Abstract
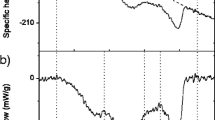
Shell-on eggs cooked by immersion in water at low and constant temperatures (∼60–70 °C) yield yolks with very particular textures. Structure development in such unique cooking conditions is far from understood. The present study shows that egg yolk, despite its compositional complexity, follows typical gelation kinetics found in many globular proteins and that it can develop structure at temperatures as low as 56 °C. It follows that yolk texture is dictated by time/temperature combinations. Under isothermal, low temperature cooking conditions, the thickening and gelation kinetics of egg yolk follow Arrhenius-type kinetic relationships. The energy of activation of these processes was ∼470 kJ mol−1, which agrees well with the values reported for the denaturation and gelation of the thermally labile chicken serum albumin and immunoglobulin Y. Results are related to common foodstuffs in order to allow chefs and home cooks to achieve a priori conceived textures in egg yolks.








Similar content being viewed by others
References
C. Vega, Egg yolk: A library of Textures, in The kitchen as a laboratory: science reflections inspired by the kitchen, ed. by C. Vega, J. Ubbink, E. van der Linden (Columbia University Press, New York, 2011)
C. Vega, J. Ubbink, Trends Food Sci. Technol. 19, 372–382 (2008)
D. Buay, S.K. Foong, D. Kiang, L. Kuppan, V.H. Liew, Eur. J. Phys. 27, 119–131 (2006)
P. Roura, P. Fort, J. Saurina, Eur. J. Phys. 21, 95–100 (2000)
P. Gadsby (2007) Cooking for eggheads. Discover magazine. Available at: http://discovermagazine.com/2006/feb/cooking-for-eggheads
H. This, Molecular Gastronomy: Exploring the Science of Flavor (Columbia University Press, New York, 2006)
K. Mann, M. Mann, Proteomics 8, 178–191 (2008)
M. Le Denmat, M. Anton, V. Beaumal, Food Hydrocolloids 14, 539–549 (2000)
V. Kiosseoglou, A. Paraskevopoulou, Food Hydrocolloids 19, 527–532 (2005)
F. Guilmineau, I. Krause, U. Kulozik, J. Agric. Food Chem. 53, 9329–9336 (2005)
D.K. Dixon, O.J. Cotterill, J. Food Sci. 46, 981–983 (1981)
M. Le Denmat, M. Anton, G. Gandemer, J. Food Sci. 64, 194–197 (1999)
N. Matsudomi, K. Ito, Y. Yoshika, Biosci. Biotechnol. Biochem. 70, 836–842 (2006)
P.F. Predki, C. Harford, P. Brar, B. Sarkar, Biochem. J. 287, 211–215 (1992)
C.W. Heizman, G. Muller, E. Jenny, K.J. Wilson, F. Landon, A. Olomucki, Proc. Natl Acad. Sci. USA 78, 74–77 (1981)
C. Giancola, C. De Sena, D. Fessas, G. Graziano, G. Barone, Int. J. Biol. Macromol. 20, 193–204 (1997)
Y. Moriyama, E. Watanabe, K. Kobayashi, H. Harano, E. Inui, K. Takeda, J. Phys. Chem. B 112, 16585–16589 (2008)
N. Matsudomi, D. Rector, J.E. Kinsella, Food Chem. 40, 55–69 (1991)
A. Tobitani, S.B. RossMurphy, Macromolecules 30, 4845–4854 (1997)
X. Cao, J. Li, X. Yang, Y. Duan, Y. Liu, C. Wang, Thermochim. Acta 467, 99–106 (2008)
S. Baier, J. McClements, J. Agric. Food Chem. 49, 2600–2608 (2001)
M. Shimizu, H. Nagashima, K. Hashimoto, Comp. Biochem. Physiol. B Biochem. Mol. Biol. 106, 255–261 (1993)
H.E. Indyk, J.W. Williams, H.A. Patel, Int. Dairy J. 18, 359–366 (2008)
A.W.P. Vermeer, W. Norde, Biophys. J. 78, 394–404 (2000)
A.W.P. Vermeer, C.E. Giacomelli, W. Norde, Biochimica et Biophysica Acta (BBA)-General Subjects 1526, 61–69 (2001)
D.J. Oldfield, H. Singh, M.W. Taylor, K.N. Pearce, Int. Dairy J. 8, 311–318 (1998)
E. Lichan, A. Kummer, J.N. Losso, D.D. Kitts, S. Nakai, Food Res. Int. 28, 9–16 (1995)
C. D. H. Williams (2010) The science of boiling an egg. Available at: http://newton.ex.ac.uk/teaching/CDHW/egg/
S. Stølen, J. Vedde, Kunsten å koke et egg (2010) Available at: http://www.kjemi.uio.no/publikum/popularkjemi/egg/
S.L. Polley, O.P. Snyder, P. Kotnour, Food Technol. 34, 76–91 (1980)
S. Almonacid, R. Simpson, A. Teixeira, J. Food Sci. 72, E508–E517 (2007)
S. Denys, J.G. Pieters, K. Dewettinck, J. Food Eng. 63, 281–290 (2004)
H. Yamashita, J. Ishibashi, Y.H. Hong, M. Hirose, Biosci. Biotechnol. Biochem. 62, 593–595 (1998)
M.A.M. Hoffmann, J.C. van Miltenburg, P.J.J.M. Van Mil, Thermochim. Acta 306, 45–49 (1997)
C. Le Bon, T. Nicolai, D. Durand, Macromolecules 32, 6120–6127 (1999)
E. Doi, Trends Food Sci. Technol. 4, 1–5 (1993)
A.H. Clark, G.M. Kavanagh, S.B. Ross-Murphy, Food Hydrocolloids 15, 383–400 (2001)
W.S. Gosal, S.B. Ross-Murphy, Curr. Opin. Colloid Interface Sci. 5, 188–194 (2000)
F. Cordobes, P. Partal, A. Guerrero, Rheologica Acta 43, 184–195 (2004)
M. Anton, M. Le Denmat, V. Beaumal, P. Pilet, Colloids Surf., B 21, 137–147 (2001)
F. Chambon, H.H. Winter, J. Rheol. 31, 683–697 (1987)
N.R. Pollen, C. Daubert, P. Prabhasankar, M. Drake, M.L. Gumpertz, J. Texture Stud. 35, 643–657 (2004)
A. Guerrero, J. Carmona, I. Martinez, F. Cordobes, P. Partal, Rheologica Acta 43, 539–549 (2004)
T. Tsutsui, J. Food Sci. 53, 1103–1106 (1988)
R. Nakamura, T. Fukano, M. Taniguchi, J. Food Sci. 47, 1449–1453 (1982)
J.M. Aguilar, F. Cordobes, A. Jerez, A. Guerrero, Rheologica Acta 46, 731–740 (2007)
F. Cordobes, J.A. Carmona, I. Martinez, P. Partal, A. Guerrero, Gums and Stabilizers for the Food Industry 12 (Springer, Berlin, 2004)
S. Jayaraman, D. Gantz, O. Gursky, Biochemistry 44, 3965–3971 (2005)
F. Speroni, M.C. Puppo, N. Chapleau, M. de Lamballerie, O. Castellani, M.C. Anon, M. Anton, J. Agric. Food Chem. 53, 5719–5725 (2005)
P. Barham, L.H. Skibsted, W.L.P. Bredie, M. Bom Frøst, P. Møller, J. Risbo, P. Snitjær, L.M. Mortensen, Chem. Rev. 110, 2313–2365 (2010)
J. Unsworth, F.J. Duarte, Am. J. Phys. 47, 981–983 (1979)
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The analytical solution of the dynamic process of heating by conduction of a sphere was provided by Unsworth and Duarte.51 The temperature at the centre of the sphere is given by
where λ = π2 α/r 2; α is the thermal diffusivity, and r is the radius of the sphere. Buay et al.3 expanded Eq. 1 for prolate spheroids not too different than a sphere, using the equivalent radius r e instead of r.
where 2a and 2b are the major and minor axes, and β = arcsin(e)/e, where e is the ellipticity \( \left( {e = \sqrt {{1 - {b^2}/{a^2}}} } \right) \).
Rights and permissions
About this article
Cite this article
Vega, C., Mercadé-Prieto, R. Culinary Biophysics: on the Nature of the 6X°C Egg. Food Biophysics 6, 152–159 (2011). https://doi.org/10.1007/s11483-010-9200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-010-9200-1