Abstract
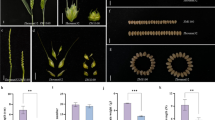
Leymus racemosus is highly resistant to wheat scab (Fusarum head bright). The transfer of scab resistant gene from L. racemosus to Triticum aestivum is of great significance for broadening the base of wheat resistance. In the present study, the pollen of T. aestivum-L. racemosus monosomic addition line with scab resistance was treated by irradiation with 1200 R 60Co-γ-rays prior to pollinating to emasculated wheat cv. Mianyang 85–45. Nine plants with a telocentric chromosome 7Lr#1S were observed in M1, and one ditelosomic substitution line 7Lr#1S was selected from selfcrossing progenies and confirmed by chromosome C-banding and GISH. Furthermore, a co-dominant EST-SSR marker CINAU 31 was employed to identify this substitution line. A pair of chromosome 7A of common wheat were found to be replaced by a pair of telocentric chromosome 7Lr#1S, and further investigation showed that chromosome configuration of the substitution line at MI of PMCs after GISH was 17.50

W + 2.19

W + 0.42

7Lr#1S + 1.08 I7Lr#1S + 0.69 IW. Two telocentric chromosomes paired as a bivalent in 59.7% of PMCs. Abnormal chromosome behaviors of telocentric chromosomes were observed in part of PMCs at anaphase I and telophase I, including the moving of two telocentric chromosomes to the same pole, lagging and earlier separation of their sister chromatid. All these abnormal behaviors can be grouped into three distinct types of tetrads according to different numbers of 7Lr#1S in their daughter cells and various micronucleus in some tetrads. However, due to the high transmission frequency of the female and male gametes with a 7Lr#1S, 84% of the selfcrossing progeny plants had ditelosomic substitution. The substitution line showed high resistance to wheat scab in a successive two-year test both in the greenhouse and field; hence, the line will be particularly valuable for alien gene mapping, small fragment translocation induction and telosomic cytological behavior analysis.
Similar content being viewed by others
References
McGuire P E, Dvorak J. High salt-tolerance potential in wheatgrasses. Crop Sci, 1981, 21: 702–705
Mujeeb-Kazi A, Bekele G T, Mirand J L. Incorporation of alien genetic information from Elymus giganteus into Triticum aestivum. In: Proc the 6th Int Wheat Genet Symp, Kyoto, Japan, 1983. 223–231
Chen P D, Wang Z T, Wang S L, et al. Transfer of useful germplasm from Leymus racemosus Lam. to common wheat. III. Development of addition lines with wheat scab resistance (in Chinese). Acta Genet Sin, 1995, 22: 206–210
Qi L L, Wang S L, Chen P D. Molecular cytogenetic analysis of Leymus racemosus chromosomes added to wheat. Theor Appl Genet, 1997, 95: 1084–1091
Sun W X, Chen P D, Liu D J. Transfer of useful germplasm from Leymus racemous Lam. to common wheat, VI. Development and identification of T. aestivum-L. racemosus telosomic addition lines (in Chinese). Acta Genet Sinica, 1998, 25(3): 259–264
Kishii M, Yamada T, Sasakuma T. Production of wheat-Leymus racemosus chromosome addition lines. Theor Appl Genet, 2004, 109: 255–260
Gill B S, Friebe B, Endo T R. Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome, 1991, 34: 830–839
Chen P D, Qi L L, Zhou B. Development and molecular cytogenetic analysis of wheat-Haynaldia villosa 6VS/6AL translocation lines specifying resistance to powdery mildew. Theor Appl Genet, 1995, 91: 1125–1128
Zhang P, Li W, Friebe B. Simultaneous painting of three genomes in hexploid wheat by BAC-FISH. Genome, 2004, 47: 979–987
Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res, 1991, 19: 1349
Chen H M, Li L Z, Wei X Y. Development, chromosome location and genetic mapping of EST-SSR markers in wheat. Chin Sci Bull, 2005, 50(20): 2328–2336
Chen J F, Ren Z L, Gao L F. Developing new SSR markers from EST of wheat. Acta Agron Sin, 2005, 31(2): 154–158
Sears E R. The transfer of leaf rust resistance from Aegilops umbellulata to wheat. Brookhaven Symp Biol 1956, 9: 1–22
Driscoll C J, Jensen N F. Characteristics of leaf rust resistance transferred from rye to wheat. Crop Sci, 1964, 4: 372–374
Driscoll C J, Jensen N F. Release of a wheat-rye translocation stock involving leaf rust and powdery mildew resistance. Crop Sci, 1965, 5: 279–280
Sharma D, Knott D R. The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can J Genet Cytol, 1966, 8: 137–143
Driscoll C J, Anderson L M. Cytogenetic studies of transec-a wheat-rye translocation line. Can J Genet Cytol, 1967, 9: 375–380
Mukai Y, Friebe B, Hatchett J H. Molecular cytogenetic analysis of radiation-induced wheat-rye terminal and intercalary chromosomal translocations and the detection of rye chromatin specifying resistance to Hessian. Chromosoma, 1993, 102: 88–95
Sears E R. Use of radiation to transfer alien chromosome segments to wheat. Crop Sci, 1993, 33: 897–901
Bie T D, Cao Y P, Chen P D. Mass Production of intergeneric chromosomal translocations through pollen irradiation of Triticum durum-Haynaldia villosa amphiploid. J Integrat Plant Biol, 2007, 49(11): 1619–1626
Pienaar R de V, van Niekerk H A. The effect of gamma-irradition on wheat gametophytes. In: Proc the 4th Int Wheat Genet Symp. Columbia, Missouri, USA, 1973. 289–294
Sears E R. The aneuploids of common wheat. Mo Agr Exp Stn Res Bull, 1954, 572: 1–58
Sears E R. Chromosome mapping with the aid of telocentrics. In: Proc the 2nd Int Wheat Genet Symp. Stockholm, 1966. 370–381
Sears E R, Sears Lotti M S. The telocentric chromosomes of common wheat. In: Proc the 4th Int Wheat Genet Symp. New Delhi, 1978. 389–407
Sears E R. Transfer of alien genetic material to wheat. In: Evans LT, Peacock W J, eds. Wheat Science-Today and Tomorrow. Cambridge: Cambridge Univerisity Press, 1981. 75–89
Gupta P K, Rustgi S, Sharma S. Transferable EST-SSR markers for the study of polymorphism and genetic diversity in bread wheat. Mol Gen Genom, 2003, 270: 315–323
Gao L F, Jing R L, Huo N X. One hundred and one new microsatellite loci derived from ESTs (EST-SSRs) in bread wheat. Theor Appl Genet, 2004, 108: 1392–1400
Ge R C, Zhao M L, Zhao B C. Study on the C banding of Leymus multicaulis, Puna chicory root tip chromosomes by HBSG method. J Hebei Normal Univ (Nat Sci Ed), 2006(30), 2: 213–216
Burnham C R, John T, Stout W. Chromosome pairing in maize. Genetics, 1972. 71: 111–126
Ding D Q, Yamamoto A, Haraguchi T. Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev Cell, 2004, 6: 329–34
Zhao M L, Li R F, Liang H X. The behavior of an alien chromosome in Triticum aestivum-Leymus multicaulis monosomic addition lines during meiosis. Prog Nat Sci, 2001, 11: 945–949
Li R F, Liang H X, Zhao M L. The meiotic behavior of an alien chromosome in Triticum aestivum-Haynaldia villosa monosomic addition lines. Scient Agricult Sin, 2002, 35(2): 127–131
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Hi-Tech Research and Development Program of China (Grant No. 2006AA10Z1F6), National Natural Science Foundation of Jiangsu Province (Grant No. BK2006720), High Tech Research Plan of Jiangsu Province (Grant No. BG2005310) and Program of Introducing Talents of Discipline to Universities (Grant No. B08025)
About this article
Cite this article
Wang, L., Chen, P. Development of Triticum aestivum-Leymus racemosus ditelosomic substitution line 7Lr#1S(7A) with resistance to wheat scab and its meiotic behavior analysis. Chin. Sci. Bull. 53, 3522–3529 (2008). https://doi.org/10.1007/s11434-008-0457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-008-0457-4