Abstract
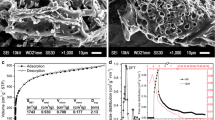
The catalytic performance of carbide slag in transesterification is investigated and the reaction kinetic parameters are calculated. After being activated at 650°C, calcium compounds of carbonate and hydroxide in the carbide slag are mainly transformed into calcium oxide. The activated carbide slag utilized as the transesterification catalyst is characterized by X-ray diffraction (XRD), attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), nitrogen adsorption-desorption and the Hammett indicator method. Compared with the carbide slag activated at 700 and 800°C, the largest surface area of 22.63 m2 g−1, the smallest particle size of 265.12 nm and the highest catalytic efficiency of the carbide slag activated at 650°C guarantee its capacity in catalyzing transesterification. Then, the influences of activated temperature (T a), molar ratio of methanol to oil (γ), catalyst added amount (ζ), reaction temperature (T r) and reaction time (τ) on the catalytic performance are investigated. Under the optimal transesterification condition of T a=650°C, γ=15, ζ=3%, T r=60°C and τ=110 min, the catalytic efficiency of 92.98% can be achieved. Finally, the kinetic parameters of transesterification catalyzed by the activated carbide slag are calculated, where activation energy (E) is 68.45 kJ mol−1 and pre-exponential factor (k 0) is 1.75×109 min−1. The activated carbide slag shows better reused property than calcium hydroxide.
Similar content being viewed by others
References
Omer A M. Energy, environment and sustainable development. Renew Sust Energ Rev, 2008, 12: 2265–2300
Yao C D, Zhang Z H, Cheung C S, et al. Experimental study on the effect of gaseous and particulate emission from an ethanol fumigated diesel engine. Sci China Tech Sci, 2010, 53: 3294–3301
Li H T, Xie W L. Transesterification of soybean oil to biodiesel with Zn/I2 catalyst. Catal Lett, 2006, 107: 25–30
Demirbas A. Biodiesel. London: Springer, 2008
Balat M. Biodiesel fuel production from vegetable oils via supercritical ethanol transesterification. Energ Source Part A, 2008, 30: 429–440
Taufiq-Yap Y H, Lee H V, Hussein M Z, et al. Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenerg, 2011, 35: 827–834
Liu F J, Wang L, Sun Q, et al. Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: Heterogeneous catalysts that are faster than homogeneous catalysts. J Am Chem Soc, 2012, 134: 16948–16950
Kansedo J, Lee K T, Bhatia S. Biodiesel production from palm oil via heterogeneous transesterification. Biomass Bioenerg, 2009, 33: 271–276
Alba-Rubio A C, Santamaría-González J, Mérida-Robles J M, et al. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal Today, 2010, 149: 281–287
Corma A, Iborra S. Optimization of alkaline earth metal oxide and hydroxide catalysts for base-catalyzed reactions. Adv Catal, 2006, 49: 239–302
Yadav G D, Kadam A A. Selective engineering using Mg-Al calcined hydrotalcite and microwave irradiation in mono-transesterification of diethyl malonate with cyclohexanol. Chem Eng J, 2013, 230: 547–557
Castro C S, Cardoso D, Nascente P A P, et al. MgAlLi mixed oxides derived from hydrotalcite for catalytic transesterification. Catal Lett, 2011, 141: 1316–1323
Xie W L, Fan M L. Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem Eng J, 2014, 239: 60–67
Kusuma R I, Hadinoto J P, Ayucitra A, et al. Natural zeolite from Pacitan Indonesia, as catalyst support for transesterification of palm oil. Appl Clay Sci, 2013, 74: 121–126
Cho Y B, Seo G, Chang D R. Transesterification of tributyrin with methanol over calcium oxide catalysts prepared from various precursors. Fuel Process Technol, 2009, 90: 1252–1258
Kouzu M, Yamanaka S, Hidaka J, et al. Heterogeneous catalysis of calcium oxide used for transesterification of soybean oil with refluxing methanol. Appl Catal A-Gen, 2009, 355: 94–99
Nurfitri I, Maniam G P, Hindryawati N, et al. Potential of feedstock and catalysts from waste in biodiesel preparation: A review. Energ Convers Manage, 2013, 74: 395–402
Boey P L, Maniam G P, Hamid S A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: a review. Chem Eng J, 2011, 168: 15–22
Liu X J, He H Y, Wang Y J, et al. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel, 2008, 87: 216–221
Ofori-Boateng C, Lee K T. The potential of using cocoa pod husks as green solid base catalysts for the transesterification of soybean oil into biodiesel: Effects of biodiesel on engine performance. Chem Eng J, 2013, 220: 395–401
Badday A S, Abdullah A Z, Lee K T. Ultrasound-assisted transesterification of crude Jatropha oil using alumina-supported heteropolyacid catalyst. Appl Energ, 2013, 105: 380–388
Niu S L, Huo M J, Lu C M, et al. An investigation on the catalytic capacity of dolomite in transesterification and the calculation of kinetic parameters. Bioresource Technol, 2014, 158: 74–80
Moradi G, Mohammadi F. Utilization of waste coral for biodiesel production via transesterification of soybean oil. Int J Environ Sci Te, 2014, 11: 805–812
Nakatani N, Takamori H, Takeda K, et al. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresource Technol, 2009, 100: 1510–1513
Diaz L, Borges M E. Low-quality vegetable oils as feedstock for biodiesel production using k-pumice as solid catalyst. Tolerance of water and free fatty acids contents. J Agric Food Chem, 2012, 60: 7928–7933
Boro J, Konwar L J, Deka D. Transesterification of non edible feedstock with lithium incorporated egg shell derived CaO for biodiesel production. Fuel Process Technol, 2014, 122: 72–78
Li Y J, Sun R Y, Liu C T, et al. CO2 capture by carbide slag from chlor-alkali plant in alcination/carbonation cycles. Int J Greenh Gas Con, 2012, 9: 117–123
Cao J X, Liu F, Lin Q, et al. Hydrothermal synthesis of xonotlite from carbide slag. Prog Nat Sci, 2008, 18: 1147–1153
Niu S L, Liu M Q, Lu C M, et al. Thermogravimetric analysis of carbide slag. J Therm Anal Calorim, 2014, 115: 73–79
Zanette A F, Barella R A, Pergher S B C, et al. Screening, optimization and kinetic of Jatropha curcas oil transesterification with heterogeneous catalysts. Renew Energ, 2011, 36: 726–731
Dossin T F, Reyniers M F, Berger R J, et al. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl Catal B-Environ, 2006, 67: 136–148
Vujicic D, Comic D, Zarubica A, et al. Kinetic of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel, 2010, 89: 2054–2061
Kouzu M, Kasuno T, Tajika M, et al. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel, 2008, 87: 2798–2806
Pradhan S, Madankar C S, Mohanty P, et al. Optimization of reactive extraction of castor seed to produce biodiesel using response surface methodology. Fuel, 2012, 97: 848–855
Taufiq-Yap Y H, Lee H V, Hussein M Z, et al. Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenerg, 2011, 35: 827–834
Lavalley J C. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal Today, 1996, 27: 377–401
Granados M L, Poves M D Z, Alonso D M, et al. Biodiesel from sunflower oil by using activated calcium oxide. Appl Catal B-Envirom, 2007, 73: 317–326
Nguyen P T, Nohair B, Mighri N, et al. TBD-functionalized mesoporous silica: Synthesis and catalytic activity in corn oil transesterification. Micropor Mesopor Mat, 2013, 180: 293–300
Kima H J, Kanga B S, Kima M J, et al. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal Today, 2004, 93: 315–320
Vedyagin A A, Volodin A M, Stoyanovskii V O, et al. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl Catal B-Environ, 2011, 103: 397–403
Birla A, Singh B, Upadhyay S N, et al. Kinetic studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Bioresource Technol, 2012, 106: 95–100
de Caland L B, Santos L S S, de Moura C V R, et al. Preparation and study of bimetallic compounds efficiency in the synthesis of biodiesel. Fuel, 2009, 128: 392–400
Peterson G R, Scarrah W P. Rapeseed oil transesterification by heterogeneous catalysis. J Am Oil Chem Soc, 1984, 61: 1593–1597
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Niu, S., Lu, C. et al. A study on the catalytic performance of carbide slag in transesterification and the calculation of kinetic parameters. Sci. China Technol. Sci. 58, 258–265 (2015). https://doi.org/10.1007/s11431-014-5691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-014-5691-1