Abstract
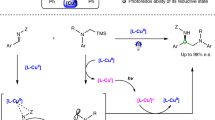
By capitalizing on the capability of photoredox catalysis to generate reactive radical intermediate under mild conditions, we established a photocatalytic cross-coupling protocol that could deliver both derivatives from 1-bromo-2-naphthols in combination with 2-naphthols or 2-naphthylamines. This distinct activation mode could overcome structural or electronic limitation associated with conventional coupling pathways. Additionally, a novel kinetic resolution protocol of unprotected BINOLs has been established with azodicarboxylates via chiral phosphoric acid (CPA) catalysis. Selectivity factor of up to 175 could be achieved and delivered to both enantiomers in atropisomerically enriched form after a simple work-up.

Similar content being viewed by others
Change history
12 December 2022
An Erratum to this paper has been published: https://doi.org/10.1007/s11426-022-1482-y
References
Chen Y, Yekta S, Yudin AK. Chem Rev, 2003, 103: 3155–3212
Kocovský P, Vyskocil S, Smrcina M. Chem Rev, 2003, 103: 3213–3246
Akiyama T. Chem Rev, 2007, 107: 5744–5758
Ding K, Li X, Ji B, Guo H, Kitamura M. COS, 2005, 2: 499–545
Zhou QL. Privileged Chiral Ligands and Catalysts. Weinheim: Wiley-VCH, 2011
Noyori R, Takaya H. Acc Chem Res, 1990, 23: 345–350
Brunel JM. Chem Rev, 2007, 107: PR1–PR45
Yang X, Toste FD. J Am Chem Soc, 2015, 137: 3205–3208
Bringmann G, Gulder T, Gulder TAM, Breuning M. Chem Rev, 2011, 111: 563–639
Kozlowski MC, Morgan BJ, Linton EC. Chem Soc Rev, 2009, 38: 3193–3207
Smyth JE, Butler NM, Keller PA. Nat Prod Rep, 2015, 32: 1562–1583
Clayden J, Moran WJ, Edwards PJ, LaPlante SR. Angew Chem Int Ed, 2009, 48: 6398–6401
Laplante SR, D Fader L, Fandrick KR, Fandrick DR, Hucke O, Kemper R, Miller SPF, Edwards PJ. J Med Chem, 2011, 54: 7005–7022
LaPlante SR, Edwards PJ, Fader LD, Jakalian A, Hucke O. ChemMedChem, 2011, 6: 505–513
Pu L. Chem Rev, 1998, 98: 2405–2494
Hartley CS, Lazar C, Wand MD, Lemieux RP. J Am Chem Soc, 2002, 124: 13513–13518
Wen K, Yu S, Huang Z, Chen L, Xiao M, Yu X, Pu L. J Am Chem Soc, 2015, 137: 4517–4524
Takaishi K, Yasui M, Ema T. J Am Chem Soc, 2018, 140: 5334–5338
Erbas-Cakmak S, Leigh DA, McTernan CT, Nussbaumer AL. Chem Rev, 2015, 115: 10081–10206
Wang YB, Tan B. Acc Chem Res, 2018, 51: 534–547
Cheng JK, Xiang SH, Li S, Ye L, Tan B. Chem Rev, 2021, 121: 4805–4902
Wencel-Delord J, Panossian A, Leroux FR, Colobert F. Chem Soc Rev, 2015, 44: 3418–3430
Lassaletta JM. Atropisomerism and Axial Chirality, Singapore: World Scientific Publishing, 2019
Tan B. Axially Chiral Compounds: Asymmetric Synthesis and Applications. Weinheim: Wiley-VCH, 2021
Wencel-Delord J, Colobert F. Synopen, 2020, 4: 107–115
Loxq P, Manoury E, Poli R, Deydier E, Labande A. Coord Chem Rev, 2016, 308: 131–190
Qi LW, Li S, Xiang SH, Wang JJ, Tan B. Nat Catal, 2019, 2: 314–323
Ding WY, Yu P, An QJ, Bay KL, Xiang SH, Li S, Chen Y, Houk KN, Tan B. Chem, 2020, 6: 2046–2059
Coombs G, Sak MH, Miller SJ. Angew Chem Int Ed, 2020, 59: 2875–2880
Wang JZ, Zhou J, Xu C, Sun H, Kürti L, Xu QL. J Am Chem Soc, 2016, 138: 5202–5205
Xu G, Fu W, Liu G, Senanayake CH, Tang W. J Am Chem Soc, 2014, 136: 570–573
Li C, Chen D, Tang W. Synlett, 2016, 27: 2183–2200
Yang H, Sun J, Gu W, Tang W. J Am Chem Soc, 2020, 142: 8036–8043
Shen D, Xu Y, Shi SL. J Am Chem Soc, 2019, 141: 14938–14945
Wang H. Chirality, 2010, 22: 827–837
Nakajima M, Miyoshi I, Kanayama K, Hashimoto S, Noji M, Koga K. J Org Chem, 1999, 64: 2264–2271
Li X, Yang J, Kozlowski MC. Org Lett, 2001, 3: 1137–1140
Hewgley JB, Stahl SS, Kozlowski MC. J Am Chem Soc, 2008, 130: 12232–12233
Li X, Hewgley JB, Mulrooney CA, Yang J, Kozlowski MC. J Org Chem, 2003, 68: 5500–5511
Luo Z, Liu Q, Gong L, Cui X, Mi A, Jiang Y. Angew Chem Int Ed, 2002, 41: 4532–4535
Guo QX, Wu ZJ, Luo ZB, Liu QZ, Ye JL, Luo SW, Cun LF, Gong LZ. J Am Chem Soc, 2007, 129: 13927–13938
Hon SW, Li CH, Kuo JH, Barhate NB, Liu YH, Wang Y, Chen CT. Org Lett, 2001, 3: 869–872
Egami H, Katsuki T. J Am Chem Soc, 2009, 131: 6082–6083
Irie R, Masutani K, Katsuki T, Synlett, 2000, 2000: 1433–1436
Egami H, Matsumoto K, Oguma T, Kunisu T, Katsuki T. J Am Chem Soc, 2010, 132: 13633–13635
Narute S, Parnes R, Toste FD, Pappo D. J Am Chem Soc, 2016, 138: 16553–16560
Tian JM, Wang AF, Yang JS, Zhao XJ, Tu YQ, Zhang SY, Chen ZM. Angew Chem Int Ed, 2019, 58: 11023–11027
Zhao XJ, Li ZH, Ding TM, Tian JM, Tu YQ, Wang AF, Xie YY. Angew Chem Int Ed, 2021, 60: 7061–7065
Hayashi H, Ueno T, Kim C, Uchida T. Org Lett, 2020, 22: 1469–1474
Hayashi H, Ueno T, Kim C, Uchida T. Org Lett, 2020, 22: 1469–1474
Yuan H, Du Y, Liu F, Guo L, Sun Q, Feng L, Gao H. Chem Commun, 2020, 56: 8226–8229
Zhang J, Qi L, Li S, Xiang S, Tan B. Chin J Chem, 2020, 38: 1503–1514
Zhang JW, Xiang SH, Li S, Tan B. Molecules, 2021, 26: 3223
Zhang JW, Jiang F, Chen YH, Xiang SH, Tan B. Sci China Chem, 2021, 64: 1515–1521
Link A, Sparr C. Chem Soc Rev, 2018, 47: 3804–3815
Tanaka K. Chem Asian J, 2009, 4: 508–518
Zhao Q, Peng C, Wang YT, Zhan G, Han B. Org Chem Front, 2021, 8: 2772–2785
Witzig RM, Fäseke VC, Häussinger D, Sparr C. Nat Catal, 2019, 2: 925–930
Takano H, Shiozawa N, Imai Y, Kanyiva KS, Shibata T. J Am Chem Soc, 2020, 142: 4714–4722
Xu K, Li W, Zhu S, Zhu T. Angew Chem Int Ed, 2019, 58: 17625–17630
Di Iorio N, Crotti S, Bencivenni G. Chem Rec, 2019, 19: 2095–2104
Yang G, Guo D, Meng D, Wang J. Nat Commun, 2019, 10: 3062
Lu S, Poh SB, Rong ZQ, Zhao Y. Org Lett, 2019, 21: 6169–6172
Munday ES, Grove MA, Feoktistova T, Brueckner AC, Walden DM, Young CM, Slawin AMZ, Campbell AD, Cheong PHY, Smith AD. Angew Chem Int Ed, 2020, 59: 7897–7905
Yao QJ, Zhang S, Zhan BB, Shi BF. Angew Chem Int Ed, 2017, 56: 6617–6621
Liao G, Yao QJ, Zhang ZZ, Wu YJ, Huang DY, Shi BF. Angew Chem Int Ed, 2018, 57: 3661–3665
Liao G, Li B, Chen HM, Yao QJ, Xia YN, Luo J, Shi BF. Angew Chem Int Ed, 2018, 57: 17151–17155
Liao G, Chen HM, Xia YN, Li B, Yao QJ, Shi BF. Angew Chem Int Ed, 2019, 58: 11464–11468
Ma G, Sibi MP. Chem Eur J, 2015, 21: 11644–11657
Liu W, Jiang Q, Yang X. Angew Chem Int Ed, 2020, 59: 23598–23602
Fang S, Tan JP, Pan J, Zhang H, Chen Y, Ren X, Wang T. Angew Chem Int Ed, 2021, 60: 14921–14930
Lu S, Ng SVH, Lovato K, Ong JY, Poh SB, Ng XQ, Kürti L, Zhao Y. Nat Commun, 2019, 10: 3061
Aoyama H, Tokunaga M, Kiyosu J, Iwasawa T, Obora Y, Tsuji Y. J Am Chem Soc, 2005, 127: 10474–10475
Lu S, Poh SB, Zhao Y. Angew Chem Int Ed, 2014, 53: 11041–11045
Ma G, Deng J, Sibi MP. Angew Chem Int Ed, 2014, 53: 11818–11821
Jones BA, Balan T, Jolliffe JD, Campbell CD, Smith MD. Angew Chem Int Ed, 2019, 58: 4596–4600
Crabtree RH. Chem Rev, 2015, 115: 127–150
Smrcina M, Vyskocil S, Maca B, Polasek M, Claxton TA, Abbott AP, Kocovsky P. J Org Chem, 1994, 59: 2156–2163
Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
Curran DP, Ko SB. Tetrahedron Lett, 1998, 39: 6629–6632
Arceo E, Montroni E, Melchiorre P. Angew Chem Int Ed, 2014, 53: 12064–12068
Esumi N, Suzuki K, Nishimoto Y, Yasuda M. Org Lett, 2016, 18: 5704–5707
Zheng D, Studer A. Angew Chem Int Ed, 2019, 58: 15803–15807
Spinnato D, Schweitzer-Chaput B, Goti G, Ošeka M, Melchiorre P. Angew Chem Int Ed, 2020, 59: 9485–9490
Wang J, Zhao Y, Gao H, Gao GL, Yang C, Xia W. Asian J Org Chem, 2017, 6: 1402–1407
Kautsky H. Trans Faraday Soc, 1939, 35: 216–219
Kuijpers KPL, Bottecchia C, Cambié D, Drummen K, König NJ, Noël T. Angew Chem Int Ed, 2018, 57: 11278–11282
Lemos A, Lemaire C, Luxen A. Adv Synth Catal, 2019, 361: 1500–1537
Lee DS, Kim CS, Iqbal N, Park GS, Son KS, Cho EJ. Org Lett, 2019, 21: 9950–9953
Jin C, Zhuang X, Sun B, Li D, Zhu R. Asian J Org Chem, 2019, 8: 1490–1494
Quintavalla A, Veronesi R, Carboni D, Martinelli A, Zaccheroni N, Mummolo L, Lombardo M. Adv Synth Catal, 2021, 363: 3267–3282
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21825105), the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002), the Guangdong Innovative Program (2019BT02Y335), the Shenzhen Special Funds (JCYJ20190812-112603598, JCYJ20210324120205016), the Shenzhen Nobel Prize Scientists Laboratory Project (C17213101) and the SUSTech Special Fund for the Construction of High-Level Universities (G02216302). The authors appreciate the assistance of SUSTech Core Research Facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Li, HH., Zhang, JY., Li, S. et al. Asymmetric synthesis of binaphthyls through photocatalytic cross-coupling and organocatalytic kinetic resolution. Sci. China Chem. 65, 1142–1148 (2022). https://doi.org/10.1007/s11426-022-1246-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1246-8