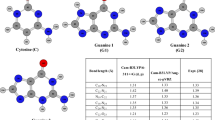
Abstract
The interacting patterns and mechanism of the catechin and cytosine have been investigated using the density functional theory B3LYP method with 6-31+G* basis set. Eleven stable structures of the catechin-cytosine complexes have been found respectively. The results indicate that the complexes are mainly stabilized by the hydrogen bonding interactions. Theories of atoms in molecules (AIM) and natural bond orbital (NBO) have been utilized to investigate the hydrogen bonds involved in all the systems. The interaction energies of all the complexes which were corrected for basis set superposition error (BSSE), are from −17.35 to −43.27 kJ/mol. The results show that the hydrogen bonding contributes to the interaction energies dominantly. The corresponding bonds stretching motions in all the complexes are red-shifted relative to that of the monomer, which is in good agreement with experimental results.
Similar content being viewed by others
References
Brant E, Billinghurst GR. Excited-state structural dynamics of cytosine from resonance Raman spectroscopy. J Phys Chem A, 2006, 110: 2353–2359
Guo JB, Zhang GW, Chen XX, Wang JJ. Studies on the interaction between catechin and DNA. J Anal Sci, 2008, 24: 507–512
Liu J, Luo GA, Wang YM, Sun HW. Research development of the interaction of small molecules with nucleic acids. Acta Pharm Sinica, 2001, 36: 74–79
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Protein kinase inhibitors: Insights into drug design from structure. Science, 2001, 293: 876–882
Zhuo L. Research progress of interactions of small molecules with DNA. J Chongqing Tech Bus Univ (Nat Sci Ed), 2005. 5: 440–445
Siavash R, Mohammad RG, Mehdi B. Theoretical investigation of interaction between Gatifloxacin and DNA: Implications for anticancer drug design. Mat Sci Eng C, 2009, 29: 1808–1813
Deepa P, Kolandaivel P, Senthilkumar K. Interactions of anticancer drugs with usual and mismatch base pairs — Density functional theory studies. Biophy Chem, 2008, 136: 50–58
Tarek M, El-Gogary, Gottfried K. Interaction of psoralens with DNA-bases (II): An ab initio quantum chemical, density functional theory and second-order Mler-Plesset perturbational study. J Mol Struct (THEOCHEM), 2009, 895: 57–64
Lv G, Chen ZX, Zheng J, Wei FD, Jiang H, Zhang RY, Wang XM. Theoretical study of the interaction pattern and the binding affinity between procaine and DNA bases. J Mol Struct (THEOCHEM) 2009, 939: 44
Yang B, Kotani A, Arai K, Kusu F. Relationship of electrochemical oxidation of catechins on their antioxidant activity in microsomal lipid peroxidation. Chem Pharm Bull, 2001, 49: 747–751
Yang CS, Chung JY, Yang GU, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr, 2000, 130: 472–478
Leanderson P, Faresjo AO, Tagesson C. Green tea polyphenols in hibit oxidant-induced DNA strand breakage in cultured lung cells. J Free Radic Biol Med, 1997, 23: 235–242
McKay DL, Blumberg JB. The role of tea in human health: An update. J Nutr, 2002, 21: 1–13
Teixeira S, Siqueta C, Alvesb C. Structure-property studies on the antioxidant activity. J Free Radic Biol Med, 2005, 39: 1099–1108
Fronczek FR, Gannuch G, Mattice WL, Tobiason FL, Broeker JL, Hemingwag RW. Dipole moment, solution, and solid state structure of (−)-epicatechin, a monomer unit of procyanidin polymers. J Chem Soc PerkinTrans II, 1984, 2: 1611–1616
Ramos-Tejada MM, Durán DG, Ontiveros-Ortega A. Investigation of alumina/(+)-catechin system properties. Colloid Surface B: Biointerf, 2002, 24: 297–308
Peng LL, Liu JL, Gou Y, Xue Y. Density functional theory study on structure and vibrational spectra of catechins. Chem Res Appl, 2008, 8: 1001–1006
Hu, F, Zhang Y, Li LL, Tian AM. Density functional theory study on hydrogen bonding interaction of catechin-(H2O)n. Chin J Chem, 2010, 28: 748
Bader RWF. Atoms in Molecules: A Quantum Theory. Oxford: Oxford University Press, 1990
Reed AE, Weinhold F, Curtiss LA, Pochatko DJ. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. J Chem Phys, 1986, 84: 5687
Boys SF, Bernardi F. Some procedures with reduced errors. J Mol Phys, 1970, 19: 553
Frisch MJ, Trucks GW, Schlegel HB, Gill PMW, Johnson BG, Robb MA, Cheeseman JR, Keith T, Petersson G.A, Montgomery JA, Raghavachari K, Al-Laham MA, Zakrzewski VG, Ortiz JV, Foresman JB, Cioslowski J, Stefanov BB, Nanayakkara A, Challacombe M, Peng CY, Ayala PY, Chen W, Wong MW, Andres JL, Replogle ES, Gomperts R, Martin RL, Stewart JP, Head-Gordon M, Gonzalez C, Pople JA. Gaussian 03, Revision A.7, Pittsburgh: Gaussian, Inc., 2003
Engle DW, Hattingh M, Hundt HKL. Structure and nuclear magnetic resonance spectra of 6-bromo-3,3′,4′,5,7-penta-0-methylcatechin. J Am Chem Soc Commun, 1978, 6: 95
Cox PJ, Kumarasamy Y, Nahar L, Sarker SD, Shoeb M. Luteolin. Acta Cryst E, 2003, 59: 975
Popelier PLA. Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A, 1998, 102: 1873
Kowalska A, Stobiecka A, Wysocki S. A computational investigation of the interactions between harmane and the functional monomers commonly used in molecular imprinting. J Mol Struct (THEOCHEM), 2009, 901: 88
Mohajeri A, Nobandegani FF. Detection and evaluation of hydrogen bond strength in nucleic acid base pairs. J Phys Chem A, 2008, 112: 281
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, W., Mao, S., Zhang, S. et al. Density functional theory study on the interaction of catechin and cytosine. Sci. China Chem. 54, 1094–1100 (2011). https://doi.org/10.1007/s11426-011-4300-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4300-4