Abstract
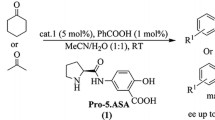
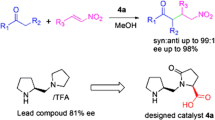
During the course of my research in asymmetric organocatalysis, inversion of enantioselectivity was observed in the asymmetric aldol reactions of acetone with different aldehydes catalyzed by amphiphilic proline derivatives in aqueous media by varying only achiral components. It was not possible to explain the explored dual stereocontrol with the existing models, therefore I proposed a new mechanism for asymmetric aldol reactions catalyzed by l-amino acid derivatives in aqueous media and explained the explored phenomenon of inversion of enantioselectivity with different structures of micelle-stabilized transition state described as a metal-free version of the Zimmerman-Traxler model with explicit participation of a water molecule. Contrary to the existing models, according to the proposed mechanism, the formation of new bonds proceeds directly in the transition state stabilized by a water molecule, without the additional step of product iminium ion hydrolysis. The proposed mechanism has universal character, it is consistent with experimental results and general theoretical conceptions, and it is applicable to all enamine-based asymmetric organocatalytic reactions carried out not only in aqueous, but in organic media as well, because the initial step of catalytic cycle, which involves the formation of an enamine from the carbonyl compound and proline (derivative), liberates one water molecule.





Similar content being viewed by others
References
List B, Lerner RA, Barbas CF III (2000) J Am Chem Soc 122:2395–2396
List B (2002) Tetrahedron 58:5573–5590
(a) Gurka AA, PhD Thesis, University of Szeged (Szeged), 2017. (b) Gurka AA, Szőri K, Bartók M, London G, Tetrahedron: Asymm. 2016; 27:936–942
Torii H, Nakadai M, Ishihara K, Saito S, Yamamoto H (2004) Angew Chem Int Ed 43:1983–1986
Nyberg AI, Usano A, Pihko PM (2004) Synlett 1891–1896
Pihko PM, Laurikainen KM, Usano A, Nyberg AI, Kaavi JA (2006) Tetrahedron 62:317–328
Rogers CJ, Dickerson TJ, Janda KD (2006) Tetrahedron 62:352–356
Gurka AA, Szőri K, Szőri M, Bartók M, London G (2017) Struct Chem 28:415–421
Author information
Authors and Affiliations
Contributions
The author contributed to all of the study conception and design. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gurka, A.A. New mechanistic approach in the enamine-based asymmetric organocatalysis. Struct Chem 34, 83–86 (2023). https://doi.org/10.1007/s11224-022-01952-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01952-w