Abstract
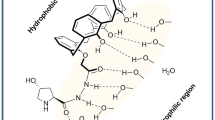
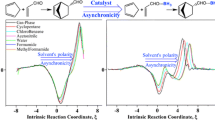
The choice of solvent plays a crucial role in aldol reactions, often affecting both the conversion rate and stereoselectivity. In this study, we investigated the influence of solvents (water, methanol and hydroalcoholic) on the proline-catalyzed aldol reactions. We focused on elucidating the solute–solvent interactions at the rate-determining step and the stereoselective step. Our theoretical finding suggests, hydroalcoholic-mediated reaction exhibits a higher conversion rate as compared to pure water and pure methanol-mediated system with the generation of most stable transition state structure. This can be attributed to the existence of strong hydrogen bonding and the formation of stable six-membered transition state structures in hydroalcoholic-mediated system. In addition to this, our research demonstrates that the choice of solvent plays a crucial role in determining the percentage of enantiomeric excess in the reaction. Theoretical finding suggest that the anti-product is preferentially formed in the presence of water and hydroalcoholic media as solvents. Pure water and hydroalcoholic solvents surprisingly showed a higher enantiomeric excess for the anti-product due to formation of strong hydrogen bonding between reaction moiety and solvents. In contrast, methanol-assisted reactions resulted in a racemic mixture, consistent with experimental observations. Results reported in the present study contribute to the broader understanding of solvent effects in organic reactions and offer valuable insights for the design of organic reactions.






Similar content being viewed by others
References
Geary LM, Hultin PG (2009) The state of the art in asymmetric induction: the aldol reaction as a case study. Tetrahedron Asymmetry 20:131–173. https://doi.org/10.1016/j.tetasy.2008.12.030
Mandal S, Mandal S, Ghosh SK, Ghosh A, Saha R, Banerjee S, Saha B (2016) Review of the aldol reaction. Synth Commun 46:1327–1342. https://doi.org/10.1080/00397911.2016.1206938
Matsuo J, Murakami M (2013) The mukaiyama aldol reaction: 40 years of continuous development. Angew Chem Int Ed 52:9109–9118. https://doi.org/10.1002/anie.201303192
Mahrwald R (2004) Modern aldol reactions. Wiley, Hoboken. https://doi.org/10.1002/9783527619566
Mukaiyama T (1982) The directed aldol reaction. In: Organic reactions. Wiley, Hoboken, pp 203–331. https://doi.org/10.1002/0471264180.or028.03
Casiraghi G, Zanardi F, Appendino G, Rassu G (2000) The vinylogous aldol reaction: a valuable, yet understated carbon−carbon bond-forming maneuver. Chem Rev 100:1929–1972. https://doi.org/10.1021/cr990247i
Sakthivel K, Notz W, Bui T, Barbas CF (2001) Amino acid catalyzed direct asymmetric aldol reactions: a bioorganic approach to catalytic asymmetric carbon−carbon bond-forming reactions. J Am Chem Soc 123:5260–5267. https://doi.org/10.1021/ja010037z
Cowden CJ, Paterson I (1997) Asymmetric aldol reactions using boron enolates. In: Organic reactions. Wiley, Hoboken, 1–200. https://doi.org/10.1002/0471264180.or051.01
Kalesse M (2005) Recent advances in vinylogous aldol reactions and their applications in the syntheses of natural products. Top Curr Chem 244:43–76. https://doi.org/10.1007/b96887
Kan SBJ, Ng KK-H, Paterson I (2013) The impact of the mukaiyama aldol reaction in total synthesis. Angew Chem Int Ed 52:9097–9108. https://doi.org/10.1002/anie.201303914
Emer E, Galletti P, Giacomini D (2009) Evaluation of 6-APA as a new organocatalyst for a direct cross-aldol reaction. Eur J Org Chem 2009:3155–3160. https://doi.org/10.1002/ejoc.200900181
Tartaggia S, Ferrari C, Pontini M, De Lucchi O (2015) A practical synthesis of rosuvastatin and other statin intermediates. Eur J Org Chem 2015:4102–4107. https://doi.org/10.1002/ejoc.201500356
Hu L, Xiong F, Chen X, Chen W, He Q, Chen F (2013) Synthetic studies on statins. Part 1: a short and cyanide-free synthesis of atorvastatin calcium via an enantioselective aldol strategy. Tetrahedron Asymmetry 24:207–211. https://doi.org/10.1016/j.tetasy.2012.12.009
Arnó M, Domingo LR (2002) Density functional theory study of the mechanism of the proline-catalyzed intermolecular aldol reaction. Theor Chem Acc Theory Comput Model (Theor Chim Acta) 108:232–239. https://doi.org/10.1007/s00214-002-0381-7
Bahmanyar S, Houk KN (2001) Transition states of amine-catalyzed aldol reactions involving enamine intermediates: theoretical studies of mechanism, reactivity, and stereoselectivity. J Am Chem Soc 123:11273–11283. https://doi.org/10.1021/ja011403h
Mestres R (2004) A green look at the aldol reaction. Green Chem 6:583. https://doi.org/10.1039/b409143b
Trost BM, Brindle CS (2010) The direct catalytic asymmetric aldol reaction. Chem Soc Rev 39:1600. https://doi.org/10.1039/b923537j
List B, Lerner RA, Barbas CF (2000) Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc 122:2395–2396. https://doi.org/10.1021/ja994280y
Ramachandran PV, Chanda PB (2012) Solvent- or temperature-controlled diastereoselective aldol reaction of methyl phenylacetate. Org Lett 14:4346–4349. https://doi.org/10.1021/ol301782s
Doyagüez EG, Calderón F, Sánchez F, Fernández-Mayoralas A (2007) Asymmetric aldol reaction catalyzed by a heterogenized proline on a mesoporous support. The role of the nature of solvents. J Org Chem 72:9353–9356. https://doi.org/10.1021/jo070992s
Kobayashi S, Wakabayashi T (1998) Scandium trisdodecylsulfate (STDS). A new type of lewis acid that forms stable dispersion systems with organic substrates in water and accelerates aldol reactions much faster in water than in organic solvents. Tetrahedron Lett 39:5389–5392. https://doi.org/10.1016/S0040-4039(98)01081-8
Rankin KN, Gauld JW, Boyd RJ (2002) Density functional study of the proline-catalyzed direct aldol reaction. J Phys Chem A 106:5155–5159. https://doi.org/10.1021/jp020079p
Amedjkouh M (2005) Primary amine catalyzed direct asymmetric aldol reaction assisted by water. Tetrahedron Asymmetry 16:1411–1414. https://doi.org/10.1016/j.tetasy.2005.02.031
Zotova N, Moran A, Armstrong A, Blackmond D (2009) A coherent mechanistic rationale for additive effects and autoinductive behaviour in proline-mediated reactions. Adv Synth Catal 351:2765–2769. https://doi.org/10.1002/adsc.200900665
Zotova N, Franzke A, Armstrong A, Blackmond DG (2007) Clarification of the role of water in proline-mediated aldol reactions. J Am Chem Soc 129:15100–15101. https://doi.org/10.1021/ja0738881
Brogan AP, Dickerson TJ, Janda KD (2006) Enamine-based aldol organocatalysis in water: are they really “all wet”? Angew Chem Int Ed 118:8278–8280. https://doi.org/10.1002/ange.200601392
Mlynarski J, Baś S (2014) Catalytic asymmetric aldol reactions in aqueous media—a 5 year update. Chem Soc Rev 43:577–587. https://doi.org/10.1039/C3CS60202H
Kobayashi S, Nagayama S, Busujima T (1999) Catalytic asymmetric Mukaiyama aldol reactions in aqueous media. Tetrahedron 55:8739–8746. https://doi.org/10.1016/S0040-4020(99)00440-8
Kitanosono T, Kobayashi S (2013) Mukaiyama aldol reactions in aqueous media. Adv Synth Catal 355:3095–3118. https://doi.org/10.1002/adsc.201300798
Mąkosza M, Fedoryński M (2003) Phase transfer catalysis. Catal Rev 45:321–367. https://doi.org/10.1081/CR-120025537
Nakayama K, Maruoka K (2008) Complete switch of product selectivity in asymmetric direct aldol reaction with two different chiral organocatalysts from a common chiral source. J Am Chem Soc 130:17666–17667. https://doi.org/10.1021/ja807807p
Emma MG, Tamburrini A, Martinelli A, Lombardo M, Quintavalla A, Trombini C (2020) A simple and efficient protocol for proline-catalysed asymmetric aldol reaction. Catal 10:649. https://doi.org/10.3390/catal10060649
Bayat S, Tejo BA, Salleh AB, Abdmalek E, Normi YM, Rahman MBA (2013) Various polar tripeptides as asymmetric organocatalyst in direct aldol reactions in aqueous media. Chirality 25:726–734. https://doi.org/10.1002/chir.22205
Yamashita Y, Ishitani H, Shimizu H, Kobayashi S (2002) Highly anti-selective asymmetric aldol reactions using chiral zirconium catalysts. Improvement of activities, structure of the novel zirconium complexes, and effect of a small amount of water for the preparation of the catalysts. J Am Chem Soc 124:3292–3302. https://doi.org/10.1021/ja016293t
Woyciechowska M, Forcher G, Buda S, Mlynarski J (2012) General switch in regioselectivity in the Mukaiyama aldol reaction of silyloxyfuran with aldehydes in aqueous solvents. Chem comm 48:11029–11031. https://doi.org/10.1039/c2cc36656h
Nagayama S, Kobayashi S (2000) A novel chiral lead(II) catalyst for enantioselective aldol reactions in aqueous media. J Am Chem Soc 122:11531–11532. https://doi.org/10.1021/ja001234l
Kobayashi S, Nagayama S, Busujima T (1999) Catalytic asymmetric mukaiyama aidol reactions in aqueous media. Tetrahedron 55:8739–8746
Frisch MJ (2013) Gaussian 09, Rev.D.01. Gaussian Inc., Wallingford
Wheeler SE, Moran A, Pieniazek SN, Houk KN (2009) Accurate reaction enthalpies and sources of error in DFT thermochemistry for aldol, Mannich, and α-aminoxylation reactions. J Phys Chem A 113:10376–10384. https://doi.org/10.1021/jp9058565
Walker M, Harvey AJA, Sen A, Dessent CEH (2013) Performance of M06, M06–2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J Phys Chem A 117:12590–12600. https://doi.org/10.1021/jp408166m
Hohenstein EG, Chill ST, Sherrill CD (2008) Assessment of the performance of the M05–2X and M06–2X exchange-correlation functionals for noncovalent interactions in biomolecules. J Chem Theory Comput 4:1996–2000. https://doi.org/10.1021/ct800308k
Nakliang P, Yoon S, Choi S (2021) Emerging computational approaches for the study of regio- and stereoselectivity in organic synthesis. Org Chem Front 8:5165–5181. https://doi.org/10.1039/D1QO00531F
Emamian S How to define a new solvent and a mix of different solvents in Gaussian 16?
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Onufriev A (2008) Implicit solvent models in molecular dynamics simulations: a brief overview. Ann Rep Comput Chem. https://doi.org/10.1016/S1574-1400(08)00007-8
Zhang J, Zhang H, Wu T, Wang Q, van der Spoel D (2017) Comparison of implicit and explicit solvent models for the calculation of solvation free energy in organic solvents. J Chem Theory Comput 13:1034–1043. https://doi.org/10.1021/acs.jctc.7b00169
Cramer CJ, Truhlar DG (1999) Implicit solvation models: equilibria, structure, spectra, and dynamics. Chem Rev 99:2161–2200. https://doi.org/10.1021/cr960149m
Acknowledgements
The author Arati S. Gavali acknowledge Mahatma Jyotiba Phule Research & Training Institute (MAHAJYOTI), Nagpur, India, for providing financial support.
Author information
Authors and Affiliations
Contributions
Dr. PB: Conceptualization, Visualization, Supervision, Writing- Review and Editing. AG: Resources, Theoretical (computational) analysis, Data curation, Investigation, Writing original draft. PJ and VN: NCI plots and reviewed manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavali, A.S., Maliekal, P.J., Naik, V.A. et al. Decoding the impact of solvents in altering the conversion rates and stereoselectivity in proline-catalyzed asymmetric aldol reaction. Theor Chem Acc 143, 14 (2024). https://doi.org/10.1007/s00214-023-03088-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03088-4