Abstract
The main goal of this work is to use a simplified idea to provide a scientifically accurate plasma device at a reasonable cost and benefit to society. It is an attempt to present designing and building one plasma system that can operate as three plasma experiments using the same instruments. These experiments are glow discharge, magnetron, and sputtering. This study focused on working the device as a DC glow discharge argon plasma device by a positive electrode made of a copper rod. The Paschen curve was measured at different distances between the cathode and the anode (4, 6, 8, 10, and 12 cm). From these results, the operating conditions of argon gas pressure and voltage that will produce plasma at each distance were determined. The investigated pressures were \(1 \times {10 }^{-1}\) and \(2 \times {10 }^{-1}\) mbar, while the operating voltage ranged from 110 to 160 V. The generated plasma was used to clean and polish the surface of two Egyptian fifty-piaster coins (still in circulation) at different exposure times as a practical application for the designed plasma device. After that, the results will be compared with cleaning and polishing a coin by a chemical method. The observed cleaning and polishing by the plasma is better and resists the environmental effect more than the chemical polishing method. This is related to the plasma treatment of the surface of the metal, while the chemical replaces the dirty layer as a result of reaction with it without treating the metal surface itself. The reversed effect of the sample (coins) on the plasma parameters (voltage and current) during its exposure time is considered one of the most novel included in this work. Besides, the temperature increases of the chamber surface during the working time were recorded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A typical DC discharge is configured with the negative cathode at one end and a positive anode at the other end, separated by a gas filled gap, placed inside a glass cylinder. A few hundred volts between the cathode and anode are required to maintain the discharge. The type of discharge that is formed between the two electrodes depends upon the pressure of the working gas, the nature of the working gas, the applied voltage, and the geometry of the discharge type [1]. The change in the geometry of the two electrodes is considered as the main novelty of this manuscript.
The low-pressure DC glow discharge plasma has a very distinctive appearance. The structures, the role of each region, and a discussion of the relationship between the discharge current and the applied voltage for the DC discharge can be found as examples in review articles by Druyvesteyn and Penning (1940), Francis (1956), and Ingold (1978). The discharge physics research is given in a brief review by Brown (1978), where some of the terminology used in discharge physics is explained [1].
The broad field of applications of plasma technologies involves several cleaning, purification, and sterilization processes of solid surfaces. DC glow discharge plasma has a wide range of applications in industry and material surface cleaning. Low-pressure plasma processes were applied for cleaning the surface of glass, metals, and polymers from contaminants such as air pollutants, fingerprints, coupling agents, oxide layers, weakly bonded surface layers, slip agents, light stabilizers, or additive enrichments at the surface. It is possible to remove contaminations by simple plasma sputtering (particle bombardment) in plasmas of noble gasses, by oxidation of organic contaminants with oxygen plasma or by reduction of oxides or sulfides by hydrogen plasma [2,3,4,5]. This work attempts to use a DC argon glow discharge plasma despite oxygen or hydrogen plasma sputtering in the cleaning process. In this case, the main cause of the cleaning processes is not only the particle bombarding but also the plasma instability. The instability of plasma is due to the interaction between plasma waves and coins.
Processing in cold plasma (cleaning and/or decontamination) represents an ecological alternative for applications in various domains of a diverse range of materials. Considering the advantages it presents, cold plasma has been employed to remove the corrosion products found on the surface of the samples (some 50 piasters of Egyptian coins) [6]. The effects of plasma treatment have been evaluated through the investigation of the coin’s surface before and after the treatment, employing different analytical techniques: scanning digital optical microscopy, digital balance, and hardness tester. The results of cold plasma cleaning and polishing are compared with the chemical method.
Plasma Device
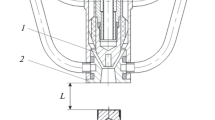
Usually, the plasma device consists of a vacuum chamber and an electrical circuit. The vacuum chamber in this study was a glass tube with a diameter of 10 cm and a length of 15 cm. The novelty of this device is that the two electrodes inside this chamber were made from different materials and also have different shapes and dimensions. This design provides us with three plasma experiments by using the same facilities. These experiments are DC glow discharge, sputtering, and magnetron to save money, effort, and place.
If an outer magnet is added under the lower electrode, the device could be used as a magnetron according to the confinement of plasma by the magnetic field. The device will work as a sputtering or glow discharge depending on the positioning of the samples inside the vessel. Along this work, the device will act as a DC argon glow discharge with two electrodes. One of them is made from stainless steel in a circular shape with a diameter of 10 cm. The other electrode is made from a copper rod with a length of 15 cm. The distance between the two electrodes could be changed by moving the copper electrode.
The electrical circuit used to generate plasma was designed and operated before in previous work [7]. The upgrade in this circuit makes it possible to change the charge of the two electrodes, and this will change the direction of the plasma generation. According to all of these variables, the plasma parameters will change. This will lead to getting benefits from different plasma properties in a wide range of applications. Figure 1 illustrates the schematic diagram of the plasma experiment and the electrical circuit, while Fig. 2 shows the device’s photographic view.
The contents of the experimental plan are as follows: first, measuring the Paschen curve at different distances between the cathode and the anode, where the cathode will be the stainless steel plate and the anode will be the copper electrode. Second, insert the coins one after one and study the effect of plasma on each one. During this exposure, the effect of coins on plasma will be observed and recorded. Finally, the coins will be cleaned chemically and the result will be compared with the plasma cleaning and polishing method.
Paschen Curve
Paschen’s law describes the characteristics of gas breakdown voltage \({(V}_{{\text{bd}}})\) between two electrodes as a function of the pd (operating pressure (p) times electrode spacing (d)). A breakdown voltage of a gas is the required voltage to start a discharge or electric arc through the gas [5, 7,8,9]. Figure 3 shows the obtained Paschen curves for each gap length from 4 to 12 cm. The shape of the obtained Paschen curves in Fig. 3 is in agreement with the ideal Paschen curve and the best one at \(d=6 {\text{cm}}\). An explanation of the shape of the Paschen curve was described in detail in previous work [10,11,12,13,14,15,16,17,18,19,20,21,22].
From the previous curves, on the left side of the minimum breakdown voltage point, pressure is low, so the mean free path is large. Due to the large mean free path, the probability of ionizing collisions of electrons with argon atoms is low and a high voltage is required for the necessary ionization on each collision. On the right side of this point, the pressure is high, so the mean free path is very low, and the collision of electrons with argon atoms is very frequent. Due to these frequent collisions, electrons cannot get the required energy to ionize argon atoms. Hence, a high voltage is required for electrons to acquire enough energy to cause ionization [5, 8, 14]. At the minimum breakdown voltage point itself, the power is the lower value which required for producing stable plasma [23]. Table 1 shows the net results for the suitable pressure and voltage to produce stable plasma at each distance between the two electrodes.
Results and Discussion
Study the Effect of Coins on the Plasma
In similar studies, the focus is usually on the effect of plasma on the surface of samples (coins). The crucial point of view in this investigation that was taken into account was noting the reversed impact of coin polishing on the plasma characteristics. Each sample—from the two coins—is exposed to plasma at different times and for different periods (this explanation will be called runs). This study will explain in detail the effect of plasma on each sample as a run. Also, at the same time, it will explain the effect of polishing on plasma parameters (voltage, current, and temperature of the vessel).
From the Paschen curve results, the distance between the cathode and the anode will be fixed at 6 cm during all work. The array of experimental work begins with putting the coin above the cathode inside the chamber of the plasma device. Then, the vacuum adjusts to 10−3 mbar. After that, argon gas flows to 10−1 mbar and increases the DC power supply voltage to 160 V. These are the operating conditions to produce plasma at that distance.
Once plasma appears the time of the run begins to record. During the time of each run, the voltage and current of the plasma are recorded in addition to the temperature of the vessel and the change in the power supply voltage output. Figures 4, 5, and 6 show the change in these parameters during each run to the first coin (S1), while Figs. 7, 8, and 9 show the changes during each run to the second coin (S2). For the plasma, the current was nearly the same value without any available change during all the work.
The interpretation of curves is as follows.
Each coin (S1 and S2) was exposed to plasma for three runs. The sample (S1) was exposed to 15 min of plasma radiation in the first run. In the second run, the sample was exposed to 10 min. The time period of the third run was 55 min. That means the first coin (S1) was exposed to 80 min of plasma in total. For S2, the first run time was 50 min, the second run was 25 min, and the third run was 45 min. That means the second coin (S2) was exposed to 120 min of plasma radiation.
Figures 4 and 7 show the change in plasma voltage for each coin (S1 and S2). From Fig. 4, it is clear that during run (1) and run (2), there is no magnificent change in plasma voltage value. While during run (3), there is a sudden decrease in its value at 40 min. From Fig. 7, it is clear that the sudden decrease appears in each run with considerable value. This noticed a decrease in the plasma voltage for each sample happened because of the sudden change in density which leads to an instability in the plasma. This happened because the sample absorbed the energy of the plasma. The plasma instability leads to a change in the amplitude of power supply voltage which is recorded by the voltammeter connected in parallel with the two ends of the power supply. This change in voltage happens to continue plasma production inside the chamber to fulfill the quasi-neutrality process. So, the similarity in power supply voltage curves (Figs. 5 and 8) with plasma voltage curves was noted. During this symphony between plasma voltage and power supply voltage to reach synchronization, the plasma current is still approximately stable at a value of 25 mA.
It was important after that to measure the temperature of the plasma. This measuring was done by using a double electric probe. From the experimental work, it was clear that there was a discharge between the probe and the sample. So, there are other effects that led to difficult uses of this diagnosis. Instead of measuring plasma temperature, the external surface temperature of the vessel was measured. During all the (6) runs for samples, this temperature was increased as shown in Figs. 6 and 9.
The Effect of Plasma Radiation on the Coins
The dimensions of the two Egyptian fifty-piaster coins used in this study are 23 mm in diameter and 2.3 mm in thickness. To study the effect of plasma radiation on each coin, a sensitive digital balance and hardness tester (HWDM-7) was used to compare the weight and hardness of the sample before and after exposure to the plasma. Also, there is a DOM (digital optical microscope) which was set at 1600 X magnification to scan the surface of the coins before and after exposure to compare them.
Table 2 shows the change in each coin weight after each run plus the weight loss calculations. Figures 10 and 11 show the change in hardness of (S1 and S2), before and after plasma exposure. Figure 12 is a photograph picture of the coin’s surface (S1 and S2) before and after finishing the polishing time. Figure 13 shows the DOM scan in different parts of the surface coins (S1 and S2).
The DOM scans to different parts of the surface coins: (a) S1 before plasma, after 25 min, and after 80 min exposed to plasma respectively. (b) Other parts of S1 before and after 80 min plasma exposure. (c) S2 before plasma, (d) S2 after 120 min irradiate, and (e) S2 before plasma, after 50 min, 75 min, and 120 min exposed to plasma, respectively
Effect of Chemical Methods on the Coin
The chemical polishing is made by using a solution of mirror universal metal polish. It is one of the quickest and easiest ways to care for brass, copper, bronze, zinc, aluminum, stainless steel, and glass ceramics. It cleans and preserves metal surfaces. It is gentle on the surface and has a long-lasting water-resistance coating that protects against rust and tarnish. There are decreases in the weight and hardness of the coin after the chemical polishing due to the removal of the metal oxide layer from the coin surface [24, 25]. Table 3 illustrates the decrease in weight and weight loss, while Fig. 14 shows the decrease in hardness. Figure 15 shows the photographic view of the coin (S3) before and after chemical cleaning.
From this study, it is clear that the DC cold plasma is better than a chemical method for cleaning and polishing coins. The results illustrated that the loss in weight of the coin by plasma was accompanied by an increase in hardness. Vice versa, loss in weight by the chemical method is accompanied by a decrease in hardness. After two months, polishing the coins by plasma is still without change while chemicals begin to return before polishing. This is related to the plasma treatment of the metal surface while the chemical replaces the dirty layer as a result to reaction with it without treating the metal surface itself.
Conclusion
Low-pressure plasma processes were applied for cleaning the surface of many types of materials from different types of contaminations or additive enrichments on the surface. By using the plasma technique, it is clear that the effect of plasma is better than the effect of the chemical method. The hardness of the coin was increased plus cleaning it by using plasma. The effect of the chemical method began to be replaced after two months, while the effect of plasma was still without change. The sample which here is the coins affects and changes the properties of plasma due to inserting it inside the chamber. The recommendation after this study is to record the change in plasma properties during the work in experimental contains applications.
Data Availability
All data generated or analyzed during this study are included in this Published article.
References
Gudmundsson JT, Hecimovic A (2017) Foundations of DC plasma sources
Bogaerts A, Neyis E, Gijbels R, van der Mullen J (2002) Spectrochimica Acta Part B 57:609
Bogaerts A, Gijbels R (1998) Spectrochimica Acta Part B 53:1
Krüger P, Knes R, Friedrich J (1999) Surface cleaning by plasma-enhanced desorption of contaminants (PEDC). Surf Coat Technol 112(1–3):240–244
Mathew P, George J, Mathews TS, Kurian PJ (2019) Experimental verification of modified Paschen’s law in DC glow discharge argon plasma. AIP Adv 9:025215
Ioanid E, Ioanid A, Rusu D, Doroftei F (2011) Surface investigation of some medieval silver coins cleaned in high-frequency cold plasma. J Cult Herit 12(2):220–226
Talab AA, Yahia A, Saudy MA, Elsayed M (2020) Characterization of a new DC-glow discharge plasma set-up to enhance the electronic circuits performance. J Mod Phys 11:1044–1057
Massarczyk R, Chu P, Dugger C, Elliott SR, Rielage K, Xu W (2017) Paschen’s law studies in cold gases. J Instrum 12
Harry JE (2010) Introduction to plasma technology: science, engineering and applications. InterScience (Online service), Wiley-VCH Verlag &Co. KGaA, Boschstr. 12, 69469 Weinheim, Germany
Radmilovic-Radjenovic M, Radjenovic B, Klas M, Bojarov A, Matejcik S (2013) The breakdown mechanisms in electrical discharges: the role of the field emission effect in direct current discharges in micro gaps. Acta Physica Slovaca 63:105
Radwan SI, El-Khabeary H, Helal AG (2016) Can J Phys 94(12):1275–1281
Raizer YP (1991) Gas discharge physics. Springer-Verlag, Berlin, Heidelberg
Husain E, Nema RS (1982) IEEE Transactions on Electrical Insulation EI-17(4)
Lisovskiy VA, Koval VA, Yegorenkov VD (2011) Phys Lett A 375:1986
Fu Y, Yang S, Zou X, Luo H, Wang X (2016) Phys Plasmas 23, 093509- 1–093509–4
Venkattraman A, Alexeenko AA (2012) Phys Plasmas 19:123515
Go DB, Pohlman DA (2010) J Appl Phys 107:103303
Loeb LB, Meek JM (1941) Mechanism of electric spark. Stanford University Press, Stanford, CA
Montijn C, Ebert U (2006) J Phys D: Appl Phys 39:2979–2992
Mathew P, Sajith Mathews T, Kurian PJ, Chattopadyay PK (2018) AIP Conf Proc 1953:060041
Fu Y, Yang S, Zou X, Luo H, Wang X (2016) High Volt 1(2):86–89
Goswami SN (2011) Elements of plasma physics (New Central Book Agency (P) Limited)
Talab AA, Abido AMRMN, Ismail MI (2022) Treatment of tantalum using argon glow discharge plasma for applied purposes. Int J Mod Phys B 36:18
Megahed MM, Youssif M, El-Shamy AM (2020) Selective formula as a corrosion inhibitor to protect the surfaces of antiquities made of leather – composite brass alloy. Egypt J Chem 63(12):5269–52287
Kannan S, Balamurugan A, Rajeswari S (2005) Electrochemical characterization of hydroxyapatite coatings on HNO3 passivated 316L SS for implant applications. Electrochim Acta 50(10):2065–2072
Acknowledgements
My thanks and gratitude to the staff on the board of the Plasma Physics and Nuclear Fusion department, Nuclear Research Center, Egyptian Atomic Energy Authority, for their great conversations about the manuscript. Special thanks to Prof. Dr. Wageh Hanafi and Prof. Dr. Bahaa Mohamed for their effective advices which lead to the change of the title of the paper to express well the physical processes in the work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission contributed to the study conception. Design and building the device were by AT and contribution from AMNA, GGM, and MF. Sample selection, operate the system, data collection, and analysis were by AT and contribution from AMNA and MF. The first draft of the manuscript was written by AT, and the other authors commented on the previous versions of the manuscript. The last review was by AT and MF. All the authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talab, A.A., Abido, A.M.N., Mohamed, G.G. et al. Comparing the Effect of Low-Temperature Plasma on Cleaning and Polishing Coins with a Chemical Method and the Reversed Effect on the Plasma Properties. Plasmonics (2024). https://doi.org/10.1007/s11468-024-02197-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11468-024-02197-5