Abstract
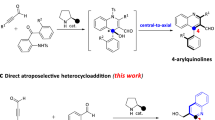
Organocatalytic dynamic kinetic resolution of configurationally labile cyclic molecules represents one of the most efficient methods for the atroposelective construction of axially chiral molecules bearing a tetra-ortho-substituted chiral axis. Notably, this privileged strategy is limited to constructing a C-C chiral axis. Herein, organocatalytic dynamic kinetic resolution of configurationally labile N-arylindole lactams has been successfully achieved at the first time, allowing for access to a structurally diverse set of axially chiral N-arylindole amino esters with a tetra-ortho-substituted C-N chiral axis in excellent yields and atroposelectivities. In addition to the N-arylindole skeleton, N-aryl thieno[3,2-b]pyrrole, furo[3,2-b]pyrrole, and pyrrolo[2,3-b] pyridine skeletons are also compatible with this transformation. This transition-metal-free facile strategy features a broad substrate scope, mild reaction conditions, easy scale-up and excellent atom economy. Several potentially valuable molecules, such as axially chiral peptides, were efficiently generated from the resulting configurationally stable axially-chiral N-arylindole amino esters, demonstrating the power of this strategy.

Similar content being viewed by others
References
Bringmann G, Gulder T, Gulder TAM, Breuning M. Chem Rev, 2011, 111: 563–639
Clayden J, Moran WJ, Edwards PJ, LaPlante SR. Angew Chem Int Ed, 2009, 48: 6398–6401
Fu W, Tang W. ACS Catal, 2016, 6: 4814–4858
Xie JH, Zhou QL. Acc Chem Res, 2008, 41: 581–593
Feuillastre S, Pauton M, Gao L, Desmarchelier A, Riives AJ, Prim D, Tondelier D, Geffroy B, Muller G, Clavier G, Pieters G. J Am Chem Soc, 2016, 138: 3990–3993
Link A, Sparr C. Chem Soc Rev, 2018, 47: 3804–3815
Cheng JK, Xiang SH, Li S, Ye L, Tan B. Chem Rev, 2021, 121: 4805–4902
Carmona JA, Rodríguez-Franco C, Fernández R, Hornillos V, Lassaletta JM. Chem Soc Rev, 2021, 50: 2968–2983
Carmona JA, Rodríguez-Franco C, López-Serrano J, Ros A, Iglesias-Sigüenza J, Fernández R, Lassaletta JM, Hornillos V. ACS Catal, 2021, 11: 4117–4124
Zhang JW, Jiang F, Chen YH, Xiang SH, Tan B. Sci China Chem, 2021, 64: 1515–1521
Xu MM, You XY, Zhang YZ, Lu Y, Tan K, Yang L, Cai Q. J Am Chem Soc, 2021, 143: 8993–9001
Yang H, Sun J, Gu W, Tang W. J Am Chem Soc, 2020, 142: 8036–8043
Witzig RM, Fäseke VC, Häussinger D, Sparr C. Nat Catal, 2019, 2: 925–930
Shen D, Xu Y, Shi SL. J Am Chem Soc, 2019, 141: 14938–14945
Patel ND, Sieber JD, Tcyrulnikov S, Simmons BJ, Rivalti D, Duvvuri K, Zhang Y, Gao DA, Fandrick KR, Haddad N, Lao KS, Mangunuru HPR, Biswas S, Qu B, Grinberg N, Pennino S, Lee H, Song JJ, Gupton BF, Garg NK, Kozlowski MC, Senanayake CH. ACS Catal, 2018, 8: 10190–10209
Yu C, Huang H, Li X, Zhang Y, Wang W. J Am Chem Soc, 2016, 138: 6956–6959
Chen GQ, Lin BJ, Huang JM, Zhao LY, Chen QS, Jia SP, Yin Q, Zhang X. J Am Chem Soc, 2018, 140: 8064–8068
Beleh OM, Miller E, Toste FD, Miller SJ. J Am Chem Soc, 2020, 142: 16461–16470
Wang G, Shi Q, Hu W, Chen T, Guo Y, Hu Z, Gong M, Guo J, Wei D, Fu Z, Huang W. Nat Commun, 2020, 11: 946
Zhao K, Duan L, Xu S, Jiang J, Fu Y, Gu Z. Chem, 2018, 4: 599–612
Zhang X, Zhao K, Li N, Yu J, Gong LZ, Gu Z. Angew Chem Int Ed, 2020, 59: 19899–19904
Zhao K, Yang S, Gong Q, Duan L, Gu Z. Angew Chem Int Ed, 2021, 60: 5788–5793
Shimada T, Cho YH, Hayashi T. J Am Chem Soc, 2002, 124: 13396–13397
Zhang J, Sun T, Zhang Z, Cao H, Bai Z, Cao ZC. J Am Chem Soc, 2021, 143: 18380–18387
Feng J, Bi X, Xue X, Li N, Shi L, Gu Z. Nat Commun, 2020, 11: 4449
Bi X, Feng J, Xue X, Gu Z. Org Lett, 2021, 23: 3201–3206
Deng R, Zhan S, Li C, Gu Z. Angew Chem Int Ed, 2020, 59: 3093–3098
Deng R, Xi J, Li Q, Gu Z. Chem, 2019, 5: 1834–1846
Kamikawa K, Kinoshita S, Matsuzaka H, Uemura M. Org Lett, 2006, 8: 1097–1100
Ototake N, Morimoto Y, Mokuya A, Fukaya H, Shida Y, Kitagawa O. Chem Eur J, 2010, 16: 6752–6755
Zhao Q, Peng C, Wang YT, Zhan G, Han B. Org Chem Front, 2021, 8: 2772–2785
Shang Q, Tang H, Liu Y, Yin MM, Su L, Xie S, Liu L, Yang W, Chen Y, Dong J, Zhou Y, Yin SF. Chem Sci, 2022, 13: 263–273
Xu WL, Zhao WM, Zhang RX, Chen J, Zhou L. Chem Sci, 2021, 12: 14920–14926
Gu XW, Sun YL, Xie JL, Wang XB, Xu Z, Yin GW, Li L, Yang KF, Xu LW. Nat Commun, 2020, 11: 2904
Wang L, Zhong J, Lin X. Angew Chem Int Ed, 2019, 58: 15824–15828
Lu S, Ng SVH, Lovato K, Ong JY, Poh SB, Ng XQ, Kürti L, Zhao Y. Nat Commun, 2019, 10: 3061
Li SL, Yang C, Wu Q, Zheng HL, Li X, Cheng JP. J Am Chem Soc, 2018, 140: 12836–12843
Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Chem Rev, 2015, 115: 11239–11300
Clayden J, Senior J, Helliwell M. Angew Chem Int Ed, 2009, 48: 6270–6273
Brückner H, Fujii N. D-Amino Acids in Chemistry, Life Sciences, and Biotechnology. Weinheim: Wiley, 2010
Saghyan AS, & Langer P. Asymmetric Synthesis of Non-Proteinogenic Amino Acids. Weinheim: Wiley, 2016
Hughes AB. Amino Acids, Peptides and Proteins in Organic Chemistry. Vol 5. Analysis and Function of Amino Acids and Peptides. Weinheim: Wiley, 2016
Zhang Y, Huang J, Guo Y, Li L, Fu Z, Huang W. Angew Chem Int Ed, 2018, 57: 4594–4598
Wang G, Zhang QC, Wei C, Zhang Y, Zhang L, Huang J, Wei D, Fu Z, Huang W. Angew Chem Int Ed, 2021, 60: 7913–7919
Bringmann G, Tasler S, Endress H, Kraus J, Messer K, Wohlfarth M, Lobin W. J Am Chem Soc, 2001, 123: 2703–2711
Zhang MZ, Chen Q, Yang GF. Eur J Medicinal Chem, 2015, 89: 421–441
Baumann T, Brückner R. Angew Chem Int Ed, 2019, 58: 4714–4719
Sindac JA, Yestrepsky BD, Barraza SJ, Bolduc KL, Blakely PK, Keep RF, Irani DN, Miller DJ, Larsen SD. J Med Chem, 2012, 55: 3535–3545
Wang R, Shi HF, Zhao JF, He YP, Zhang HB, Liu JP. Bioorg Med Chem Lett, 2013, 23: 1760–1762
Kessler A, Faure H, Petrel C, Ruat M, Dauban P, Dodd RH. Bioorg Med Chem Lett, 2004, 14: 3345–3349
Acknowledgements
This work was support by the National Key Research and Development Program of China (2017YFA0204704), the General Program of Chongqing Natural Science Foundation Project (cstc2020jcyj-msxmX0712), Ningbo Natural Science Foundation (202003N4063), the National Natural Science Foundation of China (21602105, 22174065), and the Natural Science Foundation of Jiangsu Province (BK20171460).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supplementary Information
11426_2021_1209_MOESM1_ESM.pdf
Organocatalytic Dynamic Kinetic Resolution of N-Arylindole Lactams: Atroposelective Construction of Axially Chiral Amino Acids Bearing a C-N Chiral Axis
Rights and permissions
About this article
Cite this article
Hong, X., Guo, J., Liu, J. et al. Organocatalytic dynamic kinetic resolution of N-arylindole lactams: atroposelective construction of axially chiral amino acids bearing a C-N chiral axis. Sci. China Chem. 65, 905–911 (2022). https://doi.org/10.1007/s11426-021-1209-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1209-2