pH (3–7), biopolymer ratio (r = 2:1 to 10:1) and temperature (25 and 85 °C). The total biopolymer concentration was kept constant in a dilute region at 1% (w/w). This biopolymer concentration was selected to allow a sufficient number of biopolymers to increase the probability of polymer-polymer interactions, but still allow a free movement of the molecules to undergo associative phase separation. The pH range was selected to assess the influence of different biopolymer net charge properties on the complexation behavior beyond and close to the isoelectric point (pI) of the protein. We expected that the highest electrostatic attraction and highest yield of complex coacervates would be at pH slightly below the pI of the proteins due to the high charge difference between the anionic pectin and cationic patches of the pea proteins. In case of the protein-polysaccharide ratio, we hypothesized that low protein concentrations will limit the number of positive charges available for the electrostatic interactions with the negatively charged pectin, and the electrostatic attraction will be low or not sufficient in forming stable biopolymer complexes. At high protein concentrations, on the other hand, enough positive charges are present to neutralize the negatively charged pectin molecules, which leads to associative phase separation and formation of large particles. Heating above the denaturation temperature of the proteins was hypothesized to induce protein unfolding and increase protein aggregation that would increase hydrophobic interactions, and lead to formation of smaller biopolymer complexes.
Materials & Methods
Materials
Pea protein isolate (Pisane C9) with a dry matter content of 95.7% was obtained from Cosucra (Groupe Warcoing S.A., Warcoing, Belgium), and contained 66.9 ± 0.4% proteins, 9.1 ± 0.1% moisture, 5.9 ± 0.1% ash, and 0.4 ± 0.0% fat, and had a mineral composition of 0.002% Al, 0.041% Ca, 0.016% Fe, 0.192% K, 0.045% Mg, 0.001% Mn, 1.643% Na, 0.820% P, 0.816% S, and 0.006% Zn. Apple pectin donated by Herbstreith & Fox KG (Neuenbürg, Germany) had a 62% degree of esterification (DE), a molecular weight of 73.7 kDa, and a galacturonic acid content of 74%. Hydrochloric acid, sodium hydroxide, and sodium azide (purity ≥ 99%) were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Double distilled water was used for the preparation of all solutions.
Fractionation of pea protein isolate
An aqueous pea protein isolate solution (5% w/w, pH 7.4) was hydrated at 2-5 °C for 4–8 h followed by twice performed centrifugation at 17,000 rpm at 4 °C for 30 min (Avanti J-30 I, Beckmann Coulter GmbH, Krefeld, Germany). The fractionation was carried out at the native pH of 7.4 of the pea protein isolate to (i) ensure enhanced solubility at a pH above the pI of the proteins as known from literature [8, 30] and verified by the solubility and net charge data (Fig. 1a,b), and (ii) ensure an easy and straightforward process. The supernatant was collected and freeze-dried in a Sublimator 15 freeze dryer (Zirbus technology GmbH, Bad Grund, Germany) to obtain a powder that is referred to as ‘soluble pea proteins’. The soluble pea proteins contained 57.7 ± 0.6% protein, 9.8 ± 0.1% water, 16.1 ± 0.1% ash comprising 0.001% Al, 0.079% Ca, 0.012% Fe, 0.584% K, 0.105% Mg, 0.001% Mn, 4.855% Na, 1.943% P, 1.690% S, and 0.005% Zn, and 1.0 ± 0.3% fat. The rest (~15%) of the unidentified solids in the soluble pea proteins may be attributed to carbohydrates such as starch or fiber found in pea [31, 32].
Chemical Composition Analysis of Apple Pectin
The apple pectin was analyzed for protein content by determining elemental nitrogen (Dumatherm N Pro, Gerhardt GmbH & Co. KG, Königswinter, Germany) according to Dumas method [33] by applying a nitrogen-to-protein conversion factor of N x 6.25. The ash and fat content was analyzed according to AS § 64 LFGB 06.00–4 and AS § 64 LFGB 06.00–6, respectively [34], mineral composition and quantification according to methods of 2.1.3 [35] and 8.10 [36], respectively, and total sulfur according to ISO 15178:2000 [37].
Preparation of Individual Biopolymer Solutions
Aqueous solutions of pea protein isolate, soluble pea proteins, and apple pectin (0.5% w/w) were stirred at 25 °C for 4–8 h. After hydration, the pH of the solutions was adjusted to pH 3.0–7.0 using 0.1 and/or 1 M sodium hydroxide or hydrochloric acid solutions.
Formation of Biopolymer Complexes
Pea protein isolate, soluble pea proteins, and apple pectin (1% w/w) were dissolved in water containing 0.02% sodium azide to prevent microbial growth, and stirred at 25 °C for 4–8 h. The hydrated protein and pectin solutions were initially adjusted to pH 7.0 using 0.1 or 1 M sodium hydroxide, and stirred for 30 min. Next, the biopolymer solutions were mixed at protein-to-pectin ratios r of 2:1, 4:1, 6:1, 8:1, 10:1 to obtain a final biopolymer concentration of 1% (w/w), and stirred another 30 min. Then the mixtures were adjusted to pH 3.0–7.0. Additional tests were also carried out for heat treated biopolymer mixtures. For this, 30 mL of sample was inserted in a 50 mL Schott bottle with a closed screw cap, and placed in a water bath (85 °C) for 20 min, and stirred manually a few times during the set time. Then the samples were cooled down under stirring for 1–2 h until they reached room temperature.
Protein Solubility
The pea protein isolate and soluble pea protein solutions (0.5% w/w) at pH 3–7 were centrifuged at 20,000 rpm for 30 min (Z 32 HK, Hermle Labortechnik GmbH, Wehingen, Germany). The protein content of the supernatant was measured by applying the Dumas combustion method using a Dumatherm N Pro device (Gerhardt GmbH & Co. KG, Königswinter, Germany). A nitrogen-to-protein conversion factor of 5.36 [38] was used. The protein solubility was calculated as follows (Eq. 1):
ζ-Potential
The samples were diluted 1:10 with appropriate pH-adjusted water to prevent multiple scattering effects. Electrophoretic light scattering (Nano ZS, Malvern Instruments, Malvern, UK) was used to measure the ζ-potential of the samples at 25 °C.
Transmittance
Transmittance of the samples was determined using a UV-Vis 8453 spectrophotometer (Hewlett Packard, Waldbronn, Germany) at 630 nm at 25 °C. Double distilled water was used as a blank.
Particle Size
Static light scattering (Horiba LA-950, Retsch Technology GmbH, Haan, Germany) was applied to determine the mean volume diameters (d 43) and particle size distributions of the samples that were diluted with appropriate pH-adjusted water. The refractive indices were set at 1.52 for the dispersed biopolymer phase and at 1.33 for the dispersant.
Optical Microscopy and Visual Observation
The microstructure of the samples was assessed by light microscopy using an Axio Scope A1 light microscope (Carl Zeiss AG, Jena, Germany) equipped with a Canon Powershot G10 digital camera (Canon, Tokyo, Japan) at magnification of 40x. Photographic images of the samples were taken after 24 h of preparation using an iPhone 6s (Apple Inc., Cupertino, CA, USA).
Statistical Analysis
All characterization measurements were performed in triplicate with freshly prepared solutions. Complex formation was performed with a freshly prepared mixture, and the measurements were performed twice with a minimum of three repetitions. The results are shown as means and standard deviations.
Results & Discussion
Characterization of individual biopolymers
Pea proteins. The soluble pea proteins obtained from the pea protein isolate by water fractionation showed a slightly improved protein solubility between pH 3 and pH 5 compared to the pea protein isolate, however, their solubility increased substantially at pH 6 and 7 (Fig. 1a), resulting in miscible solutions as demonstrated by the high levels of light transmission through the samples (Fig. 1c). At pH 3–5, the proteins were at or near their pI (Fig. 1b), which explains their low solubility (Fig. 1a) and high turbidity (Fig. 1c). At pI, the net charge of the proteins is zero, which favors protein-protein interactions over protein-water interactions [30], leading to formation of protein aggregates as corroborated by the transmittance measurements (Fig. 1c) and visual observations (data not shown). The pea protein isolate had a pI at pH ~ 4.6, whereas the soluble pea proteins had a lower pI at ~ 3.7 (Fig. 1b). This can be explained by the higher ionic strength (I) of 21 mM in the soluble pea protein solution (0.5% w/w) that concentrated up in the sample upon water fractionation compared to that of the pea protein isolate (I = 8 mM) and affected the charge properties via electrostatic screening. The ionic strength I was calculated from the mineral composition of the raw materials (6% minerals in pea protein isolate, and 16% minerals in the soluble pea proteins) as follows (Eq. 2):
where c i is the molar concentration of ion i (mol/m3), and z i is the charge number of the specific ion. Similar pH dependent behavior in protein solubility has been reported for pea proteins between pH 4 and 5 [8]. Adebiyi et al. [8] reported similarly high protein solubilities (80–90%) above the pI for water soluble pea protein fractions. They also reported that the protein solubility of the water-soluble pea protein fraction remained above 80% even at pH 3–5, however, the extraction included the use of 2% NaCl and a dialysis step.
Apple pectin. The compositional analysis showed that the apple pectin product contained – besides pectin − 8.8 ± 0.1 % water, 6.4 ± 0.0% minerals, 1.0 ± 0.1% protein, and 0.2 ± 0.1% fat. It had a mineral composition of 0.001% Al, 0.482% Ca, 0.007% Fe, 1.768% K, 0.017% Mg, 0.0003% Mn, 0.239% Na, 0.034% P, 0.001% S, and 0.0003% Zn. The 0.5% (w/w) aqueous solutions of apple pectin carried a negative charge over the whole pH range tested (Fig. 1b), which is typical for pectins due to their high number of galacturonic acid moieties (pKa = 3.4) [39]. The visual observations (data not shown) and transmittance measurements (Fig. 1c) showed that the aqueous pectin solutions were water soluble, which can be attributed to the charged galacturonic acid groups in the pectin molecules [28]. In addition, no sedimentation was observed in the 0.5% (w/w) aqueous apple pectin solutions.
Soluble Pea Protein – Apple Pectin Complexes
In this set of experiments, we investigated the influence of pH (3–7), protein-to-pectin ratio (r = 2:1 to 10:1), and temperature (25 and 85 °C) on the complexation behavior of soluble pea protein – apple pectin mixtures with a final biopolymer concentration set to 1% (w/w). State diagrams (Fig. 2) based on the test tube images (Fig. 3) were plotted to summarize the phase behavior of the samples.
Influence of pH
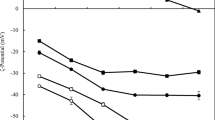
At pH 6–7, the soluble pea protein – apple pectin mixtures were miscible (Fig. 2a) and showed no aggregation, sedimentation or phase separation as determined by visual observation (Fig. 3), transmittance measurements (T = 20–65%), and optical microscopy imaging (Fig. 4a). This indicated that no electrostatic complex formation had occurred between the biopolymers or that the complexes formed were too small to be detected. This co-solubility can be attributed to the negatively charged soluble pea proteins (ζ = -32 to -33 mV) and apple pectin (ζ = -44 to -46 mV) at this pH range (Fig. 1b), resulting in overall electrostatic repulsion between the molecules that limited their association to only potential positive charged patches on the pea proteins. This was confirmed by the net negative charge of the biopolymer mixtures at pH 6 and 7 (Fig. 5a) that were in the similar range as the individual biopolymers or slightly intermediate between pectin and pea protein (Fig. 1b). Similar behavior has also been observed at pH 6–7 in pea protein isolate mixed with different types of pectins at specific mixing ratios [13, 16], alginate [12], chitosan [11], and gum arabic [14]. Although the soluble pea protein – apple pectin mixtures at pH 6–7 showed the presence of predominantly large particles with volume-based mean particle sizes (d 43) of around 300–780 μm (Figs. 5a and 6), the reliability of these measurements is poor as the static light scattering analysis based on Mie theory assumes the presence of particles with spherical shape [40]. In this case, the optical microscopy is better suited to determine the presence (and size) of materials with large aspect ratios. However, no presence of particulate matter was observed in the optical microscopy images besides some minor cellular particles (Fig. 4a), confirming that the samples were miscible.
Lowering the pH to 5 led to formation of turbid dispersions with or without sedimented layers (Figs. 2a and 3). This indicated association of the biopolymers due to electrostatic attraction between the pectin molecules (ζ = -41.5 ± 0.6 mV) and soluble pea proteins (ζ = − 19.1 ± 1.1 mV) (Fig. 1b), resulting in formation of biopolymer complexes with net charges between those of the individual biopolymers (Fig. 5a). Even though the soluble pea proteins had a net negative charge at pH 5 (Fig. 1b), the pea protein molecules carry an increasing number of positively charged amino groups upon lowering the pH closer to the pI of the protein due to protonation that allows electrostatic attraction to the negatively charged galacturonic acids in the pectin backbone. The existence of charged patches on the protein surface is more important for complex coacervation than the overall net charge [41]. The complex formation was also verified by the optical microscopy images showing the presence of some aggregates (Fig. 4a) and by transmittance analysis indicating that light could not pass through the highly turbid samples anymore (T = 0.01–0.02%). Increased turbidity and formation of soluble complexes around pH 5 has been reported for biopolymer mixtures comprising, for example, pea protein isolate and different pectins [15, 16], and pea protein isolate and alginate [12].
Upon lowering the pH to 4, the soluble pea protein – apple pectin mixtures formed stronger electrostatic complexes (ζ = -25 to -27 mV) (Fig. 5a) due to the stronger attractive forces between the almost zero-net charged (ζ = -3.3 ± 1.5 mV) soluble pea proteins close to their pI and negatively charged apple pectins (ζ = -29.3 ± 1.2 mV) (Fig. 1b). The stronger electrostatic attraction made the complexes also denser [42], and they began to sediment to a larger extent compared to pH 5 (Fig. 3). This was in agreement with the optical microscopy imaging, showing that more particles were present (Fig. 4a). In addition to the electrostatic interactions, water structuring via hydrogen bonding and hydrophobic interactions due to the presence of hydrophobic areas on the biopolymers can also play a role in increasing the density of the biopolymer complexes [42].
At pH 3, the biopolymer attraction was highest due to the opposite net charges (Fig. 1b), resulting in the formation of nearly electroneutral complexes (ζ = -4 to -8 mV) (Fig. 5a). At the pH of the electrical equivalence, the complex formation is at its optimum [3, 4]. Previous literature reported that the electroneutrality of pea protein isolate – pectin complexes occurred at pH 2.5–4.2 when the pI of the pea protein isolates was at pH ~ 4.6–4.8 [13, 16, 18]. The decreasing magnitude of the net negative charges on the biopolymer complexes upon lowering the pH (Fig. 5a) demonstrates that the protein aggregates are mostly encased by pectin molecules, which agrees with previous research on electrostatically associated protein-polysaccharide complexes [2, 6, 12,13,14, 16, 43]. Nevertheless, some proteins may also be attached on the surface of the pectin backbone as proposed previously [4, 6]. This also partly explains why the complexes can become electroneutral or positively charged at pH < < pI as the amino and acid groups in the proteins become increasingly protonated. The other reason is the protonation of the carboxyl groups in the polysaccharide at pH < pKa, resulting in the loss of the negative charges, which will also lead to dissociation of the complexes due to the loss of attractive electrostatic forces between the biopolymers.
Influence of Biopolymer Ratio
The data showed that the biopolymer ratio affected the complex behavior of the soluble pea protein – apple pectin mixtures at pH 3–5. At pH 6–7, no complexation occurred independent of the ratio applied (Figs. 2a and 3) as discussed in section “Influence of pH”. In a study by Lan et al., [13], on the other hand, the mixing ratio affected the phase behavior already at pH 6–7 when mixing 1% (w/w) pea protein isolate with 0.05-1% (w/w) high-methoxyl citrus pectin (DE 81%, galacturonic acid 38%, 110 kDa) at r = 1:1 to 20:1. For example, the biopolymer mixtures at r = 1:1 (ctotal = 2%) and 2:1 (ctotal = 1.5%) formed turbid solutions with a sedimented layer at pH 6.5-7 that the authors attributed to thermodynamic incompatibility [13]. On the other hand, transparent solutions were formed at r = 5:1 (ctotal =1.2%) to 10:1 (ctotal =1.1%) at pH 7, but lowering the pH to 6 resulted in formation of turbid biopolymer solutions [13]. This shows that the composition and properties of the individual biopolymers and applied conditions play a major role in phase behavior.
At pH 5, the pea protein – apple pectin mixtures at r = 2:1 and 6:1 showed the appearance more of a homogenous dispersion without distinct sedimented layers after 24 h (Fig. 3), suggesting that the formed biopolymer complexes may be more water soluble due to a looser complex structure attributed to weak electrostatic attraction. Indeed, the biopolymer complexes at r = 2:1 with a net charge of -37 ± 1 mV were the weakest electrostatic complexes among the samples (Fig. 5a). However, the net charges were not that different from the other biopolymer complexes at pH 5 (ζ = -34 to -36 mV) (Fig. 5a), indicating that all the biopolymer complexes at pH 5 were rather loosely bound. This homogenously turbid appearance of the biopolymer complexes at r = 2:1 showed the presence of polydisperse particles with small (d 43 ~ 200 nm), intermediate (d 43 ~ 40 μm) and large sizes (d 43 ~ 200 μm) (Fig. 6a). The optical microscopy images, however, showed no presence of large aggregates in the sample (Fig. 4a), which suggests that not all the biopolymer molecules at r = 2:1 had formed complexes, and the large sizes were due to hydrated and loosely-formed biopolymer clusters or due to protein aggregates that still had enough electrostatic forces to prevent extensive attraction to pectin molecules. The formation of such unstable soluble biopolymer complexes at pH 5 with high particle polydispersity has been reported for whey protein - citrus pectin complexes [44], which can be attributed to weak molecular interactions between the proteins and polysaccharides [5]. To our knowledge, no investigations on the particle sizes of pea protein – polysaccharide complexes are available. In the case of the biopolymer complexes at r = 6:1, only minor sedimentation occurred as the complexes had smaller mean particle sizes (d 43 = 25 ± 2 μm) (Fig. 5a) with more narrow particle size distribution compared to the other samples (Fig. 6c), which prevented gravitational separation. On the other hand, the biopolymer complexes at r = 4:1, 8:1, and 10:1 sedimented after 24 h, leaving a slightly turbid phase on top (Fig. 3). This behavior indicated that these biopolymer complexes had to be larger in size, making them more prone to gravitational separation. This was verified by the particle size measurements, showing narrowly distributed complexes (Fig. 6b,d-e) with mean sizes ranging from 33 μm at r = 4:1 to 69 μm at r = 10:1 to 81 μm at r = 8:1 (Fig. 5a).
At pH 4, the biopolymer complexes at r = 2:1 were smaller (d 43 = 21 ± 1 μm) (Fig. 5a) and less polydisperse compared to pH 5 (Fig. 6a), indicating that a lower pH was needed for complexation at this biopolymer ratio. This agrees with previous studies showing that the critical pH for forming either soluble or insoluble complexes was lower at lower pea protein-to-pectin ratios [13, 16]. This behavior has also been observed for other biopolymer complexes such as those between pea protein isolate and gum arabic [14], and pea protein isolate and alginate [12]. The reason for the biopolymer ratio dependance on the pH can be explained by self-aggregation of the proteins near their pI, and their subsequent association with individual polysaccharide molecules [14, 45]. The particle size distribution data of the pea protein – apple pectin mixtures at r = 2:1 revealed the presence of a minor number of small particles at ~ 0.45 μm and larger particles at ~23 μm (Fig. 6a) that may be attributed to differently sized biopolymer complexes. It is unlikely that these small particles were protein aggregates as the static light scattering measurements of the soluble pea proteins showed the formation of larger protein aggregates ranging between 10 and 500 μm sizes at pH 3–5 (data not shown). An earlier study on 0.4% whey protein isolate – pectin (DE 36%, 100 kDa, plant origin not given) complexes at r = 2:1 and at pH 4 reported the formation of mean complex sizes at ~ 25 and ~ 70 μm for mixtures acidified after or before mixing the biopolymers together, respectively [2]. However, no data on the polydispersity of the complex sizes was given [2]. The biopolymer complexes at other ratios formed either slightly bigger complexes (d 43 = 53 μm at r = 4:1; d 43 = 70 μm at r = 6:1) or the mean sizes did not change much (d 43 = 92 μm at r = 8:1; d 43 = 72 μm at r = 10:1) when compared to pH 5 (Figs. 5 and 6). At r = 8:1 and 10:1, the samples showed a bimodal particle size distributions with highest volume frequency peaks at ~ 20 and ~ 100 μm sizes. This suggests the formation of complexes with different sized protein aggregates embedded mostly in the “core” of the complex with pectin molecules bound around it or formation of flocs or aggregates of interacting complexes. These size variations were also visible in the optical microscopy images (Fig. 4a). Increasing the protein-to-polysaccharide mixing ratio has been reported to increase the amount of protein in whey protein – gum arabic coacervates [46]. The mixing ratio has also been shown to affect the polydispersity and size distribution of β-lactoglobulin – acacia gum complex coacervates at pH 4.2 [43] and whey protein – pectin complexes at pH 4.0 [6]. In the study of Krzemiski et al. [6], the authors suggested that the increasingly larger particles detected within the whey protein – pectin complexes from r = 2:1 to 5:1 to 8:1 were due to precipitated protein aggregates and lower availability of pectin to interact with the protein aggregates at increasing mixing ratios. Similar results were also reported in another study [16] showing that the turbidity of the pea protein isolate - pectin complexes (at pHopt) increased upon increasing the protein-to-pectin mixing ratio until a plateau was reached, with a subsequent decrease in the turbidity at higher mixing ratios that was attributed to less complex formation because of excess of proteins. Moreover, the turbidity changes as a function of mixing ratio were dependent on the pectin type applied and the turbidity generally increased with increasing the degree of methyl esterification and degree of blockiness of the pectin [16].
At pH 3, the particle sizes of the biopolymer complexes at r = 2:1 shifted to higher number of smaller sizes around 150 nm, with some larger particles still present (Fig. 6a). As these smaller particles were not affected by gravity, no sedimented layer was observed (Fig. 3a). The absence of large detectable particulate matter at r = 2:1 was also confirmed by the optical microscopy (Fig. 4a). In a study by Bedie et al. [2], mean complex sizes of 75–200 μm were reported for whey protein isolate – pectin (DE 36%, 100 kDa, no data on the plant origin was given) complexes at r = 2:1 at pH 3 depending on the sequence of mixing and pH adjustments. The soluble pea protein – apple pectin complexes at r = 4:1 to 10:1, however, showed increasing amount of sedimentation (Fig. 3), indicating the formation of increasing amounts of insoluble complexes at the pH around the electrical neutrality (Fig. 5a). This corroborated with the optical microscopy images showing the presence of more aggregates at pH 3 (Fig. 4a).
These results indicated that increasing the protein ratio from r = 2:1 to 10:1 enhanced the stability of the biopolymer complexes. This agrees with literature showing that increasing the protein concentration favored the formation of insoluble biopolymer complexes over the formation of soluble complexes [13].
Influence of Temperature
Heating is a commonly applied treatment in foods, and therefore it is important to assess the influence of elevated temperature on the biopolymer complexation. Heat treatment of the individual soluble pea proteins and apple pectin solutions showed negligible impact on their visual appearance and transmittance at the tested pH range of 3 to 7 compared to the unheated ones (data not shown). Upon heat treatment at 85 °C for 20 min, some differences in the complex behavior between the soluble pea proteins and apple pectin were observed.
At pH 6–7, the soluble pea protein – apple pectin mixtures after the heat treatment were co-soluble, and no sedimentation or phase separation was observed (Figs. 2b and 3). Nevertheless, some minor particulate matter was detected in the optical microscopy analysis (Fig. 4b). This particulate matter can most likely be attributed to either cellular particles as in the case of unheated samples, or formation of minor amounts of pea protein aggregates induced by denaturation upon heating [47]. The denaturation temperature for pea globulins has been reported to be around 78.5–82.4 °C [47]. Denaturation with subsequent unfolding of the protein structure can affect the optical appearance (i.e. turbidity) of the solutions [30], even in solutions at pH > pI [5]. However, the turbidity at pH 7 and 6 actually decreased upon heat treatment of the biopolymer mixtures by 6 and 32% at r = 2:1, by 29 and 36% at r = 4:1, by 38 and 56% at r = 6:1, by 0 and 32% at r = 8:1, and by 0 and 17% at r = 10:1, respectively, when compared to the unheated mixtures. This suggests that either small protein aggregates formed which scattered less light, or the effect originated from the pectins. In pectins, some degree of depolymerization due to β-elimination, and demethoxylation can occur already at 80-90 °C as was observed for high-methoxyl apple pectin (DE 78%) solutions at pH 7 (in 100 mM sodium phosphate buffer) heated for 160 min [48]. Depolymerization of the pectins may therefore explain the slight decrease in turbidity in the heated biopolymer mixtures as it would decrease the light scattering. Furthermore, the net charges of the heat-treated biopolymer mixtures at pH 6–7 were only slightly less negative (ζpH 7 = -31 to -37 mV; ζpH 6 = -35 to -38 mV) than those of the unheated biopolymer mixtures (ζpH 7 = -36 to -41 mV; ζpH 6 = -35 to -39 mV) (Fig. 5), indicating that heating had very little influence on the net charges, and therefore on the strength of the electrostatic repulsion. Therefore, any interactions between the biopolymers can be mainly attributed to increased hydrophobic interactions due to the exposure of more hydrophobic groups upon unfolding of the protein structures [49]. Overall, the results indicated that the heat treatment did not have a major impact of the miscibility of the biopolymer complexes or induced any complex formation at pH 6–7.
At pH 5, the complex formation between the soluble pea proteins and apple pectin was more pronounced than in the unheated samples (Fig. 3). The net charges of the heated biopolymer complexes did not differ from the unheated ones (Fig. 5), which indicated that the hydrophobic interactions played an additional role in the complex formation. At r = 2:1, the heat treatment did not have a major impact on the visual appearance of the biopolymer complexes as illustrated with the one-phase dispersion (Fig. 3), indicating the presence of soluble complex coacervates. This was verified by the optical microscopy images showing that no aggregates were present (Fig. 4b). The heat treatment also did not influence the mean particle size (Fig. 5b), and the complexes remained highly polydisperse (Fig. 6a). At r = 4:1, the optical appearance of the biopolymer mixture after heating was similar to the unheated one, displaying a two-phase system with a sedimented biopolymer complex layer and a soluble biopolymer complex phase on top (Fig. 3). The heat treatment slightly increased the mean particle sizes of biopolymer complexes at r = 4:1 from 33 μm (25 °C) to 49 μm (85 °C) (Fig. 5). The optical microscopy images confirmed that some larger aggregates were present in the sample after heating compared to the unheated system (Fig. 4). At r = 6:1 to 10:1, on the other hand, a clear phase separation to a biopolymer-rich and solvent-rich phases was detected after heat treatment (Fig. 3). At r = 6:1, the biopolymer complexes increased in size after heating from 25 μm (25 °C) to 73 μm (85 °C) (Fig. 5), that was also evident from the particle size distribution data (Fig. 6c) and optical microscopy images (Fig. 4b). Subsequently, they were also prone to sedimentation (Fig. 3). At r = 8:1 and 10:1, the mean particle size and size distribution of the soluble pea protein – apple pectin complexes did not change substantially after the heat treatment (Figs. 5 and 6).
At pH 4 − 3, heating did not influence the biopolymer complexation at r = 2:1, and the complexes remained polydisperse (Fig. 6a) and showed the appearance of homogenously turbid dispersion similar to before heating (Fig. 3). This agreed with the optical microscopy images showing the absence of aggregates (Fig. 4b), thus indicating the presence of soluble complexes. At r = 4:1, the heat treatment led to formation of some larger complexes (d 43 = 90–100 μm) (Figs. 5b and 6b) that sedimented more than the unheated ones (Fig. 3). This agreed with the optical microscopy images that showed the presence of more particulates (Fig. 4b), indicating the presence of insoluble complexes. At r = 6:1, the properties of the biopolymers complexes at pH 4 did not substantially change after heat treatment (Figs. 4b, 5b and 6c). At pH 3, on the other hand, the biopolymer complexes at r = 6:1 phase separated into clear biopolymer-rich and solvent-rich phases (Fig. 3), indicating that the complexes became either denser or larger. The static light scattering data showed that the mean size of the complexes at pH 3 increased from 45 (25 °C) to 95 μm (85 °C) upon heat treatment (Fig. 5). This was in agreement with the microstructure which showed the presence of more aggregates (Fig. 4b). At r = 8:1 and 10:1, the heat treatment did not lead to any major changes in the particle sizes and net charges (Fig. 5b), or microstructure (Fig. 4b). On the other hand, the test tube images showed that the biopolymer complexes at pH 4 had more soluble complexes present than before heat treatment (Fig. 3).
Previous studies on protein-polysaccharide complexes have shown that heating often results in formation of submicron sized complexes, especially those comprising milk proteins and pectins [1, 5] which is in contrast to our results. Nevertheless, heating may still result in larger than submicron sizes upon changing the mixing ratios of the biopolymers as demonstrated in heated (90 °C, 5 min) whey protein – citrus pectin (DE 38%) complexes with a constant protein concentration of 0.5% [6]. The authors observed submicron sized complexes at r = 5:1, however, at r = 2:1 and 8:1 larger complex sizes of 10 to 100 μm were present whether using low (DE 38%) or high-methoxyl (DE 70%) citrus pectins that were attributed to interpolymeric complexation [6]. It should be noted that the behavior of complex coacervates comprising milk proteins and different types of polysaccharides cannot be directly compared to pea proteins and pectin used in this study due to the differences in biopolymer structures, compositions and physicochemical properties. To our knowledge, however, no previous studies on heat-treated complexes made of pea proteins and pectins exist. It is possible that the generally higher molecular weight and hydrophobicity of the pea protein components such as globulins (~ 175–360 kDa) and some of the albumins (~ 5–80 kDa) [10] can restrict the formation of smaller particles compared to the more hydrophilic milk proteins with smaller molecular weights (α-lactalbumin ~ 14 kDa, β-lactoglobulin ~ 18 kDa, caseins ~ 19–25 kDa) [50].
Overall, the heat treatment increased the amount of insoluble biopolymer complexes at pH 3–5 at all ratios, except at r = 2:1 (Fig. 3). The biopolymer complex sizes also became slightly larger at r = 2:1 to 6:1, whereas only minor particle size changes were observed at r = 8:1 to 10:1 (Figs. 5 and 6). This can be attributed to increased hydrophobic interactions between the non-polar sections of the biopolymer complexes upon heating, thus leading to slightly more aggregated structures (Fig. 4) and slightly larger sizes (Fig. 5). The effect was less pronounced at higher ratios (r = 8:1 and 10:1) as more protein was available to interact via hydrophobic interactions already before heat treatment. The heat treatment can also have less of an impact on the complexes, if the proteins are already denaturated. Indeed, the pea protein isolate became denaturated during its processing as stated by the manufacturer. On the other hand, it is less likely that the heat-treatment had a major impact on the high-methoxyl apple pectins as they have been shown to be quite stable at elevated temperatures of 80-90 °C, showing negligible degree of depolymerization and demethoxylation at pH 2–5 as well as lack of acid hydrolysis at pH 2–3 [48]. Heating, however, can induce hydrophobic interactions between the nonpolar methoxyl groups of pectins and nonpolar patches of proteins [3, 29].
Conclusions
This study demonstrated that that the pH and biopolymer ratio affected the associative phase separation of soluble pea protein- high-methoxyl apple pectin mixtures as hypothesized. At pH 6–7, the biopolymer-solvent interactions dominated due to electrostatic repulsion, keeping the biopolymers in solution. At pH 5, the electrostatic attraction between the biopolymers overcame the biopolymer-solvent interactions, thereby inducing biopolymer complexation. Formation of insoluble complexes became more prominent upon lowering the pH further, but also by increasing the ratio to a higher protein concentration. Heat treatment, on the other hand, had less an effect on the biopolymer complex sizes opposed to what was hypothesized, as the complexes remained similarly sized or even became larger compared to the unheated ones and is in contrast to previous research on complex coacervation of milk proteins showing formation of typically submicron sized complexes. Knowledge of such behavior is especially important during food processing to control the stability of the food products. Furthermore, this study is the first to give insights into the sizes of pea protein – polysaccharide complexes. Overall, more research is needed to understand the complex formation with a larger set of plant-based proteins.
Availability of data and material
Not applicable.
Code Availability
Not applicable.
References
H. Salminen, J. Weiss, Food Biophys. 9, 29–38 (2014)
G.K. Bédié, S.L. Turgeon, J. Makhlouf, Food Hydrocoll. 22, 836–844 (2008)
C.L. Cooper, P.L. Dubin, A.B. Kayitmazer, S. Turksen, Curr. Opin. Colloid Interface Sci. 10, 52–78 (2005)
C.G. de Kruif, F. Weinbreck, R. de Vries, Curr. Opin. Colloid Interface Sci. 9, 340–349 (2004)
O.G. Jones, D.J. McClements, Adv. Colloid Interface Sci. 167, 49–62 (2011)
A. Krzeminski, K.A. Prell, J. Weiss, J. Hinrichs, Food Hydrocoll. 35, 332–340 (2014)
J. Weiss, H. Salminen, P. Moll, C. Schmitt, Adv. Colloid Interface Sci. 271, 101987 (2019)
A.P. Adebiyi, R.E. Aluko, Food Chem. 128, 902–908 (2011)
J. Boye, F. Zare, A. Pletch, Food Res. Int. 43, 414–431 (2010)
A. Singhal, A.C. Karaca, R. Tyler, M. Nickerson, Grain Legumes, ed. A., Goyal, (IntechOpen, 2016) p. 55–78
C. Elmer, A.C. Karaca, N.H. Low, M.T. Nickerson, Food Res. Int. 44, 1441–1446 (2011)
K.J. Klemmer, L. Waldner, A. Stone, N.H. Low, M.T. Nickerson, Food Chem. 130, 710–715 (2012)
Y. Lan, J.-B. Ohm, B. Chen, J. Rao Food Hydrocoll. 101, 105556 (2020)
S. Liu, N.H. Low, M.T. Nickerson, J. Agric. Food Chem. 57, 1521–1526 (2009)
Y. Lan, B. Chen, J. Rao, Food Hydrocoll. 80, 245–253 (2018)
S. Warnakulasuriya, P.K.S. Pillai, A.K. Stone, M.T. Nickerson, Food Chem. 264, 180–188 (2018)
A. Gharsallaoui, K. Yamauchi, O. Chambin, E. Cases, R. Saurel, Carbohydr. Polym. 80, 817–827 (2010)
Y. Lan, J.-B. Ohm, B. Chen, J. Rao Food Chem. 307, 125536 (2020)
P.K.S. Pillai, B.E. Morales-Contreras, L. Wicker, M.T. Nickerson, Food Chem. 305, 125433 (2020)
D.R. Klassen, M.T. Nickerson, Food Res. Int. 46, 167–176 (2012)
P.K.S. Pillai, Y. Ouyang, A.K. Stone, M.T. Nickerson, Food Sci. Technol. Int. 27, 3–12 (2020)
B.M. Yapo, D. Gnakri, in Polysaccharides: Bioactivity and Biotechnology, ed. by K.G. Ramawat, J.-M. Mérillon (Springer International Publishing, Cham, 2015), pp. 1729–1749
B.M. Yapo, Carbohydr. Polym. 86, 373–385 (2011)
A. Shpigelman, C. Kyomugasho, S. Christiaens, A.M. Van Loey, M.E. Hendrickx, Food Hydrocoll. 43, 189–198 (2015)
B.M. Yapo, P. Lerouge, J.-F. Thibault, M.-C. Ralet, Carbohydr. Polym. 69, 426–435 (2007)
G.A. Morris, M.-C. Ralet, E. Bonnin, J.-F. Thibault, S.E. Harding, Carbohydr. Polym. 82, 1161–1167 (2010)
C. Remoroza, H.C. Buchholt, H. Gruppen, H.A. Schols, Carbohydr. Polym. 101, 1205–1215 (2014)
L. Flutto, Encyclopedia of Food Sciences and Nutrition (Elsevier, 2003), pp. 4440–4449
B.R. Thakur, R.K. Singh, A.K. Handa, M.A. Rao, Crit. Rev. Food Sci. Nutr. 37, 47–73 (1997)
J.F. Zayas, Functionality of Proteins in Food (Springer, Berlin, Heidelberg, 1997), pp. 1–373
P. Colonna, D. Gallant, C. Mercier, J. Food Sci. 45, 1629–1639 (1980)
Z. Gao, P. Shen, Y. Lan, L. Cui, J.-B. Ohm, B. Chen, J. Rao, Food Res. Int. 131, 109045 (2020)
J. Dumas, Ann. Chim. Phys. 247, (1831)
BVL, Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB, § 35 vorläufiges Tabakgesetz, § 28b GenTG: Verfahren zur Probenahme und Untersuchung von Lebensmitteln, (Beuth Verlag, Berlin, 2011), pp. 1–10749
VDLUFA, M. Band, V.I.I. Umweltanalytik (VDLUFA-Verlag, Darmstadt, 2019) p. 1–690
VDLUFA, in, Methodenbuch Band II. 1 Die Untersuchung von Düngemittel (VDLUFA-Verlag, Darmstadt, 2007), pp. 1–1040
D.I.N. Standards, DIN ISO 15178:2001-02 (Beuth Verlag, 2001), pp. 1–5
F. Mariotti, D. Tome, P.P. Mirand, Crit. Rev. Food Sci. Nutr. 48, 177–184 (2008)
I.G. Plaschina, E.E. Braudo, V.B. Tolstoguzov, Carbohydr. Res. 60, 1–8 (1978)
G. Mie, Ann. Phys. 330, 377–445 (1908)
K. Kaibara, T. Okazaki, H.B. Bohidar, P.L. Dubin, Biomacromolecules. 1, 100–107 (2000)
V. Ducel, D. Pouliquen, J. Richard, F. Boury, Int. J. Biol. Macromol. 43, 359–366 (2008)
C. Schmitt, C. Sanchez, S. Despond, D. Renard, F. Thomas, J. Hardy, Food Hydrocoll. 14, 403–413 (2000)
C. Stenger, B. Zeeb, J. Hinrichs, J. Weiss, J. Dispers Sci. Technol. 38, 1258–1265 (2017)
F. Weinbreck, R. de Vries, P. Schrooyen, C.G. de Kruif, Biomacromolecules. 4, 293–303 (2003)
F. Weinbreck, R.H. Tromp, C.G. de Kruif, Biomacromolecules. 5, 1437–1445 (2004)
J.-L. Mession, N. Sok, A. Assifaoui, R. Saurel, J. Agric. Food Chem. 61, 1196–1204 (2013)
I. Fraeye, A. De Roeck, T. Duvetter, I. Verlent, M. Hendrickx, A. Van Loey, Food Chem. 105, 555–563 (2007)
S. Damodaran, in Food Chemistry, ed. by O.R. Fennema (Marcel Dekker, Inc., New York, 1996), pp. 321–429
P.F. Fox, in Advanced Dairy Chemistry – 1 Proteins Part A, ed. by P.F. Fox, P.L.H. McSweeney (Kluwer Academic/Plenum Publishers, New York, 2003), pp. 1–48
Funding
This study was funded by Société des Produits Nestlé S.A. (Lausanne, Switzerland).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Hanna Salminen: Conceptualization, Formal analysis, Visualization, Writing – original draft, review and editing, Project administration, Supervision. Melody Sachs: Investigation, Formal analysis, Visualization. Christophe Schmitt: Writing – review and editing, Funding. Jochen Weiss: Writing – review and editing, Resources, Overall supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salminen, H., Sachs, M., Schmitt, C. et al. Complex Coacervation and Precipitation Between Soluble Pea Proteins and Apple Pectin. Food Biophysics 17, 460–471 (2022). https://doi.org/10.1007/s11483-022-09726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-022-09726-x