Abstract
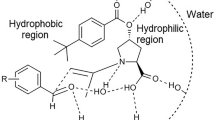
The direct asymmetric aldol reaction between various aldehydes and acetone catalyzed by l-proline catalyst was successfully carried out in supercritical CO2 (scCO2) and 1,1,1,2-tetrafluoroethane (R-134a) fluids. The enantioselectivity of 84% ee to the targeted product was achieved under 20 MPa, 40 °C, and 15 mol% of the catalyst in supercritical CO2 (scCO2) fluid. The effects of reaction parameters, such as temperature, pressure, catalyst loading and different substituted aldehydes on both enantioselectivity and aldol yield were discussed. The titled reaction was also performed in 1,1,1,2-tetrafluoroethane, and the obtained results were compared with those in scCO2. This new reaction procedure provides an environmental asymmetric aldol reaction system as compared with that in organic solvents.
Similar content being viewed by others
References
List BR, Lerner A, Barbas FCIII. Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc, 2000, 122(10): 2395–2396
Movassaghi M, Jacobsen NE. The simplest enzyme. Science, 2002, 298: 1904–1905
List B. The ying and yang of asymmetric aminocatalysis. Chem Commun, 2006, 8: 819–824
Sabitha G, Fatima N, Reddy EV, Yadav JS. First examples of proline-catalyzed domino knoevenagel/hetero-diels-alder/elimination reactions. Adv Synt Catal, 2005, 347: 1353–1355
Chen SH, Hong BC, Su CF, Sarshar S. An unexpected inversion of enantioselectivity in the proline catalyzed intramolecular Baylis-Hillman reaction. Tetrahedron Lett, 2005, 46: 8899–8903
Enders D, Seki A. Proline-catalyzed enantioselective michael additions of ketones to nitrostyrene. Synlett, 2002, 26–28
Notz W, Tanaka F, Watanabe S, Chowdari NS, Turner JM, Thayumanavan R, Barbas CFIII. The direct organocatalytic asymmetric Mannich reaction: Unmodified aldehydes as nucleophiles. J Org Chem, 2003, 68: 9624–9634
List B. Direct catalytic asymmetric alpha-amination of aldehydes. J Am Chem Soc, 2002, 124: 5656–5657
Przezdziecka A, Stepanenko W, Wicha J. Catalytic enantioselective annulation using phenylsulfanylmethyl vinyl ketone: An approach to trans-hydrindane building blocks for ent-vitamin D 3 synthesis. Tetrahedron: Asymmetry, 1999, 10(8): 1589–1598
Rispens MT, Zondervan C, Feringa BL. Catalytic enantioselective allylic oxidation. Tetrahedron: Asymmetry, 1995, 6(3): 661–664
Mestres R. A green look at the aldol reaction. Green Chem, 2004, 6: 583–603
Bellis E, Kokotos G. Proline-modified poly(propyleneimine) dendrimers as catalysts for asymmetric aldol reactions. J Mol Catal A: Chem, 2005, 241: 166–174
Chandrasekhar S, Narsihmulu C, Reddy RN; Sultana SS. Asymmetric aldol reactions in poly(ethylene glycol) catalyzed by l-proline. Tetrahedron Lett, 2004, 45: 4581–4582
Chandrasekhar S, Reddy RN, Sultana SS, Narsihmulu C, Reddy VK. L-proline catalysed asymmetric aldol reactions in PEG-400 as recyclable medium and transfer aldol reactions. Tetrahedron, 2006, 62: 338–345
Loh PT, Feng CL, Yang YH, Yang YJ. L-Proline in an ionic liquid as an efficient and reusable catalyst for direct asymmetric aldol reactions. Tetrahedron Lett, 2002, 43: 8741–8743
Hayashi Y, Aratake S, Itoh T, Okano T, Sumiya T, Shoji M. Dry and wet prolines for asymmetric organic solvent-free aldehyde-aldehyde and aldehyde-ketone aldol reactions. Chem Commun, 2007, 957–959
Kotrusz P, Kmentová I, Gotov B, Toma Š, SolČániová E. Proline-catalysed asymmetric aldol reaction in the room temperature ionic liquid [bmim]PF6. Chem Commun, 2002, 2510–2511
Fujita S, Banage MB, Ikushima Y, Arai M. Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of methyl iodide and base catalysts under mild conditions: effect of reaction conditions and reaction mechanism. Green Chem, 2001, 3: 87–91
Kawanami H, Ikushima Y. Chemical fixation of carbon dioxide to styrene carbonate under supercritical conditions with DMF in the absence of any additional catalysts. Chem Commun, 2000, 2089–2090
Kawanami H, Ikushima Y. Regioselectivity and selective enhancement of carbon dioxide fixation of 2-substituted aziridines to 2-oxazolidinones under supercritical conditions. Tetrahedron Lett, 2002, 43: 3841–3844
Kawanami H, Ikushima Y. Synthesis of 2-oxazolidinone from BETA-aminoalcohol using supercritical carbon dioxide. J Jpn Petrol Inst, 2002, 45: 321–324
Kawanami H, Sasaki A, Matsui K, Ikushima Y. A rapid and effective synthesis of propylene carbonate using a supercritical CO2-ionic liquid system. Chem Commun, 2003, 896–897
Mikami K, Matsukawa S, Kayaki Y, Ikariya T. Asymmetric mukaiyama aldol reaction of a ketene silyl acetal of thioester catalyzed by a binaphthol-titanium complex in supercritical fluoroform. Tetrahedron Lett, 2000, 41: 1931–1934
Hayashi Y, Tsuboi W, Shojia M, Suzukib N. Application of high pressure induced by water freezing to the direct asymmetric aldol reaction. Tetrahedron Lett, 2004, 45: 4353–4356
Bahmanyar S, Houk KN. Transition states of amine-catalyzed aldol reactions involving enamine intermediates: Theoretical studies of mechanism, reactivity, and stereoselectivity. J Am Chem Soc, 2001, 123(45): 11273–11283
Bahmanyar S, Houk KN. The origin of stereoselectivity in proline-catalyzed intramolecular aldol reactions. J Am Chem Soc, 2001, 123(51): 12911–12912
Hoang L, Bahmanyar S, Houk KN, List B. Kinetic and stereochemical evidence for the involvement of only one proline molecule in the transition states of proline-catalyzed intra and intermolecular aldol reactions. J Am Chem Soc, 2003, 125: 16–17
Bahmanyar S, Houk KN. Origins of opposite absolute stereoselectivities in proline-catalyzed direct mannich and aldol reactions. Org Lett, 2003, 5: 1249–1251
Bahmanyar S, Houk KN, Martin HJ, List B. Quantum mechanical predictions of the stereoselectivities of proline-catalyzed asymmetric intermolecular aldol reactions. J Am Chem Soc, 2003, 125: 2475–2479
Sekiguchi YAR, Sasaoka A, Shimomoto A, Fujioka S, Kotsuki H. High-pressure-promoted asymmetric aldol reactions of ketones with aldehydes catalyzed by L-proline. Synlett, 2003, 11: 1655–1658
Misumi Y, Bulman RA, Matsumoto K. High pressure mediated asymmetric henry reaction of nitromethane with carbonyl compounds catalyzed by cinchona alkaloids. Heterocycles, 2002, 56: 599–605
Markó IE, Giles PR, Hindley NJ. Catalytic enantioselective Baylis-Hillman reactions. Correlation between pressure and enantiomeric excess. Tetrahedron, 1997, 53: 1015–1024
Abbott AP, Corr S, Durling NE, Hope EG. Hydrogen bond interactions in liquid and supercritical hydrofluorocarbons. J Phys Chem B, 2003, 107(38): 10628–10633
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Liu, ZT., Liu, ZW. et al. L-Proline catalyzed aldol reactions between acetone and aldehydes in supercritical fluids: An environmentally friendly reaction procedure. Sci. China Chem. 53, 1586–1591 (2010). https://doi.org/10.1007/s11426-010-3197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-010-3197-7