Abstract
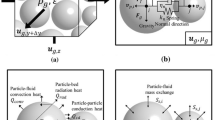
Intra-particle mass and heat transfer plays an important role in performance of the exothermic fixed-bed reactor for an isopropanol–acetone–hydrogen chemical heat pump. In this work, an exothermic fixed-bed reactor model, taking into account the actual packing structure, is established in the commercial software Fluent. A 120° segment of a tube with tube-to-particle diameter ratio (n) of 4, where realistic particles are packed and set to porous media, is used to simulate the 3D external flow, concentration and temperature fields in the exothermic packed-bed reactor. The influence of catalyst particle diameter (d p) and micropore diameter (d 0) on the intra-particle temperature, species distribution, reaction rate and selectivity is discussed. The appropriate d p and d 0 are obtained. Simulation results showed that intra-particle temperature gradient is not obvious. Large d p and small d 0 lead to remarkable gradient of reaction rate inside the catalyst particle and the decrease in the catalyst efficiency and reduce the acetone conversion and the selectivity in isopropanol. The optimal results reveal that the spherical catalyst with d p of 1 mm and d pore of 10 nm is appropriate for high-temperature acetone hydrogenation.







Similar content being viewed by others
References
Guo JF, Huai XL (2012) The application of entransy theory in optimization design of Isopropanol–Acetone–Hydrogen chemical heat pump. Energy 43:355–360
Gu QH, Yang DH (1992) Reversible catalytic reactions in chemical heat pumps. Energy Res Inform 8:35–48 (in Chinese)
Bloomquist RG (2003) Geothermal space heating. Geothermics 32:513–526
Ozgener L, Hepbasli A, Dincer I (2005) Energy and exergy analysis of geothermal district heating systems: an application. Build Environ 40:1309–1322
Faninger G (2000) Combined solar–biomass district heating in Austria. Sol Energy 69:425–435
Wongsuwan W, Kumar S, Neveu P et al (2001) A review of chemical heat pump technology and applications. Appl Therm Eng 21:1489–1519
Klinsoda I, Piumsomboon P (2007) Isopropanol–acetone–hydrogen chemical heat pump: A demonstration unit. Energy Convers Manag 48:1200–1207
Lerou JJ, Ng KM (1996) Chemical reaction engineering: A multiscale approach to a multiobjective task. Chem Eng Sci 51:1595–1614
Dixon AG, Nijemeisland M, Stitt H (2006) Packed tubular reactor modeling and catalyst design using computational fluid dynamics. In: Marin Guy B (ed) Advances in chemical engineering. Academic Press, Amsterdam, pp 307–389
Dixon AG, Boudreau J, Rocheleau A et al (2012) Flow, transport, and reaction interactions in shaped cylindrical particles for steam methane reforming. Ind Eng Chem Res 51:15839–15854
Chen WH, Lin MR, Jiang TL et al (2008) Modeling and simulation of hydrogen generation from high-temperature and low-temperature water gas shift reactions. Int J Hydrog Energy 33:6644–6656
Chen WH, Jheng JG (2007) Characterization of water gas shift reaction in association with carbon dioxide sequestration. J Power Sources 172:368–375
Nijemeisland M, Dixon AG (2004) CFD study of fluid flow and wall heat transfer in a fixed bed of spheres. AIChE J 50:906–921
Dixon AG, Taskin ME, Nijemeisland M et al (2010) CFD method to couple three-dimensional transport and reaction inside catalyst particles to the fixed bed flow field. Ind Eng Chem Res 49:9012–9025
Dixon AG, Nijemeisland M, Stitt H (2003) CFD simulation of reaction and heat transfer near the wall of a fixed bed. Int J Chem React Eng 1:A22
Dixon AG, Ertan TM, Stitt H et al (2007) 3D CFD simulations of steam reforming with resolved intraparticle reaction and gradients. Chem Eng Sci 62:4963–4966
Cheng SH, Chang H, Chen YH et al (2010) Computational fluid dynamics-based multiobjective optimization for catalyst design. Ind Eng Chem Res 49:11079–11086
Duan YJ, Xu M, Huai XL (2014) High temperature catalytic hydrogenation of acetone over raney Ni for chemical heat pump. J Therm Sci 23:85–90
Gao X, Zhu YP, Luo ZH (2011) CFD modeling of gas flow in porous medium and catalytic coupling reaction from carbon monoxide to diethyl oxalate in fixed-bed reactors. Chem Eng Sci 66:6028–6038
Long HL, Xu MJ, Yu DH et al (2012) Two-temperature model of water gas shift reaction in porous media based on FLUENT. Comp Appl Chem 29:981–985 (in Chinese)
Guo K, Tang XH, Zhou XM (2000) Chemical reaction engineering. Chemical Industry Press, Beijing (in Chinese)
Acknowledgment
This work was supported by the National Basic Research Program of China (2011CB710705) and the National Natural Science Foundation of China (21306192, 51276181).
Author information
Authors and Affiliations
Corresponding authors
Additional information
SPECIAL TOPIC: Deep Utilization of Boiler Low-Temperature Flue Gas
Appendix
Appendix
- C :
-
Mole concentration
- D :
-
Integrated diffusion coefficient (m2 s−1)
- d :
-
Diameter of catalyst particle (m)
- ∆H :
-
Reaction heat (kJ mol−1)
- I :
-
Identity matrix
- J-:
-
Diffusive mass flux (kg m−2 s−1)
- k :
-
Thermal conductivity (W m−1 K−1)
- M :
-
Molecular weight (kg kmol−1)
- n :
-
Tube-to-particle diameter ratio
- N :
-
Number of moles (mol)
- p :
-
Pressure (Pa)
- R :
-
Gas constant (J mol−1 K−1)
- r :
-
Reaction rate in kinetic equations (mol m−3 s−1)
- R :
-
Mass reaction rate (kg m−3 s−1)
- S i :
-
Selectivity of species i
- S :
-
Gas–solid momentum exchange rate
- T :
-
Temperature (K)
- v :
-
Velocity (m s−1)
- V :
-
Molecule diffusion volume (m3 mol−1)
- X :
-
Conversion
- Y :
-
Mass fraction
- ε :
-
Porosity
- η :
-
Rate exponent
- μ :
-
Viscosity (Pa s)
- ν :
-
Stoichiometric coefficient
- ρ :
-
Density (kg m−3)
- τ :
-
Tortuosity factor
- τ− :
-
Stress tensor (Pa)
- 0:
-
Micropore
- A:
-
Acetone
- CO:
-
Carbon monoxide
- e:
-
Effective diffusion
- g:
-
Gas
- H:
-
Hydrogen
- I:
-
Isopropanol
- i, j :
-
Chemical species
- in:
-
Inlet condition
- k:
-
Knudson diffusion
- m:
-
Mixture
- p:
-
Catalyst particle
- ‘:
-
Initial condition
- “:
-
Final condition
About this article
Cite this article
Duan, Y., Xu, M., Huai, X. et al. Acetone hydrogenation in exothermic reactor of an isopropanol–acetone–hydrogen chemical heat pump: effect of intra-particle mass and heat transfer. Chin. Sci. Bull. 59, 4436–4443 (2014). https://doi.org/10.1007/s11434-014-0610-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-014-0610-1