Abstract
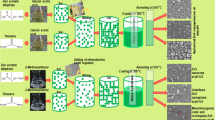
Zinc sulfide (ZnS) nanostructures with various morphologies play an imperative role in optoelectronic applications. In this study, different ZnS nanostructures with well-defined morphologies were synthesized in a controlled manner by a low-temperature solvothermal method using the binary solvent mixtures of ethylenediamine and water (EN/W). Controlling the content of EN and the growth temperature, ZnS nanostructures including nanoflowers, nanoflakes, nanorods, and hexagonal nanoplates were produced at a very low temperature ranging from 100 °C to 180 °C during short reaction times of 2 h and 6 h with excellent reproducibility. X-ray diffraction patterns of the nanostructures considerably revealed the single crystalline nature with a pure wurtzite phase of ZnS even at the low growth temperature having the average crystallite size in the range of 12.8–25.0 nm. The morphology evolution of the samples showed that there is a strong correlation between the morphologies of the ZnS nanostructures and the variations of both the growth temperature and reaction solvent. Based on the experimental results, a growth mechanism was also proposed for all the ZnS nanostructures with different morphologies. A sharp absorption band-edge was found for the ZnS nanostructures, in which the optical bandgap energy was laid ranging from 3.97 eV to 4.09 eV due to the quantum confinement effect. All the samples featured a broad asymmetrical photoluminescence emission with multiple peaks, corresponding to excitonic and trapped luminescence centers. The effect of morphology on the optoelectronic performance resulted in a tremendous photoresponsivity and an excellent time-response switching behavior in UV region.












Similar content being viewed by others
References
Acharya SA, Maheshwari N, Tatikondewar L, Kshirsagar A, Kulkarni SK (2013) Ethylenediamine-mediated wurtzite phase formation in ZnS. Crystal Growth & Design 13:1369–1376
Bacherikov YY, Lytvyn PM, Okhrimenko OB, Zhuk AG, Kurichka RV, Doroshkevich AS (2018) Surface potential of meso-dimensional ZnS: Mn particles obtained using SHS method. Journal of Nanoparticle Research 20:316
Biswas S, Kar S (2008) Fabrication of ZnS nanoparticles and nanorods with cubic and hexagonal crystal structures: a simple solvothermal approach. Nanotechnology 19:045710
Chandran A, Francis N, Jose T, George KC (2010) Synthesis, structural characterization and optical bandgap determination of ZnS nanoparticles. Acad Rev 17:17–21
Chen X, Xu H, Xu N, Zhao F, Lin W, Lin G, Yunlong F, Huang Z, Wang H, Mingmei W (2003) Kinetically controlled synthesis of wurtzite ZnS nanorods through mild thermolysis of a covalent organic− inorganic network. Inorganic Chemistry 42:3100–3106
Cullity, BD, and SR Stock. 2001. "Elements of X-ray diffraction third edition prentice hall upper saddle river." In.: NJ.
Demazeau G (2010) Solvothermal processes: definition, key factors governing the involved chemical reactions and new trends. Zeitschrift für Naturforschung B 65:999–1006
Deng Z-X, Cheng W, Sun X-M, Li Y-D (2002) Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorganic Chemistry 41:869–873
Ebrahimi S, Yarmand B, Naderi N (2019) Enhanced optoelectrical properties of Mn-doped ZnS films deposited by spray pyrolysis for ultraviolet detection applications. Thin Solid Films 676:31–41
Fang X-S, Ye C-H, Zhang L-D, Wang Y-H, Yu-Cheng W (2005) Temperature-controlled catalytic growth of ZnS nanostructures by the evaporation of ZnS nanopowders. Advanced Functional Materials 15:63–68
Fang, Xiaosheng, Yoshio Bando, Changhui Ye, and Dmitri Golberg. 2007a. 'Crystal orientation-ordered ZnS nanobelt quasi-arrays and their enhanced field-emission', Chemical Communications: 3048-50.
Fang X, Bando Y, Ye C, Shen G, Golberg D (2007b) Shape-and size-controlled growth of ZnS nanostructures. The Journal of Physical Chemistry C 111:8469–8474
Fang XS, Yoshio B, Shen GZ, Ye CH, Gautam UK, Costa PMFJ, Zhi CY, Tang CC, Golberg D (2007c) Ultrafine ZnS nanobelts as field emitters. Advanced Materials 19:2593–2596
Fang X, Gautam UK, Bando Y, Dierre B, Sekiguchi T, Golberg D (2008) Multiangular branched ZnS nanostructures with needle-shaped tips: potential luminescent and field-emitter nanomaterial. The Journal of Physical Chemistry C 112:4735–4742
Fang X, Wu L, Linfeng H (2011a) ZnS nanostructure arrays: a developing material star. Advanced Materials 23:585–598
Fang X, Zhai T, Gautam UK, Li L, Wu L, Bando Y, Golberg D (2011b) ZnS nanostructures: from synthesis to applications. Progress in Materials Science 56:175–287
Han S, Liu W, Sun K, Xiaotao Z (2016) Experimental evidence of ZnS precursor anisotropy activated by ethylenediamine for constructing nanowires and single-atomic layered hybrid structures. CrystEngComm 18:2626–2631
Huang X, Li J, Zhang Y, Mascarenhas A (2003) From 1D chain to 3D network: tuning hybrid II-VI nanostructures and their optical properties. Journal of the American Chemical Society 125:7049–7055
Jang JS, Yu C-J, Choi SH, Ji SM, Kim ES, Lee JS (2008) Topotactic synthesis of mesoporous ZnS and ZnO nanoplates and their photocatalytic activity. Journal of Catalysis 254:144–155
Joshi RK, Schneider JJ (2012) Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chemical Society Reviews 41:5285–5312
Khomutov GB, Kislov VV, Gainutdinov RV, Gubin SP, Obydenov AY, Pavlov SA, Sergeev-Cherenkov AN, Soldatov ES, Tolstikhina AL, Trifonov AS (2003) The design, fabrication and characterization of controlled-morphology nanomaterials and functional planar molecular nanocluster-based nanostructures. Surface science 532:287–293
Kole AK, Tiwary CS, Kumbhakar P (2014) Morphology controlled synthesis of wurtzite ZnS nanostructures through simple hydrothermal method and observation of white light emission from ZnO obtained by annealing the synthesized ZnS nanostructures. Journal of Materials Chemistry C 2:4338–4346
Li LS, Pradhan N, Wang Y, Peng X (2004a) High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano letters 4:2261–2264
Li Y, Li X, Yang C, Li Y (2004b) Ligand-controlling synthesis and ordered assembly of ZnS nanorods and nanodots. The Journal of Physical Chemistry B 108:16002–16011
Li Z, Wang J, Xu X, Ye X (2008) The evolution of optical properties during hydrothermal coarsening of ZnS nanoparticles. Materials letters 62:3862–3864
Liang Y, Liang H, Xiao X, Hark S (2012) The epitaxial growth of ZnS nanowire arrays and their applications in UV-light detection. Journal of Materials Chemistry 22:1199–1205
Liu H, Hu L, Watanabe K, Hu X, Dierre B, Kim B, Sekiguchi T, Fang X (2013) Cathodoluminescence modulation of ZnS nanostructures by morphology, doping, and temperature. Advanced Functional Materials 23:3701–3709
Lixiong Y, Wang D, Jianfeng H, Liyun C, Haibo O, Wu J, Xiang Y (2015) Microwave hydrothermal synthesis and photocatalytic activities of morphology-controlled ZnS crystallites. Ceramics International 41:3288–3292
Mendil R, Ben Ayadi Z, Djessas K (2016) Effect of solvent medium on the structural, morphological and optical properties of ZnS nanoparticles synthesized by solvothermal route. Journal of Alloys and Compounds 678:87–92
Mi L, Han M, Li Z, Wang Y, Shen C, Zheng Z (2010) Transformation of a zinc inclusion complex to wurtzite ZnS microflowers under solvothermal conditions. Crystal Research and Technology 45:973–976
Mir IA, Alam H, Priyadarshini E, Meena R, Rawat K, Rajamani P, Rizvi MS, Bohidar HB (2018) Antimicrobial and biocompatibility of highly fluorescent ZnSe core and ZnSe@ ZnS core-shell quantum dots. Journal of Nanoparticle Research 20:174
Mosca R, Ferro P, Calestani D, Nasi L, Besagni T, Licci F (2011) Solvothermal synthesis of ZnS [C2H4 (NH2) 2] 0.5 nanosheets. Crystal Research and Technology 46:818–822
O'neil M, Marohn J, McLendon G (1990) Dynamics of electron-hole pair recombination in semiconductor clusters. Journal of Physical Chemistry 94:4356–4363
Ouyang, Xiang, Tsung-Yen Tsai, Dong-Hwang Chen, Qi-Jie Huang, Wu-Hsun Cheng, and Abraham Clearfield. 2003. 'Ab initio structure study from in-house powder diffraction of a novel ZnS (EN) 0.5 structure with layered wurtzite ZnS fragment', Chemical Communications: 886-87.
Qi S, Zhang M, Guo X, Yue L, Wang J, Shao Z, Xin B (2017) Controlled extracellular biosynthesis of ZnS quantum dots by sulphate reduction bacteria in the presence of hydroxypropyl starch as a mediator. Journal of Nanoparticle Research 19:212
Rao, CNR, Achim Muller, and Anthony K Cheetham. 2004. The chemistry of nanomaterials (Wiley Online Library).
Senthilkumaar, S, and R Thamiz Selvi. 2008. 'Synthesis and characterization of one dimensional ZnS nanorods', synthesis and reactivity in inorganic, metal-organic, and nano-metal chemistry, 38: 710-15.
Shakouri-Arani M, Salavati-Niasari M (2014) Synthesis and characterization of wurtzite ZnS nanoplates through simple solvothermal method with a novel approach. Journal of industrial and engineering chemistry 20:3179–3185
Suchanek WL, Riman RE (2006) Hydrothermal synthesis of advanced ceramic powders. In: In Advances in Science and Technology, 184-93. Publ, Trans Tech
Tiwari A, Dhoble SJ (2016) Critical analysis of phase evolution, morphological control, growth mechanism and photophysical applications of ZnS nanostructures (zero-dimensional to three-dimensional): a review. Crystal Growth & Design 17:381–407
Varshni, YP. 1967. 'Band-to-band radiative recombination in groups IV, VI, and III-V semiconductors (I)', physica status solidi (b), 19: 459-514.
Viswanath R, Bhojya Naik HS, Yashavanth Kumar GS, Prashanth Kumar PN, Arun Kumar G, Praveen R (2014) EDTA-assisted hydrothermal synthesis, characterization and photoluminescent properties of Mn2 + -doped ZnS. Journal of Luminescence 153:446–452
Wang H, Chen Z, Cheng Q, Yuan L (2009) Solvothermal synthesis and optical properties of single-crystal ZnS nanorods. Journal of Alloys and Compounds 478:872–875
Wang L, Dai J, Liu X, Zhu Z, Huang X, Pingwei W (2012a) Morphology-controlling synthesis of ZnS through a hydrothermal/solvothermal method. Ceramics International 38:1873–1878
Wang X, Xie Z, Huang H, Liu Z, Chen D, Shen G (2012b) Gas sensors, thermistor and photodetector based on ZnS nanowires. Journal of Materials Chemistry 22:6845–6850
Xi G, Wang C, Wang X, Zhang Q, Xiao H (2008) From ZnS ⊙ en0. 5 nanosheets to wurtzite ZnS nanorods under solvothermal conditions. The Journal of Physical Chemistry C 112:1946–1952
Xu X, Li S, Chen J, Cai S, Long Z, Fang X (2018) Design principles and material engineering of ZnS for optoelectronic devices and catalysis. Advanced Functional Materials 28:1802029
Yue GH, Yan PX, Yan D, Liu JZ, Qu DM, Yang Q, Fan XY (2006) Synthesis of two-dimensional micron-sized single-crystalline ZnS thin nanosheets and their photoluminescence properties. Journal of crystal growth 293:428–432
Zhang Y, Liu W, Wang R (2012) From ZnS nanoparticles, nanobelts, to nanotetrapods: the ethylenediamine modulated anisotropic growth of ZnS nanostructures. Nanoscale 4:2394–2399
Zheng XJ, Chen YQ, Zhang T, Jiang CB, Yang B, Yuan B, Mao SX, Li W (2010a) A photoconductive semiconductor switch based on an individual ZnS nanobelt. Scripta Materialia 62:520–523
Zheng XJ, Chen YQ, Zhang T, Yang B, Jiang CB, Yuan B, Zhu Z (2010b) Photoconductive semiconductor switch based on ZnS nanobelts film. Sensors and Actuators B: Chemical 147:442–446
Acknowledgment
Authors are grateful to Iran National Science Foundation (INSF) for providing financial support to undertake this work by Grant No. 95822972.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebrahimi, S., Yarmand, B. Morphology engineering and growth mechanism of ZnS nanostructures synthesized by solvothermal process. J Nanopart Res 21, 264 (2019). https://doi.org/10.1007/s11051-019-4714-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4714-z