Abstract
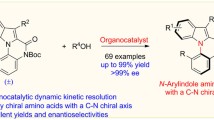
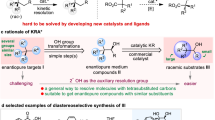
A novel kinetic resolution (KR) method has been developed for 3,3-disubstituted indolines, whose catalytic asymmetric synthesis remains a significant challenge in organic synthesis. The key to the success of this KR protocol lies in the utilization of chiral phosphoric acid-catalyzed triazane formation reaction with azodicarboxylates, which enables the enantioselective synthesis of various substituted indolines bearing C3-quaternary stereocenters with good to high enantioselectivities (with s-factors up to 70). Moreover, an intriguing parallel kinetic resolution (PKR) has been developed by combining triazane formation and dehydrogenation reactions using different azodicarboxylates. Experimental studies have provided insight into the mechanism of this PKR reaction, demonstrating stereoselectivity in both triazane formation and dehydrogenation steps, favoring the opposite enantiomers. The large-scale synthesis and diverse derivatizations of the products, particularly the imine group-containing 3H-indoles, demonstrate the value of these (P)KR methods.
Similar content being viewed by others
References
Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ. N Engl J Med, 2008, 359: 31–42
Gonzalez-Lopez de Turiso F, Shin Y, Brown M, Cardozo M, Chen Y, Fong D, Hao X, He X, Henne K, Hu YL, Johnson MG, Kohn T, Lohman J, McBride HJ, McGee LR, Medina JC, Metz D, Miner K, Mohn D, Pattaropong V, Seganish J, Simard JL, Wannberg S, Whittington DA, Yu G, Cushing TD. J Med Chem, 2012, 55: 7667–7685
Zhang W, Ma L, Li S, Liu Z, Chen Y, Zhang H, Zhang G, Zhang Q, Tian X, Yuan C, Zhang S, Zhang W, Zhang C. J Nat Prod, 2014, 77: 1887–1892
Wilson JE, Kurukulasuriya R, Reibarkh M, Reiter M, Zwicker A, Zhao K, Zhang F, Anand R, Colandrea VJ, Cumiskey AM, Crespo A, Duffy RA, Murphy BA, Mitra K, Johns DG, Duffy JL, Vachal P. ACS Med Chem Lett, 2016, 7: 261–265
Li M, Woods PA, Smith MD. Chem Sci, 2013, 4: 2907–2911
Torigoe T, Ohmura T, Suginome M. Angew Chem Int Ed, 2017, 56: 14272–14276
Tian ZX, Qiao JB, Xu GL, Pang X, Qi L, Ma WY, Zhao ZZ, Duan J, Du YF, Su P, Liu XY, Shu XZ. J Am Chem Soc, 2019, 141: 7637–7643
Zhang ZM, Xu B, Wu L, Wu Y, Qian Y, Zhou L, Liu Y, Zhang J. Angew Chem Int Ed, 2019, 58: 14653–14659
Zhang ZM, Xu B, Wu L, Zhou L, Ji D, Liu Y, Li Z, Zhang J. J Am Chem Soc, 2019, 141: 8110–8115
Cao M, Wang H, Ma Y, Tung CH, Liu L. J Am Chem Soc, 2022, 144: 15383–15390
Lackner AD, Samant AV, Toste FD. J Am Chem Soc, 2013, 135: 14090–14093
Vedejs E, Jure M. Angew Chem Int Ed, 2005, 44: 3974–4001
Müller CE, Schreiner PR. Angew Chem IntEd, 2011, 50: 6012–6042
Pellissier H. Adv Synth Catal, 2011, 353: 1613–1666
Krasnov VP, Gruzdev DA, Levit GL. EurJ Org Chem, 2012, 2012: 1471–1493
Kreituss I, Bode JW. Acc Chem Res, 2016, 49: 2807–2821
Petersen KS. Asian J Org Chem, 2016, 5: 308–320
Liu W, Yang X. Asian J Org Chem, 2021, 10: 692–710
Liu W, Wang D, Zhang D, Yang X. Synlett, 2022, 33: 1788–1812
Li HH, Zhang JY, Li S, Wang YB, Cheng JK, Xiang SH, Tan B. Sci China Chem, 2022, 65: 1142–1148
Hong X, Guo J, Liu J, Cao W, Wei C, Zhang Y, Zhang X, Fu Z. Sci China Chem, 2022, 65: 905–911
Hang QQ, Wu SF, Yang S, Wang X, Zhong Z, Zhang YC, Shi F. Sci China Chem, 2022, 65: 1929–1937
Arai S, Bellemin-Laponnaz S, Fu GC. Angew Chem Int Ed, 2001, 40: 234–236
Birman VB, Jiang H, Li X, Guo L, Uffman EW. J Am Chem Soc, 2006, 128: 6536–6537
De CK, Klauber EG, Seidel D. J Am Chem Soc, 2009, 131: 17060–17061
Fowler BS, Mikochik PJ, Miller SJ. J Am Chem Soc, 2010, 132: 2870–2871
Klauber EG, De CK, Shah TK, Seidel D. J Am Chem Soc, 2010, 132: 13624–13626
Binanzer M, Hsieh SY, Bode JW. J Am Chem Soc, 2011, 133: 19698–19701
Yang X, Bumbu VD, Liu P, Li X, Jiang H, Uffman EW, Guo L, Zhang W, Jiang X, Houk KN, Birman VB. J Am Chem Soc, 2012, 134: 17605–17612
Wanner B, Kreituss I, Gutierrez O, Kozlowski MC, Bode JW. J Am Chem Soc, 2015, 137: 11491–11497
Arp FO, Fu GC. J Am Chem Soc, 2006, 128: 14264–14265
Hou XL, Zheng BH. Org Lett, 2009, 11: 1789–1791
Murray JI, Flodén NJ, Bauer A, Fessner ND, Dunklemann DL, Bob-Egbe O, Rzepa HS, Bürgi T, Richardson J, Spivey AC. Angew Chem Int Ed, 2017, 56: 5760–5764
Saito K, Shibata Y, Yamanaka M, Akiyama T. J Am Chem Soc, 2013, 135: 11740–11743
Saito K, Akiyama T. Angew Chem Int Ed, 2016, 55: 3148–3152
Wang G, Lu R, He C, Liu L. Nat Commun, 2021, 12: 2512
Guan H, Tung CH, Liu L. J Am Chem Soc, 2022, 144: 5976–5984
Yang Z, Chen F, He Y, Yang N, Fan QH. Angew Chem Int Ed, 2016, 55: 13863–13866
Liu C, Wang M, Xu Y, Li Y, Liu Q. Angew Chem Int Ed, 2022, 61: e202202814
Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem Int Ed, 2004, 43: 1566–1568
Uraguchi D, Terada M. J Am Chem Soc, 2004, 126: 5356–5357
Akiyama T. Chem Rev, 2007, 107: 5744–5758
Terada M. Synthesis, 2010, 2010: 1929–1982
Parmar D, Sugiono E, Raja S, Rueping M. Chem Rev, 2014, 114: 9047–9153
Li X, Song Q. Chin Chem Lett, 2018, 29: 1181–1192
Rahman A, Lin X. Org Biomol Chem, 2018, 16: 4753–4777
Chen Y, Zhu C, Guo Z, Liu W, Yang X. Angew Chem Int Ed, 2021, 60: 5268–5272
Pan Y, Wang D, Chen Y, Zhang DK, Liu W, Yang X. ACS Catal, 2021, 11: 8443–8448
Xie J, Guo Z, Liu W, Zhang D, He YP, Yang X. Chin J Chem, 2022, 40: 1674–1680
Jiang Q, Xie W, Yang X. Chem Commun, 2023, 59: 4762–4765
Egger N, Hoesch L, Dreiding AS. Helv Chim Acta, 1983, 66: 1599–1607
Kanzian T, Mayr H. Chem Eur J, 2010, 16: 11670–11677
Tang R-J, Milcent T, Crousse B. Eur J Org Chem, 2017, 2017: 4753–4757
Liu W, Jiang Q, Yang X. Angew Chem Int Ed, 2020, 59: 23598–23602
Yu S, Bao H, Zhang D, Yang X. Nat Commun, 2023, 14: 5239
Vedejs E, Chen X. J Am Chem Soc, 1997, 119: 2584–2585
Eames J. Angew Chem Int Ed, 2000, 39: 885–888
Russell TA, Vedejs E. Enantiodivergent reactions: divergent reactions on a racemic mixture and parallel kinetic resolution. In: Todd M, Ed. Separation of Enantiomers: Synthetic Methods. Weinheim: Wiley-VCH, 2014. 217–266.
Kagna HB, Fiaud JC. Topics in stereochemistry. In: Eliel EL, Ed. Kinetic Resolution. New York: Wiley, 1988. 249–330
Yin L, Xing J, Wang Y, Shen Y, Lu T, Hayashi T, Dou X. Angew Chem Int Ed, 2019, 58: 2474–2478
Zhang D, Shao YB, Xie W, Chen Y, Liu W, Bao H, He F, Xue XS, Yang X. ACS Catal, 2022, 12: 14609–14618
Bariwal J, Voskressensky LG, Van der Eycken EV. Chem Soc Rev, 2018, 47: 3831–3848
James MJ, O’Brien P, Taylor RJK, Unsworth WP. Chem Eur J, 2016, 22: 2856–2881
See Supporting Informations for details
Nandi RK, Guillot R, Kouklovsky C, Vincent G. Org Lett, 2016, 18: 1716–1719
Sabat N, Zhou W, Gandon V, Guinchard X, Vincent G. Angew Chem Int Ed, 2022, 61: e202204400
Wu KJ, Dai LX, You SL. Org Lett, 2012, 14: 3772–3775
Wang G, Piva de Silva G, Wiebe NE, Fehr GM, Davis RL. RSC Adv, 2017, 7: 48848–48852
Ertugrul B, Kilic H, Lafzi F, Saracoglu N. J Org Chem, 2018, 83: 9018–9038
Singh P, Mritunjay P. Asian J Org Chem, 2021, 10: 964–979
Jackson AH, Smith AE. Tetrahedron, 1965, 21: 989–1000
Ibaceta-Lizana JSL, Jackson AH, Prasitpan N, Shannon PVR. J Chem Soc Perkin Trans 2, 1987, 1221–1226
Fedoseev P, Coppola G, Ojeda GM, Van der Eycken EV. Chem Commun, 2018, 54: 3625–3628
Deposition Numbers 2285455 (for 4a′) contains the supplementary crystallographic data for this paper These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171186, 22222107), ShanghaiTech University Start-up Funding, and Analytical Instrumentation Center (# SPSTAIC10112914), SPST, ShanghaiTech University. The authors thank Mr. Huanchao Gu for the assistance with X-ray crystallographic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Jiang, Q., Zhang, D., Tang, M. et al. (Parallel) kinetic resolution of 3,3-disubstituted indolines via organocatalyzed reactions with azodicarboxylates. Sci. China Chem. 67, 973–980 (2024). https://doi.org/10.1007/s11426-023-1810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1810-9