Abstract
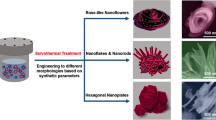
ZnO micro/nanostructures with various morphologies were grown via hydrothermal etching of Zn foil. Controlling the reaction temperature and time, rod-like, pencil-like, tube-like and flowerlike ZnO micro/nanostructures could be prepared directly on the Zn foil surface at temperatures 100–180 °C with excellent reproducibility. X-ray diffraction patterns indicated that these ZnO micro/nanostructures were hexagonal. Possible mechanisms for the variation of morphology are discussed. Moreover, photoluminescence spectra of the as-grown samples revealed that all of them consist of UV emission band at around 392 nm.
Similar content being viewed by others
References
Soh CB, Tay CB, Chua SJ, Le HQ, Ang NSS, Teng JH. Optimization of hydrothermal growth ZnO Nanorods for enhancement of light extraction from GaN blue LEDs. J Cryst Grow, 2010, 312: 1848–1854
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H, Weber E, Russo R, Yang P. Room-temperature ultraviolet nanowire nanolasers. Science, 2001, 292: 1897–1899
Wang ZL, Song JH. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science, 2006, 312: 242–246
He JH, Hsin CL, Liu J, Chen LJ, Wang ZL. Piezoelectric gated diode of a single ZnO nanowire. Adv Mater, 2007, 19: 781–784
Qian X, Liu H, Guo Y, Song Y, Li Y. Effect of aspect ratio on field emission properties of ZnO nanorod arrays. Nanoscale Res Lett, 2008, 3: 303–307
Bansal V, Jani H, DuPlessis J, Coloe PJ, Bhargava SK. Ni-Cu composite porous nanostructures obtained by galvanic replacement reactions. Adv Mater, 2008, 20: 713–717
Wu WY, Ting JM, Huang PJ. Electrospun ZnO nanowires as gas sensors for ethanol detections. Nanoscale Res Lett, 2009, 4: 513–517
Wang X, Summers CJ, Wang ZL. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett, 2004, 4: 423–426.
Arico AS, Bruce P, Scrosati B, Tarascon JM, Schalkwijk W Van. MnO2/Poly(3,4-ethyenedioxythiophene) coaxial nanowires by one-step coelectrodeposition for electrochemical energy storage. Nat Mater, 2005, 4: 366–337
Greyson EC, Babayan Y, Odom TW. Directed growth of ordered arrays of small-diameter ZnO nanowires. Adv Mater, 2004, 16: 1348–1352
Fan HJ, Fleischer F, Lee W, Nielsch K, Scholz R, Zacharias M, Gösele U, Dadgar A, Krost A. Patterned growth of aligned ZnO nanowire arrays on sapphire and GaN layers. Supperlattice Microst, 2004, 36: 95–105
Chik H, Liang J, Cloutier SG, Kouklin N, Xu JM. Periodic array of uniform ZnO nanorods by second-order self-assembly. Appl Phys Lett, 2004, 84: 3376–3378
Pan ZW. Dai ZR, Wang ZL. Nanobelts of semiconducting oxides. Science, 2001, 291: 1947–1949
Guo L, Ji YL, Xu HB, Simon P, Wu ZY. Regularly shaped, single-crystallione ZnO nanorods with wurtzite structure. J Am Chem Soc, 2002, 124: 14864–14865
Wei A, Sun XW, Xu CX, Dong ZL, Yang Y, Tan ST, Huang W, Growth mechanism of tubular ZnO formed in aqueous solution. Nanotechnology, 2006,17: 1740–1744
Yu HD, Zhang ZP, Han MY, Hao XT, Zhu FR. ZnO nanotube ethanol gas sensor. J Am Chem Soc, 2005, 127: 2378–2379
Li Y, Feng HY, Zhang N, Liu CS. Solvo-thermal synthesis and characterization of nest-like zinc oxide. Trans Nonferrous Met Soc China, 2010, 20: 119–122
Gao PX, Lee JL, Zhang ZL. Multicolored ZnO nanowire architectures of trenched silicon substrates. J Phys Chem C, 2007, 111: 13763–13769
Liu YM, Fang QQ, Wu MZ, Li Y, Lv QR, Zhou J, Wang BM. Structure and photoluminescence of arrayed Zn1−x CoxO nanorods grown via hydrothermal method. J Phy D Appl Phys, 2007, 40: 4592–4596
Tam KH, Cheung CK, Leung YH, Djurišic AB, Ling CC, Beling CD, Fung S, Kwok WM, Chan WK, Phillips DL, Ding L, Ge WK. Defects in ZnO nanorods prepared by a hydrothermal method. J Phy Chem B, 2006, 110: 20865–20871
Zhang YH, Li XJ, Zheng L, Chen QW. Nondegrading photoluminescence in porous silicon. Phys Rev Lett, 1998, 81: 1710–1713
Lee SM, Cho SN, Cheon JW. Anisotropic shape control of colloidal inorganic nanocrystals. Adv Mater, 2003, 15: 441–444
Ghosh KS, Tsujii K. Effect of pressure on colloidal behavior in hydrothermal water. J Phys Chem B, 2008, 112: 6906–6913
Marshall WL and Frank EU. Ion product of water substance, 0-1000 °C, 1-10,000 bars, new international formulation and its background. J Phys Chem Ref Data, 1981, 10: 295–304
Lioudmila ND, Dmitriy VK. Mechanism of zinc single crystal growth under hydrothermal conditions. Annu Chim Sci Mat, 2001, 26: 193–198
Guo M, Diao P, Cai SM. Hydrothermal growth of well-aligned ZnO nanorod arrays: Dependence of morphology and alignment ordering upon preparing conditions. J Solid State Chem, 2005, 178: 1864–1873
Huang MH, Wu Y, Feick H, Tran N, Weber E, Yang P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv Mater, 2001, 13: 113–116
Wu JJ,. Liu SC. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv Mater, 2002, 14: 215–218
Wang YC, Leu IC, Hon MH. Effect of colloid characteristics on the fabrication of ZnO nanowire arrays by electrophoretic deposition. J Mater Chem, 2002, 12: 2439–2444
Vanheusden K, Warren WL, Seager CH, Tallant DR, Voigt JA, Gnade BE. Mechanisms behind green photoluminescence in ZnO phosphor powders. J Appl Phys, 1996, 79: 7983–7790
Hsu CL, Chang SJ, Hung HC, Lin YR, Huang CJ, Tseng YK, Chen IC. Vertical single-crystal ZnO nanowires grown on ZnO: Ga/glass templates. IEEE Trans Nanotechnol, 2005, 4: 649–654
Lupan O, Chow L, Chai G.Y, Roldan B, Naitabdi A, Schulte A, Heinrich H. Nanofabrication and characterization of ZnO nanorod arrays and branched microrods by aqueous solution route and rapid thermal processing. Mater Sci Eng B, 2007, 145: 57–66
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, M., Lu, X., Liu, Y. et al. A facile hydrothermal preparation and photoluminescence study of ZnO micro/nanostructures on Zn foils. Sci. China Chem. 54, 1547–1551 (2011). https://doi.org/10.1007/s11426-011-4322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4322-y