Abstract
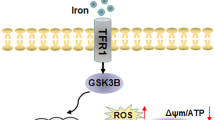
Iron is important for life, and iron deficiency impairs development, but whether the iron level regulates neural differentiation remains elusive. In this study, with iron-regulatory proteins (IRPs) knockout embryonic stem cells (ESCs) that showed severe iron deficiency, we found that the Pax6- and Sox2-positive neuronal precursor cells and Tuj1 fibers in IRP1−/−IRP2−/− ESCs were significantly decreased after inducing neural differentiation. Consistently, in vivo study showed that the knockdown of IRP1 in IRP2−/− fetal mice remarkably affected the differentiation of neuronal precursors and the migration of neurons. These findings suggest that low intracellular iron status significantly inhibits neurodifferentiation. When supplementing IRP1−/−IRP2−/− ESCs with iron, these ESCs could differentiate normally. Further investigations revealed that the underlying mechanism was associated with an increase in reactive oxygen species (ROS) production caused by the substantially low level of iron and the down-regulation of iron-sulfur cluster protein ISCU, which, in turn, affected the proliferation and differentiation of stem cells. Thus, the appropriate amount of iron is crucial for maintaining normal neural differentiation that is termed ferrodifferentiation.
Similar content being viewed by others
Data availability
All relevant data in this study are available from the corresponding author (yzchang@hebtu.edu.cn) upon reasonable request.
References
Anderson, C.P., Shen, M., Eisenstein, R.S., and Leibold, E.A. (2012). Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823, 1468–1483.
Androutsellis-Theotokis, A., Leker, R.R., Soldner, F., Hoeppner, D.J., Ravin, R., Poser, S.W., Rueger, M.A., Bae, S.K., Kittappa, R., and McKay, R.D.G. (2006). Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826.
Armitage, A.E., and Drakesmith, H. (2014). The battle for iron. Science 346, 1299–1300.
Ashraf, A., Clark, M., and So, P.W. (2018). The aging of iron man. Front Aging Neurosci 10, 65.
Bai, X., Yan, Y., Canfield, S., Muravyeva, M.Y., Kikuchi, C., Zaja, I., Corbett, J.A., and Bosnjak, Z.J. (2013). Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg 116, 869–880.
Braymer, J.J., Freibert, S.A., Rakwalska-Bange, M., and Lill, R. (2021). Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim Biophys Acta 1868, 118863.
Camaschella, C. (2019). Iron deficiency. Blood 133, 30–39.
Celotto, A.M., Liu, Z., VanDemark, A.P., and Palladino, M.J. (2012). A novel Drosophila SOD2 mutant demonstrates a role for mitochondrial ROS in neurodevelopment and disease. Brain Behav 2, 424–434.
Che, Y., Tian, Y., Chen, R., Xia, L., Liu, F., and Su, Z. (2021). IL-22 ameliorated cardiomyocyte apoptosis in cardiac ischemia/reperfusion injury by blocking mitochondrial membrane potential decrease, inhibiting ROS and cytochrome C. Biochim Biophys Acta 1867, 166171.
Chen, Y., Fan, J., Zhang, Z., Wang, G., Cheng, X., Chuai, M., Lee, K.K.H., and Yang, X. (2013). The negative influence of high-glucose ambience on neurogenesis in developing quail embryos. PLoS ONE 8, e66646.
Ci, Y.Z., Li, H., You, L.H., Jin, Y., Zhou, R., Gao, G., Hoi, M.P.M., Wang, C., Chang, Y.Z., and Yu, P. (2020). Iron overload induced by IRP2 gene knockout aggravates symptoms of Parkinson’s disease. Neurochem Int 134, 104657.
Cooke, M.S., Evans, M.D., Dizdaroglu, M., and Lunec, J. (2003). Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17, 1195–1214.
Daley, G.Q. (2007). Gametes from embryonic stem cells: a cup half empty or half full? Science 316, 409–410.
Dlouhy, A.C., and Outten, C.E. (2013). The iron metallome in eukaryotic organisms. In: Banci, L., ed. Metallomics and the Cell. Metal Ions in Life Sciences. Dordrecht: Springer. 241–278.
Evans, M.D., Dizdaroglu, M., and Cooke, M.S. (2004). Oxidative DNA damage and disease: induction, repair and significance. Mutat Res Rev Mutat Res 567, 1–61.
Favaro, E., Ramachandran, A., McCormick, R., Gee, H., Blancher, C., Crosby, M., Devlin, C., Blick, C., Buffa, F., Li, J.L., et al. (2010). MicroRNA-210 regulates mitochondrial free radical response to hypoxia and Krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS ONE 5, e10345.
Fu, X., He, Q., Tao, Y., Wang, M., Wang, W., Wang, Y., Yu, Q.C., Zhang, F., Zhang, X., Chen, Y.G., et al. (2021). Recent advances in tissue stem cells. Sci China Life Sci 64, 1998–2029.
Galaris, D., Barbouti, A., and Pantopoulos, K. (2019). Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta 1866, 118535.
Galy, B., Ferring-Appel, D., Kaden, S., Gröne, H.J., and Hentze, M.W. (2008). Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab 7, 79–85.
Galy, B., Ferring-Appel, D., Sauer, S.W., Kaden, S., Lyoumi, S., Puy, H., Kölker, S., Gröne, H.J., and Hentze, M.W. (2010). Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab 12, 194–201.
Ganz, T. (2013). Systemic iron homeostasis. Physiol Rev 93, 1721–1741.
Gao, G., Li, J., Zhang, Y., and Chang, Y.Z. (2019). Cellular Iron Metabolism and Regulation. In: Chang, Y.Z., ed. Brain Iron Metabolism and CNS Diseases. Advances in Experimental Medicine and Biology. Singapore: Springer. 21–32.
Ghosh, M.C., Zhang, D.L., and Rouault, T.A. (2015). Iron misregulation and neurodegenerative disease in mouse models that lack iron regulatory proteins. Neurobiol Dis 81, 66–75.
Hentze, M.W., Muckenthaler, M.U., Galy, B., and Camaschella, C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38.
Hitoshi, S., Alexson, T., Tropepe, V., Donoviel, D., Elia, A.J., Nye, J.S., Conlon, R.A., Mak, T.W., Bernstein, A., and van der Kooy, D. (2002). Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16, 846–858.
Holmes-Hampton, G.P., Ghosh, M.C., Rouault, T.A., and David, S.S.P. (2018). Methods for studying iron regulatory protein 1: an important protein in human iron metabolism. Methods in Enzymology. New York: Academic Press. 139–155.
Jeong, S.Y., Crooks, D.R., Wilson-Ollivierre, H., Ghosh, M.C., Sougrat, R., Lee, J., Cooperman, S., Mitchell, J.B., Beaumont, C., and Rouault, T.A. (2011). Iron insufficiency compromises motor neurons and their mitochondrial function in Irp2-null mice. PLoS ONE 6, e25404.
Katsarou, A., and Pantopoulos, K. (2020). Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med 75, 100866.
Kim, H.Y., LaVaute, T., Iwai, K., Klausner, R.D., and Rouault, T.A. (1996). Identification of a conserved and functional iron-responsive element in the 5′-untranslated region of mammalian mitochondrial aconitase. J Biol Chem 271, 24226–24230.
LaVaute, T., Smith, S., Cooperman, S., Iwai, K., Land, W., Meyron-Holtz, E., Drake, S.K., Miller, G., Abu-Asab, M., Tsokos, M., et al. (2001). Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27, 209–214.
Li, H., Zhao, H., Hao, S., Shang, L., Wu, J., Song, C., Meyron-Holtz, E.G., Qiao, T., and Li, K. (2018). Iron regulatory protein deficiency compromises mitochondrial function in murine embryonic fibroblasts. Sci Rep 8, 5118.
Liu, P., Chen, S., Wang, Y., Chen, X., Guo, Y., Liu, C., Wang, H., Zhao, Y., Wu, D., Shan, Y., et al. (2021). Efficient induction of neural progenitor cells from human ESC/iPSCs on Type I Collagen. Sci China Life Sci 64, 2100–2113.
Meyron-Holtz, E.G., Ghosh, M.C., Iwai, K., LaVaute, T., Brazzolotto, X., Berger, U.V., Land, W., Ollivierre-Wilson, H., Grinberg, A., Love, P., et al. (2004). Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J 23, 386–395.
Mleczko-Sanecka, K., and Silvestri, L. (2021). Cell-type-specific insights into iron regulatory processes. Am J Hematol 96, 110–127.
Muckenthaler, M.U., Galy, B., and Hentze, M.W. (2008). Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 28, 197–213.
Nakamura, T., Naguro, I., and Ichijo, H. (2019). Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta 1863, 1398–1409.
Orrenius, S., Nicotera, P., and Zhivotovsky, B. (2011). Cell death mechanisms and their implications in toxicology. Toxicol Sci 119, 3–19.
Páll, E., Lichner, Z., Bontovics, B., and Gócza, E. (2013). Differentiation of embryonic stem cells: lessons from embryonic development. Lucrari Stiintifice Zootehnie Si Biotehnol 41, 130–137.
Pantopoulos, K. (2004). Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci 1012, 1–13.
Romero, A., Ramos, E., de Los Ríos, C., Egea, J., del Pino, J., and Reiter, R.J. (2014). A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56, 343–370.
Rouault, T.A. (2006). The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2, 406–414.
Rouault, T.A. (2013). Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14, 551–564.
Rouault, T.A. (2019). The indispensable role of mammalian iron sulfur proteins in function and regulation of multiple diverse metabolic pathways. Biometals 32, 343–353.
Saha, P.P., Kumar, S.K.P., Srivastava, S., Sinha, D., Pareek, G., and D’Silva, P. (2014). The presence of multiple cellular defects associated with a novel G50E Iron-Sulfur Cluster Scaffold Protein (ISCU) mutation leads to development of mitochondrial myopathy. J Biol Chem 289, 10359–10377.
Schiavi, A., Strappazzon, F., and Ventura, N. (2020). Mitophagy and iron: two actors sharing the stage in age-associated neuronal pathologies. Mech Ageing Dev 188, 111252.
Silva, J., and Smith, A. (2008). Capturing pluripotency. Cell 132, 532–536.
Siriwardana, G., and Seligman, P.A. (2013). Two cell cycle blocks caused by iron chelation of neuroblastoma cells: separating cell cycle events associated with each block. Physiol Rep 1, e00176.
Smith, S.R., Cooperman, S., Lavaute, T., Tresser, N., Ghosh, M., Meyronholtz, E., Land, W., Ollivierre, H., Jortner, B., Switzer III, R., et al. (2004). Severity of neurodegeneration correlates with compromise of iron metabolism in mice with iron regulatory protein deficiencies. Ann N Y Acad Sci 1012, 65–83.
Smith, S.R., Ghosh, M.C., Ollivierre-Wilson, H., Hang Tong, W., and Rouault, T.A. (2006). Complete loss of iron regulatory proteins 1 and 2 prevents viability of murine zygotes beyond the blastocyst stage of embryonic development. Blood Cells Mol Dis 36, 283–287.
Subramanian, P., Rodrigues, A.V., Ghimire-Rijal, S., and Stemmler, T.L. (2011). Iron chaperones for mitochondrial Fe-S cluster biosynthesis and ferritin iron storage. Curr Opin Chem Biol 15, 312–318.
Tan, D.Q., and Suda, T. (2018). Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal 29, 149–168.
Tewari, R.K., Hadacek, F., Sassmann, S., and Lang, I. (2013). Iron deprivation-induced reactive oxygen species generation leads to non-autolytic PCD in Brassica napus leaves. Environ Exp Bot 91, 74–83.
Thiriveedi, V.R., Mattam, U., Pattabhi, P., Bisoyi, V., Talari, N.K., Krishnamoorthy, T., and Sepuri, N.B.V. (2020). Glutathionylated and Fe-S cluster containing hMIA40 (CHCHD4) regulates ROS and mitochondrial complex III and IV activities of the electron transport chain. Redox Biol 37, 101725.
Turrens, J.F. (2003). Mitochondrial formation of reactive oxygen species. J Physiol 552, 335–344.
Volz, K. (2021). Conservation in the iron responsive element family. Genes 12, 1365.
von der Mark, C., Ivanov, R., Eutebach, M., Maurino, V.G., Bauer, P., and Brumbarova, T. (2020). Reactive oxygen species coordinate the transcriptional responses to iron availability in Arabidopsis. J Exp Bot 72, 2181–2195.
Wang, H., Liu, C., Zhao, Y., and Gao, G. (2019). Mitochondria regulation in ferroptosis. Eur J Cell Biol 99, 151058.
Wang, X., Tsai, J.W., LaMonica, B., and Kriegstein, A.R. (2011). A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci 14, 555–561.
Warthon-Medina, M., Qualter, P., Zavaleta, N., Dillon, S., Lazarte, F., and Lowe, N. (2015). The long term impact of micronutrient supplementation during infancy on cognition and executive function performance in pre-school children. Nutrients 7, 6606–6627.
Wilkinson, N., and Pantopoulos, K. (2014). The IRP/IRE system in vivo: insights from mouse models. Front Pharmacol 5, 176.
Wilson, K., and Sloan, J.M. (2015). Iron-deficiency anemia. N Engl J Med 373, 485.
Wu, T., Liang, X., Liu, X., Li, Y., Wang, Y., Kong, L., and Tang, M. (2020). Induction of ferroptosis in response to graphene quantum dots through mitochondrial oxidative stress in microglia. Part Fibre Toxicol 17, 30.
Zeng, W., Yang, F., Shen, W.L., Zhan, C., Zheng, P., and Hu, J. (2022). Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci 65, 1929–1958.
Zhang, C. (2014). Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell 5, 750–760.
Zhang, D.L., Ghosh, M.C., and Rouault, T.A. (2014). The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front Pharmacol 5, 124.
Zhang, J., Chen, X., Hong, J., Tang, A., Liu, Y., Xie, N., Nie, G., Yan, X., and Liang, M. (2021). Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis and oxidative responses. Sci China Life Sci 64, 352–362.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30871260), the Natural Science Foundation of Hebei Province (E2021205003, C2019206575) and the Science and Technology Project of Hebei Education Department (QN2019005, BJK2022049).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Chang, S., Wang, P., Han, Y. et al. Ferrodifferentiation regulates neurodevelopment via ROS generation. Sci. China Life Sci. 66, 1841–1857 (2023). https://doi.org/10.1007/s11427-022-2297-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-022-2297-y