Abstract
Time perception received growing interest in psychiatry for its psychopathological implications. Cannabis use can cause a subjective experience of temporal perception alteration and increases the risk of emergence of mental illnesses such as psychotic and mood disorders. In this framework, we systematically reviewed the findings regarding the clinical, cognitive, and neurobiological correlates of time alterations due to cannabis consumption. According to preclinical results, cannabis exerts a dose-dependent time overestimation, associated with motor inhibition and circadian alterations. Clinical results reported that cannabis impair time estimation and time reproduction abilities, causing subjective temporal fragmentation and depersonalization symptoms. The alteration of timing mediated by cannabis use might depend on a dopaminergic indirect action and on structural, functional, and metabolic alterations of the cerebello-thalamo-cortical circuit. Despite the potential interest, however, only few studies explored the link between cannabis-induced alterations of time processing and psychiatric symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cannabis is among the most widespread, popular substances worldwide (Degenhardt et al., 2013). Among its various psychotropic effects, cannabis has long been known to change the perception of time. Time perception is a fundamental dimension of experience, and its relationship with psychopathology is well-recognized (Amadeo et al., 2022; Kent et al., 2023). Given the evidence that suggests the potential implications of cannabinoids compound on mental health (Escelsior et al., 2020), this review aims to summarize the available evidence on cannabis, time perception, and psychopathology.
The Endocannabinoid System and Cannabis Effects
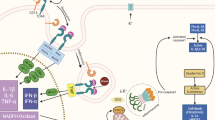
Cannabis is a plant that contains several psychotropic active compounds (Degenhardt et al., 2010). Among them, delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are of particular importance and display opposite effects in many domains: while Δ9-THC increase in pulse rate, alters time judgment, or might induce psychiatric outcomes like anxiety or psychosis, CBD, when administered together with Δ9-THC, prevents most of its adverse effects. In addition, CBD made the Δ9-THC effects more pleasurable for the subjects (Atakan, 2012). Cannabis mainly exerts its manifold effects on the body and brain through the endocannabinoid system (ECBs), which has been characterized only recently as a widespread system that regulates different neurobiological functions (Curran et al., 2016). The ECBs act as a neuromodulatory system that regulates the integration of internal stimuli with the perception of the external environment. In this framework, the ECBs modulates the sensation of pain and influences motivation, reward, emotional homeostasis, synaptic plasticity, learning, and memory; these levels of integration are essential for the adaptation of behavioral responses such as fearful reactions, anxiety, and stress response (Lutz et al., 2015; Lu and Mackie, 2016). The ECBs act mainly through the cannabinoid receptors CB1 and CB2 (Devane et al., 1988; Munro et al., 1993; Console-Bram et al., 2012). Whereas CB1 is expressed in different brain areas such as the cingulate gyrus, frontal cortex, secondary somatosensory and motor cortex, hippocampus, amygdala, basal ganglia, and cerebellum (Mackie, 2005), CB2 is is primarily expressed on immune system cells, including microglia and astrocytes, lymphocytes, macrophages, and NK cells (Matias and Di Marzo, 2007; Stella, 2010). Other receptors within the ECBs are transient receptor potential (TRP), especially TRPV1, G protein-coupled receptor 55 (GPR55), and peroxisome proliferator-activated receptor (PPAR) α and γ (Zygmunt et al., 2002; O’Sullivan, 2007; Ryberg et al., 2007; Puente et al., 2011; Escelsior et al., 2020). The ECBs receptors are the target for endogenous ligands such as anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) (Atsak et al., 2015; Curran et al., 2016; Lu and Mackie, 2016) or a plethora exogenous ligand like the Cannabis sativa phytocannabinoids Δ9-THC, cannabidiol, or Δ8-THC (Ashton, 2001; Console-Bram et al., 2012). The Δ9-THC exerts its action by activating presynaptic CB1 also inducing a differentiated modulation of other neurotransmitter systems (Fig. 1).
The intake of Cannabis sativa induces a wide variety of effects spanning from euphoria, time and space perceptual changes, decreased alertness and anxiety, analgesia, sedation, hunger and impairments in cognitive and psychomotor performance (Ashton, 2001; Andrade, 2016) to severe anxiety, panic, dysphoric states, manic or mixed episodes, paranoia, suicidal thoughts, and psychosis (Ashton, 2001; Henquet et al., 2006; Moore et al., 2007; Agrawal et al., 2011). Furthermore, a large amount of literature confirms that cannabis consumption can predict specific harmful behaviors, including suicide attempts, self-injurious behaviors (Escelsior et al., 2021), or restlessness and aggressive behaviors (Ashton, 2001; Curran et al., 2016), especially in subjects with a history of psychiatric disorders.
Among the diverse effects of cannabinoid compounds, alterations in time perception induced by cannabis are intriguing in terms of a potential link between fundamental perceptual changes and psychopathology. These were highlighted as early as the foundational study by Weil and colleagues, which was the first to objectively measure the effects of cannabis on time estimation. The study was conducted on nine marijuana näive subjects and specifically found that the main active constituent of cannabis, Δ9-THC, could modify subjective time perception in the range of seconds to minutes in a dose-dependent way (Weil et al., 1979). This finding first prompted a line of research that, however, has received little attention (Tinklenberg et al., 1976; McDonald et al., 2003).
Time Perception, Psychopathology, and Neurobiology
Our understanding of the world is based on a hierarchy of dimensions, whereby time holds the first level (Navon, 1978). One of the primary keys to survival and adaptation to the world is the ability to time the sequence of events and make predictions in a seconds-to-minute range (Meck and Benson, 2002). This capacity influences the reach of motor goals and, in humans, is crucial for organizing thoughts in language and communication (Atakan, 2012). Usually, the ability to “time” and coordinate actions and thoughts is pre-reflective and unconscious. Thus, the existence of an “internal clock” setting the pace and tuning peripheral clocks in several organs was postulated (Madison, 2001; Ivry and Schlerf, 2008). Drugs, organic and psychiatric diseases, context, and perceptual modalities can influence time perception, causing motor, emotional, behavioral, and cognitive disturbances (Coull et al., 2011).
Time Perception and Psychopathology
The distortion of the sense of time, with the loss of the regular continuity of past, present, and future, has been described in the twentieth century by phenomenologists as one central subjective experience of schizophrenia (Binswanger, 1960; Erwin Straus, 1960; Minkowski E., 1970; Blankenburg W, 1971; Kimura Bin, 1985; K. Jaspers, 1997; ELVEVÅG et al., 2003). More recently, the “cognitive dysmetria theory” postulated that cognitive and behavioral symptoms of schizophrenia might be underpinned by the alteration of the cerebello-thalamo-cortical-circuit, well known for its implication in temporal processes; this pattern of dysconnectivity would lead to the loss of the fluidity and temporal sequence of thoughts and actions characterizing “normal cognition and behavior” (Andreasen et al., 1998; Cao and Cannon, 2019; Escelsior et al., 2019).
Consistent with these theories, recent studies have confirmed that schizophrenia is associated with a wide range of difficulties in time measurement, including those related to time perception and time production (Carroll et al., 2009a, 2009b, 2009c; Bolbecker et al., 2014). Stanghellini and colleagues reported that the fragmentation of time experience (disruption of time flowing, déjà vu/vecu, premonitions about oneself and the external world) specifically characterizes anomalous time experience (ATE) in schizophrenia (Stanghellini et al., 2015). A recent meta-analysis showed that the perception of time in schizophrenia might be prolonged or shortened compared to objective time; this finding may reflect a more variable internal clock in patients with schizophrenia (Thoenes and Oberfeld, 2017). Similarly, disturbances in temporal processes are also found in individuals with high schizotypy scores or diagnosed with a prodromal syndrome (i.e., clinical high risk; CHR) and may constitute a specific vulnerability trait (Penney et al., 2005; Lee et al., 2006; Reed and Randell, 2014). Consistent with this hypothesis, a recent study found that individuals at clinical high risk for psychosis had poorer temporal accuracy compared with control subjects, and the degree of temporal inaccuracy was associated with abnormal connectivity in the cerebellar circuitry (Osborne et al., 2021).
Neurobiology of Time Perception
Time perception is thought to result from a network-like activity rather than the consequence of a single area’s activity (Droit-Volet and Meck, 2007; Gómez et al., 2014; Hass and Durstewitz, 2016). The neuronal processes of different temporal scales depend on the differential recruitment of a distributed brain network (Paton and Buonomano, 2018) that permits rapid adaptations of an organism to environmental temporal characteristics, thus modeling cognitive functions and motor activity (Praamstra, 2006; Avanzino et al., 2016). Two detailed reviews highlight the role of cortical activity localizing it in specific areas such as the dorsolateral prefrontal cortex (dlPFC), supplementary motor area (SMA), and right inferior frontal cortex (IFC) (Droit-Volet et al., 2013; Lake et al., 2016). Other brain areas involved in time perception are the cerebellum, left inferior insula, left putamen, and hippocampus (Droit-Volet et al., 2013; Gómez et al., 2014; Thoenes and Oberfeld, 2017; Ptacek et al., 2019). Time processing seems to rely on different areas or networks depending on the different durations, with cerebello-thalamo-cortical circuit mainly involved in sub-second intervals and cortico-striatal circuitry mainly involved in second-to-minute intervals processing (Schubotz et al., 2000; Pouthas and Macar, 2005; Wiener et al., 2010; van Wassenhove et al., 2011; Carvalho et al., 2016; Lošák et al., 2016; Apaydın et al., 2018; Mioni et al., 2020). The perception of time involves brain areas crucial for motor behavior. Therefore, damage in regions such as the cerebellum and basal ganglia can affect motor and perceptual timing, thus proving that spatial and temporal processing work together to shape accurate actions, thoughts, or emotions in specific temporal windows (Atakan, 2012).
Several neurotransmitter systems participate in that function, especially the dopaminergic (Droit-Volet et al., 2013; Gómez et al., 2014; Wiener et al., 2014a; Hass and Durstewitz, 2016; Lake et al., 2016; Thoenes and Oberfeld, 2017). Not surprisingly, among drugs, those affecting the dopaminergic activity seem to influence timing: particularly, dopamine-antagonists like first-generation antipsychotics (i.e., haloperidol) decrease the inner clock speed, while dopamine-agonists, including amphetamine, cocaine, or cannabis, increase it (Rammsayer and Vogel, 1992). Other examples of dopamine involvement in time perception come from studies on neurological conditions, such as Parkinson’s disease and Huntington’s chorea, which can alter time perception (O’Boyle et al., 1996; Rowe et al., 2010), as well as from pharmacological studies which reported improvements in time perception after tolcapone (COMT inhibitor that induces an increased dopaminergic tone in the PFC) administration (Mitchell et al., 2018) or genetic studies that reported a worsening in time perception performances associated to D2/ANKK1-Taq1a A1 polymorphism (Wiener et al., 2014b).
Aims and Objectives
Unlike the well-recognized role of cannabis exposure in promoting the onset of psychiatric symptoms and illnesses such as psychotic disorders, the field of cannabis-induced alterations of temporal perception and their psychopathological, cognitive, and behavioral correlates is still poorly characterized. Considering that distortions of time perception have been suggested to be a fundamental alteration linked to psychotic disorders, this systematic review aims to reassume and present the current evidence concerning the alteration of time perception induced by cannabis exposure and their cognitive, psychopathological, and neurobiological correlates.
Methods
Data Sources
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed for the present review (Page et al., 2021). A literature search on PubMed, Cochrane Library, and Embase with no year restriction was conducted on April 3, 2023. We searched for a combination of the following search string: “cannabi* AND (temporality OR “time estimation” OR “time perception” OR “time processing” OR timing OR “interval timing” OR “motor timing” OR “perceptual timing” OR “implicit timing” OR “explicit timing”).”
Eligibility Criteria
We sought to include all studies (1) written in English, (2) with an experimental or observational design, and (3) directly investigating the effects of cannabis on timing behavior in clinical and preclinical samples. Since the number/type of studies proved insufficient to conduct a meta-analysis, we extracted and tabulated data relative to sample characteristics, study design, type of intervention, outcome measures, and main findings. We excluded from data collection: (1) reviews and meta-analyses and (2) case reports. Titles and abstracts were screened for inclusion by two blinded researchers (A.E. and M.B.M.), who determined if the studies met the inclusion criteria. Manuscripts were assessed independently by the two researchers, and discrepancies were clarified by consent in a meeting with another researcher (G.S.). Firstly, the researchers screened articles by title and abstract and after by full text. Duplicates, review articles, and articles not fulfilling the search criteria were removed.
Results
Study Selection
Our search strategy yielded 1332 studies, out of which 38 satisfied our inclusion criteria, and full texts were screened (Fig. 2).
Study Characteristics
Table 1 reports study characteristics. Of these, 11 articles were about experiments conducted on animals (rats, mice, hamsters, rabbits, chimpanzees) (Carlini et al., 1974; Han and Robinson, 2001; Crystal et al., 2003; de Solages et al., 2008; Pietr et al., 2010; Sanford et al., 2008; Steinmetz and Freeman, 2010; Oleson et al., 2014). The remainder of the studies on human subjects included 3743 cannabis users, 1121 individuals who do not use cannabis, 68 occasional or non-smoking cannabis users, and 10 former cannabis users.
Study designs were the following: 37 studies had an experimental design, and one was observational (anonymous online survey) (Cuttler, Mischley, and Sexton 2016).
In most studies, marijuana cigarettes with different levels of Δ9-THC were used, although in some studies, CBD was assessed. Most of the studies did not consider the differences among the ways of administration: most of the studies used inhalation, others oral administration or i.v. administration. Placebo and alcohol were mainly used in controlled trials. A double-blind method was used in 9 of the studies. In clinical samples, most of the subjects involved were male healthy volunteers with cannabis frequency use going from occasional to regular.
Cognitive Assessments
Cognitive functions were assessed with Wechsler adult intelligence scale (WAIS) (Dornbush and Kokkevi, 1976; Mendhiratta et al., 1978), WAIS-revised (WAIS-R) (OʼLeary et al., 2003a), American National Adult Reading Test (ANART) (Boggs et al., 2018), national adult reading test (NART) (Sewell et al., 2013a), word association test (Borg et al., 1975), Cambridge Neuropsychological Test Automated Battery Rapid Visual Processing (CANTAB-RVP) (Boggs et al., 2018), Cambridge Neuropsychological Test Automated Battery Motor Screening Task (CANTAB-MOT) (Boggs et al., 2018), and full scale IQ (FSIQ) (Takagi et al., 2011).
Time Processing Assessments
The most common research modality we found assesses “interval timing,” which refers to perception and behavioral control with respect to time in the seconds to minutes range (Matell and McGovern, 2018). Most research on the effects of cannabis on timing has involved timing intervals using three different methods: (1) in “time estimation” tasks, the subject is asked to estimate how long an interval is (for example, 7 s); (2) in “time production” tasks, the subject is asked to produce the interval set by the experimenter (for example, 7 s); (3) in “time reproduction” tasks, the subject is asked to reproduce the interval presented by the interviewer (for example, counting out 7 s).
Time processing was assessed with eStroop task (Asmaro et al., 2014), standard Stroop task (TAKAGI et al., 2011), Go-NoGo task (TAKAGI et al., 2011), car simulator task (Bech et al., 1973), Grooved Pegboard (Boggs et al., 2018), paced fingertapping task (Boggs et al., 2018), simple reaction time task (SRT) (Borg et al., 1975), complex reaction time task (CRT) (Borg et al., 1975), time estimation task (TE) (Weil et al., 1979; Jones and Stone, 1970; Dornbush et al., 1971; Vachon et al., 1974; Borg et al., 1975; Dornbush and Kokkevi, 1976; Soueif, 1976; Han and Robinson, 2001; Wenzel and Cheer, 2014), time production task (TPT) (Carlini et al., 1974; Borg et al., 1975; Tinklenberg et al., 1976), corneal reflex (Carlini et al., 1974), rope climbing (Carlini et al., 1974), goal-directed serial alternation (Casswell and Marks 1973), typing (Chopra, 1973), A-B interresponse (Conrad et al., 1979), reaction time task (RT) (Dornbush et al., 1971; Mendhiratta et al., 1978), time perception task (TP) (Mendhiratta et al., 1978; Heishman et al., 1997), whisking task (Pietr et al., 2010), fixed consecutive number task (McLaughlin et al., 2017), temporal integration inventory (TII) (Melges, 1970), temporal extension inventory (Melges, 1971), pencil tapping task (Mendhiratta et al., 1978), speed and accuracy test (Mendhiratta et al., 1978), conditioned eyeblink response (Steinmetz and Freeman, 2010; Steinmetz et al., 2012a), fixed interval schedules (Oleson et al., 2014), continuous performance test (CPT) (Vachon et al., 1974), automated digit symbol substitution test (ADSST) (Vachon et al., 1974), digit symbol substitution test (DSST) (Weil et al., 1979; Jones and Stone, 1970; Borg et al., 1975).
Symptom Assessment
Psychiatric symptoms were assessed with Likert scale (Asmaro et al., 2014), Beck depression inventory version II (BDI-II) (Asmaro et al., 2014), state-trait anxiety inventory (STAI) (Asmaro et al., 2014), Structured Clinical Interview for DSM-IV Axis I (SCID-I) (Steinmetz et al., 2012a; Boggs et al., 2018), Structured Clinical Interview for DSM-IV Axis II (SCID-II) (Melges, 1970, 1971; Steinmetz et al., 2012a), Dixon depersonalization index (DDI) (Melges, 1970), Nowlis-Green mood adjective check list (MACL) (Melges, 1970, 1971), Maudsley personality inventory short scale (MPI) (Mendhiratta et al., 1978), Child Behavior Check List Youth Self Report (CBCL-YSR) (Takagi et al., 2011), and Positive and Negative Affect Schedule (PANAS) (Takagi et al., 2011).
Neuroimaging Assessment
O’Leary and colleagues used water-PET (OʼLeary et al., 2003b), whereas Klumpers et al. used RS-fMRI (Klumpers et al., 2012).
Effects of Cannabis on Time Perception in Preclinical Models
In their study, Carlini and colleagues found that, depending on its pharmacological potency, marijuana determines different levels of corneal areflexia, reduction of motor activity, and catatonic behaviors in rabbits (Carlini et al., 1974). Pietr and colleagues, while studying the effects of cannabinoids on whisking in rats, found that channels controlling amplitude, but not timing, were modulated by CB1 receptor (Pietr et al., 2010). Acuna-Goycolea, Obrietan, and van den Pol found that, besides the motor alteration, cannabinoid receptor activation markedly alters the capacity of light to entrain the core clock timing process. The release of endogenous cannabinoids (endocannabinoids) influences clock phasing in dark-adapted mice (Acuna-Goycolea et al., 2010). These results were replicated in hamsters (Sanford et al., 2008). Considering the alterations of motor and circadian responses induced by cannabis administration, it is unsurprising that different authors reported alterations of time perceptions in preclinical models. In this framework, an early study by Conrad and colleagues described a dose-dependent time overestimation induced by Δ9-THC oral administration in a population of chimpanzees with highly efficient and stable schedule-controlled timing behavior (Conrad et al., 1979). Similarly, in rats, the administration of WIN55,212-2, a CB1 receptor agonist, induced dose-related decreases in sensitivity to time (Crystal et al., 2003) and in conditioned blink response amplitude (Steinmetz and Freeman 2010). Opposite results of shortened and lengthened time responses were obtained respectively after administering CB1 receptor agonist or antagonist in rats (Han and Robinson, 2001). Similarly, McLaughlin et al. showed that the blockage of the CB1 receptor-induced underestimation of time perception (McLaughlin et al., 2017), whereas Oleson et al. demonstrated that CB1 receptor agonist-induced a dose-dependent increase in dopamine release and accelerated temporal processing in mice (Oleson et al., 2014). Using a differential reinforcement of low rate (DRL) task in rats, Wan-Ting Liao and colleagues demonstrated that activation of CB1 receptors in the medial entorhinal cortex (MEC) disrupts the functional connectivity between the hippocampal CA1 region and MEC, essential for the transmission of temporal-associated information (Liao et al., 2020).
Effects of Cannabis on Time Perception in Clinical Samples
In a PET study, O’Leary and colleagues assessed brain perfusion during an internal timing task before and after smoking marijuana and placebo cigarettes in 12 recreational cannabis users compared with a group of 12 daily users. After smoking cannabis, counting rates and self-paced tapping increased in both groups. This was also associated with increased blood flow in the cerebellum. This finding may indicate the existence of a single internal clock in the cerebellum that controls different activities, such as counting, and that this clock is affected by cannabis use (OʼLeary et al., 2003b). In an RS-fMRI study on healthy volunteers, Klumpers and colleagues found that Δ9-THC administration determines both increases and decreases in functional brain connectivity in brain regions with high densities of CB1 receptors, such as cerebellum and dorsal frontal cortical regions. These regions may be linked to Δ9-THC-induced CNS effects, such as postural stability, feeling high, and altered time perception (Klumpers et al., 2012).
In a study comparing the effects of alcohol and cannabis on the estimation of time and distance, Δ9-THC showed a more marked effect than alcohol on time estimation in a dose-dependent way. Specifically, the subjective estimation worsened further than the objective measurement (Bech et al., 1973).
Similarly, Carlini et al. compared the cognitive and behavioral effects of three samples of Brazilian marihuana with different ∆9-THC content, finding that the higher the Δ9-THC content, the higher the disruption of the time production task was (Carlini et al., 1974). In a study on recreational cannabis users, McDonald and colleagues found that, in a time estimation task, Δ9-THC increased estimates of the duration of short intervals while not affecting estimates of longer intervals (McDonald et al., 2003).
Boggs and colleagues found that the acute i.v. administration of Δ9-THC led to significant deficits in fine motor performance and timing, while gross motor performance was unaltered (Boggs et al., 2018). Other authors tested simple and complex reaction time, time estimation, and time production and found that cannabis significantly worsened subjects’ performances in all test scores (Casswell and Marks, 1973; Borg et al., 1975). Similarly, subjects that smoked cannabis took more time to complete a typing task (Chopra, 1973). Dornbush and colleagues produced two articles about the association between Δ9-THC and time: in the first one, they found that auditory or visual reaction time was positively related to Δ9-THC dose, while time estimation was unaffected (Dornbush et al., 1971); in the second study, they noticed a significant overestimation in a time estimation task (Dornbush and Kokkevi, 1976). Likewise, another article reported time overestimation in time estimation but not in time production tasks, although experienced users showed some sort of pharmacologic sensitization (Jones and Stone, 1970). Moreover, in one study, subjects had to judge time by duration production after smoking cigarettes with or without Δ9-THC; Δ9-THC increased the subjective time rate (Hicks et al., 1984a). In a study comparing the effects of Δ9-THC and cannabinol (CBN) on time perception and heart rate, Karniol and colleagues found that Δ9-THC produced increased heart rate and time underestimation, while CBN had no effect. In combination, the two drugs produced no further effect on heart rate; on the contrary, they induced significant overestimates and underestimates of the passage of 1 min (Karniol et al., 1975). Interestingly, Melges et al. reported that Δ9-THC impaired the results of cognitive tasks by causing, in a dose-dependent way, “temporal disintegration”: this expression refers to the difficulty of the individual in retaining, keeping track of, and sequencing memories, perceptions, and expectations that are relevant to his goals. Subjectively, this state is perceived as confusion of past, present, and future, thus contributing to the sensation of the self as unreal or weird typical of depersonalization, another common symptom of cannabis intoxication (Melges, 1970). Casswell and colleagues compared cannabis-experienced and naïve smokers: a temporal disintegration following cannabis abuse was found in both groups (Casswell and Marks, 1973). In another study on that topic, subjects exposed to oral marijuana reported in a time concentration inventory a discontinuous subjective experience of time passing with an overfocus on to present and a foreshortening of the awareness span into the future (Melges, 1971).
In contrast, other results reported that cannabis users were significantly more oriented toward the past (King and Manaster, 1975). Mendhiratta et al. found that subjects who smoked cannabis scored more poorly in reaction time and time perception tests (Mendhiratta et al., 1978). Similarly, another article reported that the current cannabis consumption impaired both the acquisition and the timing of the conditioned eyeblink reflex and that former cannabis utilizers still had shorter latencies. Thus, it has been demonstrated that heavy cannabis use negatively influences timing-related synaptic plasticity within the cerebellum (Steinmetz et al., 2012a). Sewell et al. reported that cannabis intake induced time overestimation and underproduction in a non-dose-dependent correlation; moreover, like Jones and colleagues previously reported, the authors found that chronic cannabis consumption did not alter baseline time perception (Sewell et al., 2013b). Previously, another paper found that cannabis use induced a significant time underproduction, corresponding to the increase in heart rate and the onset of subjective feelings of drug effects (Tinklenberg et al., 1976). These findings may indicate that Δ9-THC causes an increase in the internal clock speed. Likewise, Vachon and colleagues assessed a shorter time production due to Δ9-THC use (Vachon et al., 1974). Weil and colleagues reported increased time estimations after cannabis administration (Weil et al., 1979). Another article evidenced that cannabis use had a significant impact on every speed task and initial reaction time (Soueif, 1976). In contrast, Takagi and colleagues found no difference in reaction time between cannabis and a control group (Takagi et al., 2011). Similarly, in another study comparing the effects of alcohol and smoked marijuana effects, it was found that none of the drugs affected time perception and reaction time tests (Heishman et al., 1997).
Interestingly, indomethacin pre-treatment abolished the profound effect of Δ9-THC on time estimation and production, suggesting a role of prostaglandins in the neurophysiologic mechanisms responsible for some of the typical effects of Δ9-THC, particularly the alteration of time perception (Perez-Reyes et al., 1991a). Finally, in a study conducted through an online survey addressed to cannabis users, men participants reported a subjective alteration in time perception more frequently than women (Cuttler et al., 2016).
Discussion
Since the 1970s, studies have shown that cannabis can modify the experience of time, a very important part of how we experience the world. By modifying the perception of time, cannabis may elicit various forms of psychopathology. We reviewed the existing literature on the effects of cannabis use on time perception.
We found that cannabis use is associated with a distorted sense of time, and this alteration is strictly linked with impaired short-term memory, motor behavior, and decision-making. Studies evaluating time estimation evidence that cannabis intoxication, both in clinical and preclinical models, mainly conducts to time overestimation in a dose-dependent way. Otherwise, results on time production and reproduction failed to converge on homogeneous evidence. These latter inconclusive results may be attributed to the significant limitations of most of the studies. Some limitations specifically regard the study populations: in fact, most of the studies have small sample sizes; moreover, being participants mainly men volunteers, gender differences in cannabis-induced symptoms were not evaluated (Pattij et al., 2008; Prieto-Arenas et al., 2022); some studies included chronic cannabis users, not considering the potential impact of factors such as CB1 downregulation and tolerance (Weil et al., 1979; Dornbush and Kokkevi, 1976; Heishman et al., 1997; OʼLeary et al., 2003b), while others did not assess previous exposure (Dornbush et al., 1971; Karniol et al., 1975; Hicks et al., 1984b; Perez-Reyes et al., 1991b; McDonald et al., 2003).
In many studies, the pharmacokinetics of cannabinoids are not taken into account: despite the well-known nonlinear and biphasic effects of cannabinoids, in most of the research, only one dose was administered to participants (Jones and Stone, 1970; Vachon et al., 1974; Tinklenberg et al., 1976; Hicks et al., 1984b; Heishman et al., 1997; OʼLeary et al., 2003b). Notwithstanding, studies used different ways of administration, not considering that both inhalation and oral administration are associated with individual and intra-individual differences in pharmacokinetics. For example, oral administration is associated with lower pick plasma levels and protracted Δ9-THC effects compared to inhaled or i.v. administration (Ohlsson et al., 1980).
Other limitations concern timing tasks: time perception was evaluated only in a few studies using time estimation and time production tasks (Jones and Stone, 1970; Perez-Reyes et al., 1991a). Additionally, only a few studies used the prevention of “internal counting” techniques, which are helpful in increasing the accuracy of timing tasks (Weil et al., 1979; McDonald et al., 2003). Another considerable limit is the inability to discriminate, through simple timing tasks, the effects of Δ9-THC on clock, memory, and decision phases of temporal discrimination. Indeed, motor timing and time estimation may be indivisible functions, as they mainly involve the same brain regions (Ranganathan and D’Souza, 2006). Differentiating temporal stimuli may be necessary to disentangle the Δ9-THC effects on timing from those on memory and decision-making.
Unfortunately, only scarce literature explored the linkage between the cannabis-induced alterations of time processing and the emergence of specific psychiatric symptoms, such as depersonalization or psychosis (Melges, 1970).
Impairment in time processing seems to involve brain networks and areas rich in CB1 receptors, known to be implicated in the pathogenesis of schizophrenia. There is evidence that the endocannabinoid system is a widespread system implicated in executive functions by modulating dopaminergic and glutamatergic transmission on cortical and striatal networks (Pattij et al., 2008). Furthermore, it has been proposed that the interaction between the endocannabinoid system and DA receptors may indirectly influence time perception. Indeed, DA transmission has been linked to the speed of the internal clock (Cheng et al., 2016), with DA receptor agonists speeding it up and DA receptor antagonists slowing it down (Frederick, 1996; Meck, 2006). It has been found that cannabinoids induce an augmentation in dopamine concentrations within the striatum via CB1 and CB2 receptors influencing time perception (Oleson et al., 2014; Wenzel and Cheer, 2014). As previously pointed out, cannabis users and schizophrenia patients share the tendency to overestimate time intervals, probably induced by dopamine-related accelerated time procession (Droit-Volet and Meck, 2007). Acceleration of the internal clock has been related to the hypervigilant state typical of the delusional mood and the productive symptoms of psychosis (Peterburs et al., 2013; Lošák et al., 2016). Hypervigilance would lead to the “aberrant salience” phenomenon by inducing cognitive and perceptual biases in interpreting external stimuli.
In conclusion, further studies about the effects of cannabis on time perception and executive and cognitive functions are needed to understand better the neurobiology of timing and its implication in debilitating disorders such as schizophrenia. To this purpose, it would be worth studying how exactly dopamine exerts its actions in altering time perception, knowing which step is affected.
Moreover, cannabis-related timing alterations seem to be associated with alterations of synaptic plasticity (Steinmetz et al., 2012b), activity (Klumpers et al., 2012), and metabolism (OʼLeary et al., 2003) of the cerebellum. Given its widely recognized role both in schizophrenia and sub-second time perception, we believe it would be essential to investigate the role of the cerebello-thalamo-cortical circuit in temporal alterations, particularly in cannabis-induced psychosis.
These goals would not only be of significant theoretical value but would pave the way for new therapeutic strategies for treating schizophrenia based on the modulation of the endocannabinoid system.
References
Acuna-Goycolea, C., Obrietan, K., & van den Pol, A. N. (2010). Cannabinoids excite circadian clock neurons. The Journal of Neuroscience, 30, 10061–10066.
Agrawal, A., Nurnberger, J. I., & Lynskey, M. T. (2011). Cannabis involvement in individuals with bipolar disorder. Psychiatry Resesrch, 185, 459–461.
Amadeo, M. B., Esposito, D., Escelsior, A., Campus, C., Inuggi, A., Pereira Da Silva, B., Serafini, G., Amore, M., & Gori, M. (2022). Time in schizophrenia: A link between psychopathology, psychophysics and technology. Translational Psychiatry, 12, 331.
Andrade, C. (2016). Cannabis and neuropsychiatry, 1: Benefits and risks. The Journal of Clinical Psychiatry, 77, e551–e554.
Andreasen, N. C., Paradiso, S., & O’Leary, D. S. (1998). “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin, 24, 203–218.
Apaydın, N., Üstün, S., Kale, E. H., Çelikağ, İ., Özgüven, H. D., Baskak, B., & Çiçek, M. (2018). Neural mechanisms underlying time perception and reward anticipation. Frontiers in Human Neuroscience, 12.
Ashton, C. H. (2001). Pharmacology and effects of cannabis: A brief review. British Journal of Psychiatry, 178, 101–106.
Asmaro, D., Carolan, P. L., & Liotti, M. (2014). Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addictive Behaviors, 39, 114–121.
Atakan, Z. (2012). Cannabis, a complex plant: Different compounds and different effects on individuals. Ther Adv Psychopharmacol, 2, 241–254.
Atsak, P., Hauer, D., Campolongo, P., Schelling, G., Fornari, R. V., & Roozendaal, B. (2015). Endocannabinoid signaling within the basolateral amygdala integrates multiple stress hormone effects on memory consolidation. Neuropsychopharmacology, 40, 1485–1494.
Avanzino, L., Pelosin, E., Vicario, C. M., Lagravinese, G., Abbruzzese, G., & Martino, D. (2016). Time processing and motor control in movement disorders. Frontiers in Human Neuroscience, 10, 631.
Bech, P., Rafaelsen, L., & Rafaelsen, O. J. (1973). Cannabis and alcohol: Effects on estimation of time and distance. Psychopharmacologia, 32, 373–381.
Bin, K. (1985). Time and psychosis. In D. Kruger (Ed.), The changing reality of modern man: Essays in honour of J.H. van den Berg. Duquesne University Press.
Binswanger L (1960) Melancholie und Manie. phänomenologische Studien. (Pfullingen N, ed).
Blankenburg, W. (1971). Der Verlust der natürlichen Selbstverständlichkeit. Ein Beitrag zur Psycho- pathologie symptomarmer Schizophrenien ((Enke, ed). ed.).
Boggs, D. L., Cortes-Briones, J. A., Surti, T., Luddy, C., Ranganathan, M., Cahill, J. D., Sewell, A. R., D’Souza, D. C., & Skosnik, P. D. (2018). The dose-dependent psychomotor effects of intravenous delta-9-tetrahydrocannabinol (Δ9-THC) in humans. Journal of Psychopharmacology, 32, 1308–1318.
Bolbecker, A. R., Westfall, D. R., Howell, J. M., Lackner, R. J., Carroll, C. A., O’Donnell, B. F., & Hetrick, W. P. (2014). Increased timing variability in schizophrenia and bipolar disorder. PLoS One, 9, e97964.
Borg, J., Gershon, S., & Alpert, M. (1975). Dose effects of smoked marihuana on human cognitive and motor functions. Psychopharmacologia, 42, 211–218.
Cao, H., & Cannon, T. D. (2019). Cerebellar dysfunction and schizophrenia: From “cognitive dysmetria” to a potential therapeutic target. American Journal of Psychiatry, 176, 498–500.
Carlini, E. A., Karniol, I. G., Renault, P. F., & Schuster, C. R. (1974). Effects of marihuana in laboratory animals and in man. British Journal of Pharmacology, 50, 299–309.
Carroll, C. A., O’Donnell, B. F., Shekhar, A., & Hetrick, W. P. (2009a). Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain and Cognition, 71, 345–353.
Carroll, C. A., O’Donnell, B. F., Shekhar, A., & Hetrick, W. P. (2009b). Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain and Cognition, 70, 181–190.
Carvalho, F. M., Chaim, K. T., Sanchez, T. A., & de Araujo, D. B. (2016). Time-perception network and default mode network are associated with temporal prediction in a periodic motion task. Frontiers in Human Neuroscience, 10, 268.
Casswell S, Marks DF (1973) Cannabis and temporal disintegration in experienced and naive subjects. Science 179: (4075) 803–805
Cheng, R.-K., Tipples, J., Narayanan, N. S., & Meck, W. H. (2016). Clock speed as a window into dopaminergic control of emotion and time perception. Timing & Time Perception, 4, 99–122.
Chopra, G. S. (1973). Studies on psycho-clinical aspects of long-term marihuana use in 124 cases. International Journal of the Addictions, 8, 1015–1026.
Conrad, D. G., & Elsmore, T. F. (1979). Sodetz FJ (1972) Δ9-tetrahydrocannabinol: Dose-related effects on timing behavior in chimpanzee. Science, 175, 547–550.
Console-Bram, L., Marcu, J., & Abood, M. E. (2012). Cannabinoid receptors: Nomenclature and pharmacological principles. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 38, 4–15.
Coull, J. T., Morgan, H., Cambridge, V. C., Moore, J. W., Giorlando, F., Adapa, R., Corlett, P. R., & Fletcher, P. C. (2011). Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology, 218, 543–556.
Crystal, J. D., Maxwell, K. W., & Hohmann, A. G. (2003). Cannabinoid modulation of sensitivity to time. Behavioural Brain Research, 144, 57–66.
Curran, H. V., Freeman, T. P., Mokrysz, C., Lewis, D. A., Morgan, C. J. A., & Parsons, L. H. (2016). Keep off the grass? Cannabis, cognition and addiction. Nature Reviews Neuroscience, 17, 293–306.
Cuttler, C., Mischley, L. K., & Sexton, M. (2016). Sex differences in cannabis use and effects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res, 1, 166–175.
de Solages, C., Szapiro, G., Brunel, N., Hakim, V., Isope, P., Buisseret, P., Rousseau, C., Barbour, B., & Léna, C. (2008). High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron, 58, 775–788.
Degenhardt, L., Coffey, C., Carlin, J. B., Swift, W., Moore, E., & Patton, G. C. (2010). Outcomes of occasional cannabis use in adolescence: 10-Year follow-up study in Victoria, Australia. British Journal of Psychiatry, 196, 290–295.
Degenhardt, L., Whiteford, H. A., Ferrari, A. J., Baxter, A. J., Charlson, F. J., Hall, W. D., Freedman, G., Burstein, R., Johns, N., Engell, R. E., Flaxman, A., Murray, C. J., & Vos, T. (2013). Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. The Lancet, 382, 1564–1574.
Devane, W. A., Dysarz, F. A., Johnson, M. R., Melvin, L. S., & Howlett, A. C. (1988). Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology, 34, 605–613.
Dornbush, R. L., Fink, M., & Freedman, A. M. (1971). Marijuana, memory, and perception. American Journal of Psychiatry, 128, 194–197.
Dornbush, R. L., & Kokkevi, A. (1976). Acute effects of cannabis on cognitive, perceptual, and motor performance in chronic hashish users. Annals of the New York Academy of Sciences, 282, 313–322.
Droit-Volet, S., Fayolle, S., Lamotte, M., & Gil, S. (2013). Time, emotion and the embodiment of timing. Timing & Time Perception, 1, 99–126.
Droit-Volet, S., & Meck, W. H. (2007). How emotions colour our perception of time. Trends in Cognitive Sciences, 11, 504–513.
Elvevåg, B., Mccormack, T., Gilbert, A., GDA, B., Weinberger, D. R., & Goldberg, T. E. (2003). Duration judgements in patients with schizophrenia. Psychological Medicine, 33, 1249–1261.
Escelsior, A., Belvederi Murri, M., Calcagno, P., Cervetti, A., Caruso, R., Croce, E., Grassi, L., & Amore, M. (2019). Effectiveness of cerebellar circuitry modulation in schizophrenia. The Journal of Nervous and Mental Disease, 207(11), 977–986.
Escelsior, A., Belvederi Murri, M., Pietro, C. G., Serafini, G., Aguglia, A., Zampogna, D., Cattedra, S., Nebbia, J., Trabucco, A., Prestia, D., Olcese, M., Barletta, E., Pereira da Silva, B., & Amore, M. (2021). Cannabinoid use and self-injurious behaviours: A systematic review and meta-analysis. Journal of Affective Disorders, 278, 85–98.
Escelsior, A., Sterlini, B., Murri, M. B., Serafini, G., Aguglia, A., da Silva, B. P., Corradi, A., Valente, P., & Amore, M. (2020). Red-hot chili receptors: A systematic review of TRPV1 antagonism in animal models of psychiatric disorders and addiction. Behavioural Brain Research, 393, 112734.
Frederick, D. (1996). Effects of selective dopamine D1- and D2-agonists and antagonists on timing performance in rats. Pharmacology, Biochemistry, and Behavior, 53, 759–764.
Gómez, J., Jesús Marín-Méndez, J., Molero, P., Atakan, Z., & Ortuño, F. (2014). Time perception networks and cognition in schizophrenia: A review and a proposal. Psychiatry Research, 220, 737–744.
Han, C. J., & Robinson, J. K. (2001). Cannabinoid modulation of time estimation in the rat. Behavioral Neuroscience, 115, 243–246.
Hass, J., & Durstewitz, D. (2016). Time at the center, or time at the side? Assessing current models of time perception. Current Opinion in Behavioral Sciences, 8, 238–244.
Heishman, S. J., Arasteh, K., & Stitzer, M. L. (1997). Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacology, Biochemistry, and Behavior, 58, 93–101.
Henquet, C., Krabbendam, L., de Graaf, R., ten Have, M., & van Os, J. (2006). Cannabis use and expression of mania in the general population. Journal of Affective Disorders, 95, 103–110.
Hicks, R. E., Gualtieri, T., Mayo, J. P., Jr., & Perez-Reyes, M. (1984a). Cannabis, atropine, and temporal information processing. Neuropsychobiology, 12, 229–237.
Ivry, R. B., & Schlerf, J. E. (2008). Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences, 12, 273–280.
Jaspers, K. (1997). General psychopathology. JHU Press.
Jones, R. T., & Stone, G. C. (1970). Psychological studies of marijuana and alcohol in man. Psychopharmacologia, 18, 108–117.
Karniol, I. G., Shirakawa, I., Takahashi, R. N., Knobel, E., & Musty, R. E. (1975). Effects of delta9-tetrahydrocannabinol and cannabinol in man. Pharmacology, 13, 502–512.
Kent, L., Nelson, B., & Northoff, G. (2023). Can disorders of subjective time inform the differential diagnosis of psychiatric disorders? A transdiagnostic taxonomy of time. Early Intervention in Psychiatry, 17, 231–243.
King, M. R., & Manaster, G. J. (1975). Time perspective correlates of collegiate marijuana use. Journal of Consulting and Clinical Psychology, 43, 99–99.
Klumpers, L. E., Cole, D. M., Khalili-Mahani, N., Soeter, R. P., te Beek, E. T., Rombouts, S. A. R. B., & van Gerven, J. M. A. (2012). Manipulating brain connectivity with δ9-tetrahydrocannabinol: A pharmacological resting state FMRI study. Neuroimage, 63, 1701–1711.
Lake, J. I., LaBar, K. S., & Meck, W. H. (2016). Emotional modulation of interval timing and time perception. Neuroscience and Biobehavioral Reviews, 64, 403–420.
Lee, K.-H., Dixon, J. K., Spence, S. A., & Woodruff, P. W. R. (2006). Time perception dysfunction in psychometric schizotypy. Personality and Individual Differences, 40, 1363–1373.
Liao, W., Chang, C., & Hsiao, Y. (2020). Activation of cannabinoid type 1 receptors decreases the synchronization of local field potential oscillations in the hippocampus and entorhinal cortex and prolongs the interresponse time during a differential-reinforcement-of-low-rate task. European Journal of Neuroscience, 52, 4249–4266.
Lošák, J., Hüttlová, J., Lipová, P., Mareček, R., Bareš, M., Filip, P., Žůbor, J., Ustohal, L., Vaníček, J., & Kašpárek, T. (2016). Predictive motor timing and the cerebellar vermis in schizophrenia: An fMRI study. Schizophrenia Bulletin, 42, 1517–1527.
Lu, H.-C., & Mackie, K. (2016). An introduction to the endogenous cannabinoid system. Biological Psychiatry, 79, 516–525.
Lutz, B., Marsicano, G., Maldonado, R., & Hillard, C. J. (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nature Reviews Neuroscience, 16, 705–718.
Mackie, K. (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. In Cannabinoids (pp. 299–325). Springer-Verlag.
Madison, G. (2001). Variability in isochronous tapping: Higher order dependencies as a function of intertap interval. Journal of Experimental Psychology Human Perception and Performance, 27, 411–422.
Matell, M. S., & McGovern, D. J. (2018). Interval timing. In Encyclopedia of animal cognition and behavior (pp. 1–8). Springer International Publishing.
Matias, I., & Di Marzo, V. (2007). Endocannabinoids and the control of energy balance. Trends in Endocrinology and Metabolism, 18, 27–37.
McDonald, J., Schleifer, L., Richards, J. B., & de Wit, H. (2003). Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology, 28, 1356–1365.
McLaughlin, P. J., Jagielo-Miller, J. E., Plyler, E. S., Schutte, K. K., Vemuri, V. K., & Makriyannis, A. (2017). Differential effects of cannabinoid CB1 inverse agonists and antagonists on impulsivity in male Sprague Dawley rats: Identification of a possibly clinically relevant vulnerability involving the serotonin 5HT1A receptor. Psychopharmacology, 234, 1029–1043.
Meck, W. H. (2006). Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109, 93–107.
Meck, W. H., & Benson, A. M. (2002). Dissecting the brain’s internal clock: How frontal–striatal circuitry keeps time and shifts attention. Brain and Cognition, 48, 195–211.
Melges, F. T. (1970). Temporal disintegration and depersonalization during marihuana intoxication. Archives of General Psychiatry, 23, 204.
Melges, F. T. (1971). Marihuana and the temporal span of awareness. Archives of General Psychiatry, 24, 564.
Mendhiratta, S. S., Wig, N. N., & Verma, S. K. (1978). Some psychological correlates of long-term heavy cannabis users. British Journal of Psychiatry, 132, 482–486.
Minkowski, E. (1970). Lived time. Northwestern University.
Mioni, G., Grondin, S., Bardi, L., & Stablum, F. (2020). Understanding time perception through non-invasive brain stimulation techniques: A review of studies. Behavioural Brain Research, 377, 112232.
Mitchell, J. M., Weinstein, D., Vega, T., & Kayser, A. S. (2018). Dopamine, time perception, and future time perspective. Psychopharmacology, 235, 2783–2793.
Moore, T. H., Zammit, S., Lingford-Hughes, A., Barnes, T. R., Jones, P. B., Burke, M., & Lewis, G. (2007). Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. The Lancet, 370, 319–328.
Munro, S., Thomas, K. L., & Abu-Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365, 61–65.
Navon, D. (1978). On a conceptual hierarchy of time, space, and other dimensions. Cognition, 6, 223–228.
O’Boyle, D. J., Freeman, J. S., & Cody, F. W. J. (1996). The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain, 119, 51–70.
O’Sullivan, S. E. (2007). Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. British Journal of Pharmacology, 152, 576–582.
Ohlsson, A., Lindgren, J.-E., Wahlen, A., Agurell, S., Hollister, L. E., & Gillespie, H. K. (1980). Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology and Therapeutics, 28, 409–416.
OʼLeary, D. S., Block, R. I., Turner, B. M., Koeppel, J., Magnotta, V. A., Ponto, L. B., Watkins, G. L., Hichwa, R. D., & Andreasen, N. C. (2003). Marijuana alters the human cerebellar clock. Neuroreport, 14, 1145–1151.
Oleson, E. B., Cachope, R., Fitoussi, A., Tsutsui, K., Wu, S., Gallegos, J. A., & Cheer, J. F. (2014). Cannabinoid receptor activation shifts temporally engendered patterns of dopamine release. Neuropsychopharmacology, 39, 1441–1452.
Page, M. J., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Medicine, 18, e1003583.
Paton, J. J., & Buonomano, D. V. (2018). The neural basis of timing: Distributed mechanisms for diverse functions. Neuron, 98, 687–705.
Pattij, T., Wiskerke, J., & Schoffelmeer, A. N. M. (2008). Cannabinoid modulation of executive functions. European Journal of Pharmacology, 585, 458–463.
Penney, T. B., Meck, W. H., Roberts, S. A., Gibbon, J., & Erlenmeyer-Kimling, L. (2005). Interval-timing deficits in individuals at high risk for schizophrenia. Brain and Cognition, 58, 109–118.
Perez-Reyes, M., Burstein, S. H., White, W. R., McDonald, S. A., & Hicks, R. E. (1991a). Antagonism of marihuana effects by indomethacin in humans. Life Sciences, 48, 507–515.
Peterburs, J., Nitsch, A. M., Miltner, W. H. R., & Straube, T. (2013). Impaired representation of time in schizophrenia is linked to positive symptoms and cognitive demand. PLoS One, 8, e67615.
Pietr, M. D., Knutsen, P. M., Shore, D. I., Ahissar, E., & Vogel, Z. (2010). Cannabinoids reveal separate controls for whisking amplitude and timing in rats. Journal of Neurophysiology, 104, 2532–2542.
Pouthas, V., & Macar, F. (2005). Les bases neurales de la perception du temps et de la régulation temporelle de l’action. Psychologie Française, 50, 27–45.
Praamstra, P. (2006). Neurophysiology of implicit timing in serial choice reaction-time performance. Journal of Neuroscience, 26, 5448–5455.
Prieto-Arenas, L., Díaz, I., & Arenas, M. C. (2022). Gender differences in dual diagnoses associated with cannabis use: A review. Brain Sciences, 12, 388.
Ptacek, R., Weissenberger, S., Braaten, E., Klicperova-Baker, M., Goetz, M., Raboch, J., Vnukova, M., & Stefano, G. B. (2019). Clinical implications of the perception of time in attention deficit hyperactivity disorder (ADHD): A review. Medical Science Monitor, 25, 3918–3924.
Puente, X. S., et al. (2011). Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature, 475, 101–105.
Rammsayer, T. H., & Vogel, W. H. (1992). Pharmacologic properties of the internal clock underlying time perception in humans. Neuropsychobiology, 26, 71–80.
Ranganathan, M., & D’Souza, D. C. (2006). The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology, 188, 425–444.
Reed, P., & Randell, J. (2014). Altered time-perception performance in individuals with high schizotypy levels. Psychiatry Research, 220, 211–216.
Rowe, K. C., Paulsen, J. S., Langbehn, D. R., Duff, K., Beglinger, L. J., Wang, C., O’Rourke, J. J. F., Stout, J. C., & Moser, D. J. (2010). Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology, 24, 435–442.
Ryberg, E., Larsson, N., Sjögren, S., Hjorth, S., Hermansson, N.-O., Leonova, J., Elebring, T., Nilsson, K., Drmota, T., & Greasley, P. J. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152, 1092–1101.
Sanford, A. E., Castillo, E., & Gannon, R. L. (2008). Cannabinoids and hamster circadian activity rhythms. Brain Research, 1222, 141–148.
Schubotz, R. I., Friederici, A. D., & Yves von Cramon, D. (2000). Time perception and motor timing: A common cortical and subcortical basis revealed by fMRI. Neuroimage, 11, 1–12.
Sewell, R. A., Schnakenberg, A., Elander, J., Radhakrishnan, R., Williams, A., Skosnik, P. D., Pittman, B., Ranganathan, M., & D’Souza, D. C. (2013a). Acute effects of THC on time perception in frequent and infrequent cannabis users. Psychopharmacology, 226, 401–413.
Soueif, M. I. (1976). Differential association between chronic cannabis use and brain function deficits. Annals of the New York Academy of Sciences, 282, 323–343.
Stanghellini, G., Ballerini, M., Presenza, S., Mancini, M., Raballo, A., Blasi, S., & Cutting, J. (2015). Psychopathology of lived time: Abnormal time experience in persons with schizophrenia. Schizophrenia Bulletin, 42(1), 45–55.
Steinmetz, A. B., Edwards, C. R., Vollmer, J. M., Erickson, M. A., O’Donnell, B. F., Hetrick, W. P., & Skosnik, P. D. (2012a). Examining the effects of former cannabis use on cerebellum-dependent eyeblink conditioning in humans. Psychopharmacology, 221, 133–141.
Steinmetz, A. B., & Freeman, J. H. (2010). Central cannabinoid receptors modulate acquisition of eyeblink conditioning. Learning & Memory, 17, 571–576.
Stella, N. (2010). Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia, 58, 1017–1030.
Erwin Straus (1960) Das Zeiterlebnis in der endogenen Depression und in der psychopathischen Verstimmung (Psychologie der Menschlichen Welt, ed).
Takagi, M., Lubman, D. I., Cotton, S., Fornito, A., Baliz, Y., Tucker, A., & Yücel, M. (2011). Executive control among adolescent inhalant and cannabis users. Drug and Alcohol Review, 30, 629–637.
Thoenes, S., & Oberfeld, D. (2017). Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clinical Psychology Review, 54, 44–64 https://pubmed.ncbi.nlm.nih.gov/28391027/ [Accessed June 30, 2022]
Tinklenberg, J. R., Roth, W. T., & Kopell, B. S. (1976). Marijuana and ethanol: Differential effects on time perception, heart rate, and subjective response. Psychopharmacology, 49, 275–279.
Vachon, L., Sulkowski, A., & Rich, E. (1974). Marihuana effects on learning, attention and time estimation. Psychopharmacologia, 39, 1–11.
van Wassenhove, V., Wittmann, M., Craig, A. D. B., & Paulus, M. P. (2011). Psychological and neural mechanisms of subjective time dilation. Frontiers in Neuroscience, 5, 56.
Weil, A. T., & Zinberg, N. E. (1979). Nelsen JM (1968) Clinical and psychological effects of marihuana in man. Science, 162, 1234–1242.
Wenzel, J. M., & Cheer, J. F. (2014). Endocannabinoid-dependent modulation of phasic dopamine signaling encodes external and internal reward-predictive cues. Frontiers in Psychiatry, 5, 118.
Wiener, M., Lee, Y.-S., Lohoff, F. W., & Coslett, H. B. (2014a). Individual differences in the morphometry and activation of time perception networks are influenced by dopamine genotype. Neuroimage, 89, 10–22.
Wiener, M., Turkeltaub, P., & Coslett, H. B. (2010). The image of time: A voxel-wise meta-analysis. Neuroimage, 49, 1728–1740.
Zygmunt, P. M., Andersson, D. A., & Hogestatt, E. D. (2002). Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. The Journal of Neuroscience, 22, 4720–4727.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Escelsior, A., Trabucco, A., Radicati, M. et al. Clinical, Cognitive, and Neurobiological Correlates of Impaired Timing Abilities Associate to Cannabis Use: a Systematic Review. Int J Ment Health Addiction (2023). https://doi.org/10.1007/s11469-023-01125-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11469-023-01125-8