Abstract
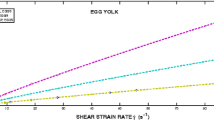
The evolution of native egg yolk undergoing a thermal-induced sol-gel transition was studied by using temperature controlled small amplitude oscillatory shear measurements. The critical gel point was determined according to Winter’s criterion: 1) from the measurements of storage and loss moduli as a function of heating time at different frequencies, and 2) from the exponents of the power law mechanical spectra obtained after cure experiments performed up to a maximum temperature (60–90 °C) followed by a sudden decrease in temperature up to 20 °C. Differential Scanning Calorimetry (DSC) was performed in order to investigate thermal transitions in egg yolk. Microstructure of gels was evaluated by Transmission and Scanning Electron Microscopy. The results obtained were discussed in terms of the processes involved in protein gelation: change in the protein system, aggregation of partially denaturated protein molecules and association of aggregates. As a result, an elastic gel network was always obtained. The influence of frequency, heating rate, solids concentration and maximum temperature of processing, was analysed. Most of the transformations found during thermal processing were found to be basically irreversible, even at the sol state and gel point. However, some reversible phenomena were detected during constant temperature processing depending on the maximum temperature performed.











Similar content being viewed by others
References
Adam M, Lairez D (1996) In: Cohen Addad JP (ed) Physical properties of polymeric gels. Wiley, New York, pp 88–139
Aymard P, Gimel JC, Nicolai T, Durand D (1996) Experimental evidence for a two-step process in the aggregation of β-lactoglobulin at pH 7. J Chim Phys 93:987–997
Bauer R, Carrotta R, Rischel C, Ogendal L (2000) Characterization and isolation of intermediates in b-lactoglobulin heat aggregation at high pH. Biophys J 79:1030–1038
Bell LN, Touma DE (1996) Glass transition temperatures determined using a temperature-cycling differential scanning calorimeter. J Food Sci 61:807–810, 828
Belloque J, Smith GM (1998) Thermal denaturation of β-lactoglobulin. a 1H-NMR study. J Agric Food Chem 46:1805–1813
Bourauoi M, Nakai S, Li-Chan E (1997) In situ Investigation of protein structure in Pacific whiting surimi and gels using Raman spectroscopy. Food Res Int 30:65–72
Boye JI, Ma CY, Ismail A, Harwalkar VR, Kalab M (1997) Molecular and microstructural studies of thermal denaturation and gelation of β-lactoglobulins, A and B. J Agric Food Chem 45:1608–1618
Burley RW, Cook WH (1961) Isolation and composition of avian egg yolk granules and their constituent α- and β-lipovitellins. Can J Biochem Physiol 39:1295–1307
Cabana A, Aït-Kadi A, Juhász J (1997) Study of the gelation process of polyethylene oxide-polypropylene oxide copolymer (Poloxamer 407) aqueous solutions. J Colloid Interface Sci 190:307–312
Chambon F, Winter HH (1987) Linear viscoelasticity at the gel point of a crosslinking PDMS with imbalanced stoichiometry. J Rheol 31:683–697
Chang CM, Powrie WD, Fennema O (1977) Microstructure of egg yolk. J Food Sci 42:1193–1200
Clark AH (1998) In: Hill SE, Ledward DA, Mitchell JR (eds) Functional properties of food macromolecules. Aspen Publishers, Gaithersburg, pp 77–142
Clark AH, Lee-Tuffnell CD (1986) In: Mitchell JR, Ledward AD (eds) Functional properties of food macromolecules. Elsevier Applied Science, Barking, pp 203–272
Clark AH, Saunderson DHP, Suggett A (1981a) Infrared and laser-Raman spectroscopic studies of thermally-induced globular proteins. Int J Peptide Protein Res 17:353–364
Clark AH, Judge FJ, Richards JB, Stubbs JM, Suggett A (1981b) Electron microscopy of network structures in thermally-induced globular protein gels. Int J Peptide Protein Res 17:380–392
Clark AH, Kavanagh GM, Ross-Murphy SB (2001) Globular protein gelation-theory and experiment. Food Hydrocolloid 15:383–400
Cocard S, Tassin JF, Nicolai T (2000) Dynamical mechanical properties of gelling colloidal disks. J Rheol 44:585–594
Damodaran S (1997) In: Damodaran S, Paraf A (eds) Food proteins and their applications. Marcel Dekker, New York, pp 1–24
de Gennes PG (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca
Di Gioia L, Cuq B, Guilbert S (1999) Thermal properties of corn gluten meal and its proteic components. Int J Biological Macromol 24:341–350
Doi E (1993) Gels and gelling of globular proteins. Trends Food Sci Technol 4:1–5
Donovan JW, Mapes CJ, Davis JG, Garibaldi JA (1975) A differential scanning calorimetric study of the stability of egg white to heat denaturation. J Sci Food Agric 26:73–83
Doublier JL, Launay B, Cuvelier G (1992) In: Rao MA, Steffe JF (eds) Viscoelastic properties of foods. Elsevier Applied Science, London, pp 371–434
Egelandsdal B, Fretheim K, Harbirt O (1986) Dynamic rheological measurements on heat-induced myosin gels: an evaluation of the method’s suitability for the filamentous gels. J Sci Food Agric 37:944–954
Fernández-Martín F, Fernández P, Carballo J, Jiménez Colmenero F (1997) Pressure/heat combinations on pork meat batters: protein thermal behavior and product rheological properties. J Agr Food Chem 45:4440–4445
Ferry JD (1948) Protein gels. Adv Protein Chem 4:1–78
Fox, PF, Mulvihill DM (1990) In: Harris P (ed) Casein in food gels. Elsevier Applied Science, London, pp 121–173
Gilsenan PM, Ross-Murphy SB (2000) Viscoelasticity of thermoreversible gelatin gels from mammalian and piscine collagens. J Rheol 44:871–883
Gimel JC, Durand D, Nicolai T (1994) Structure and distribution of aggregates formed after heat-induced denaturation of globular-proteins. Macromolecules 257:583–589
Harrison LJ, Cunningham FE (1986) Influence of frozen storage time on properties of salted yolk and its functionality in mayonnaise. J Food Quality 9:167–174
Hatta H, Kitabatake N, Doi E (1986) Turbidity and hardness of a heat-induced gel of hen egg ovoalbumin. Agric Biol Chem 50:2083–2089
Hegg P(1982) Conditions for the formation of heat-induced gels of some globular food proteins. J Food Sci 47:1241–1244
Hsu S (1999) Rheological studies on gelling behavior of soy protein isolates. J Food Sci 64:136–140
Hsu S, Lu S, Huang C (2000) Viscoelastic changes of rice starch suspensions during gelatinization. J Food Sci 65:215–220
Kavanagh GM, Clark AH, Ross-Murphy SB (2000) Heat-induced gelation of globular proteins. 4. Gelation kinetics of low pH β-lactoglobulin gels. Langmuir 16:9584–9594
Kinsella JE (1976) Functional properties of proteins in foods: a survey. CRC Crit Rev Food Sci Nutr 7:219–280
Kiosseoglou VD, Sherman P (1983) The influence of egg yolk lipoproteins on the rheology and stability of O/W emulsions and mayonnaise. Colloid Polym Sci 261:502–507
Lairez D, Adam M, Raspaud E, Emery JR, Durand D (1992) Do local motions influence rheological properties near the gelation threshold? Progr Colloid Polym Sci 90:37–42
Langton M, Hermansson AM (1992) Fine-stranded and particulate gels of β-lactoglobulin and whey-protein at varying pH. Food Hydrocolloid 5:523–539
Le Bon C, Nicolai T, Durand D (1999a) Growth and structure of aggregates of heat-denatured β-lactoglobulin. Int J Food Sci Technol 34:451–465
Le Bon C, Nicolai T, Durand D (1999b) Kinetics of aggregation and gelation of globular proteins after heat-induced denaturation. Macromolecules 32:6120–6127
Martin WG, Augustyniak J, Cook W H (1964) Fractionation and characterization of the low-density lipoproteins of hen’s egg yolk. Biochim Biophys Acta 84:714–720
McCully KA, Mok CC, Common RH (1962) Paper electrophoresis characterization of proteins and lipoproteins of hen’s yolk, Can J Biochem Physiol 40:937–952
Michon C, Cuvelier G, Launay B (1993) Concentration dependence of the critical viscoelastic properties of gelatin at the gel point. Rheol Acta 32:94–103
Miranda J, Cordobés F, Partal P, Guerrero A (2002) Rheological characterization of egg yolk processed by spray-drying and lipid-cholesterol extraction with CO2. J Am Oil Chem Soc 79:183–190
Ndi EE, Swanson BG, Barbosa-Canovas GV, Luedecke LO (1996) Rheology and microstructure of β-lactoglobulin/sodium polypectate gels, J Agric Food Chem 44:86–92
Nicolai T, Urban C, Schurtenberger P (2001) Light scattering study of turbid heat-set globular protein gels using cross-correlation dynamic light scattering. J Colloid Interface Sci 240:419–424
Nishinari K (1997) Rheological and DSC study of sol-gel transition in aqueous dispersions of industrially important polymers and colloids. Colloid Polym Sci 275:1093–1107
Noble RC (1987) In: Well RG, Belyavin CG (eds) Egg quality—current problems and recent advances. Poultry Science Symposium Series 20. Butterworths, London, pp 159–191
Oakenfull D, Pearce J, Burley RW (1997) In: Damodaran S, Paraf A (eds) Food proteins and their applications. Marcel Dekker, New York, pp 111–142
Pilosof AMR (2000) In: Pilosof AMR, Bartholomai GB (eds) Caracterización funcional y estructural de proteínas. Eudeba, Buenos Aires, pp 75–95
Puppo MC, Añon MC (1998) Structural properties of heat-induced soy protein gels as affected by ionic strength and pH. J Agric Food Chem 46:3583–3589
Puppo MC, Añon MC (1999) Soybean protein dispersions at acid pH. Thermal and rheological properties. J Food Sci 64:50–56
Qi XL, Holt C, McNulty D, Clarke DT, Brownlow S, Jones GR (1997) Effect of temperature on the secondary structure of β-lactglobulin at pH 6.7, as determined by CD and IR spectroscopy: a test of the molten globule hypothesis. Biochem J 324:341–346
Richardson, PH Norton IT (1998) Gelation behavior of concentrated locust bean gum solutions. Macromol 31:1575–1583
Richardson RK, Ross-Murphy SB (1981) Mechanical properties of globular protein gels. I. Incipient gelation behaviour. Int J Biol Macromol 3:315–322
Ross-Murphy SB (1991) Incipient behaviour of gelatin gels. Rheol Acta 30:401–411
Sánchez C, Burgos J (1997) Gelation of sunflower globulin hydrolysates: rheological and calorimetric studies. J Agric Food Chem 45:2407–2412
Scanlan JC, Winter HH (1991) Composition dependence of the viscoelasticity of end-linked poly(dimethylsiloxane) at the gel point. Macromolecules 24:47–54
Shay JS, Raghavan SR, Khan SA (2001) Thermoreversible gelation in aqueous dispersions of colloidal particles bearing grafted PEO chains. J Rheol 45:913–927
Shenstone FS (1968) In: Carter TC (ed) Egg quality: a study of the hen’s egg. Oliver and Boyd, Edinburgh, pp 26–66
Standing M, Langton M, Hermansson AM (1992) Inhomogeneous fine-stranded β-lactoglubulin gels. Food Hydrocolloid 6:455–470
Standing M, Langton M, Hermansson AM (1995) Small and large deformation studies of protein gels. J Rheol 39:1445–1450
Stauffer D, Coniglio A, Adam M (1982) Gelation and critical phenomena. Adv Polym Sci 44:103–158
Te Nijenhuis K (1981) Investigation into the aging process in gels of gelatin/water systems by the measurements of their dynamic moduli. I. Phenomenology. Colloid Polym Sci 259:522–535
Te Nijenhuis K, Winter HH (1989) Mechanical properties at the gel point of a crystallizing poly(vinylchloride) solution. Macromolecules 22:411–414
Tobitany A, Ross-Murphy SB (1997) Heat-induced gelation of globular proteins. 1. Model for the effects of time and temperature on the gelation time of BSA gels. Macromolecules 30:4845–4854
Vardhanabhuti B, Foegeding EA, McGuffey MK, Daubert CR, Swaisgood HE (2001) Gelation properties of dispersions containing polymerized and native whey protein isolate. Food Hydrocolloid 15:165–175
Verheul M, Roefs SPFM (1998) Structure of particulate whey protein gels: effect of NaCl concentration, pH, heating temperature, and protein composition. J Agric Food Chem 46:4909–4916
Winter HH (1987) Can the gel point of a cross-linking polymer be detected by the G′–G″ crossover? Polym Eng Sci 27:1698–1702
Winter HH, Chambon F (1986) Analysis of the linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol 30:367–382
Woodward SA (1990) In: Harris P (ed) Food gels. Elsevier Science Publishers, Barking, pp 175–199
Wright DJ (1986) In: Hudson BJF (ed) Developments in food proteins, vol 4. Applied Science Publishers, London, pp 61–90
Acknowledgments
This work is part of a research project sponsored by the CICYT, Spain (research project ALI 99-0502). The authors gratefully acknowledge its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cordobés, F., Partal, P. & Guerrero, A. Rheology and microstructure of heat-induced egg yolk gels. Rheol Acta 43, 184–195 (2004). https://doi.org/10.1007/s00397-003-0338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-003-0338-3