Abstract
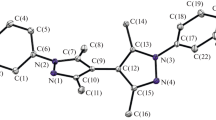
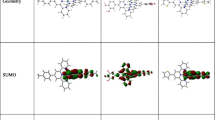
A series of ruthenium(II) complexes Ru(fppz)2(CO)L [fppz = 3-trifluoromethyl-5(2-pyridyl)pyrazole; L = pyridine (1), 4-dimethylaminopyridine (2), 4-cyanopyridine (3)] were designed and investigated theoretically to explore their electronic structures, absorption, and emissions as well as the solvatochromism. The singlet ground state and triplet excited state geometries were fully optimized at the B3LYP/LANL2DZ and CIS/LANL2DZ level, respectively. The HOMO of 1–3 is composed of dyz(Ru) atom and π(fppz). The LUMO of 1 and 2 is dominantly contributed by π*(fppz) orbital, but that of 3 is contribute by π*(L). Absorption and phosphorescence in vacuo, C6H12, and CH3CN media were calculated using the TD-DFT level of theory with the PCM model based on the optimized ground and excited state geometries, respectively. The lowest-lying absorption of 1 and 2 at 387 and 391 nm is attributed to {[dyz(Ru) + π(fppz)] → [π*(fppz)]} transition, but that of 3 at 479 nm is assigned to {[dyz(Ru) + π(fppz)] → [π*(L)]} transition. The phosphorescence of 1 and 2 at 436 and 438 nm originates from 3{[dyz(Ru) + π(fppz)] [π*(fppz)]} excited state, while that of 3 at 606 nm is from 3{[dyz(Ru) + π(fppz)] [π*(L)]} excited state. The calculation results showed that the absorption and emission transition character can be changed from MLCT/ILCT to MLCT/LLCT transition by altering the substituent on the L ligand. The phosphorescence of 1 and 2 does not have solvatochromism, but that of 3 at 606 nm (vacuo), 584 nm (C6H12), and 541 nm (CH3CN) is strongly dependent on the solvent polarity, so introducing electron-withdrawing group on ligand L will induce remarkable solvatochromism.
Similar content being viewed by others
References
Balzani V, Juris A, Venturi M, Campagna S, Serroni S. Luminescent and redox-active polynuclear transition metal complexes. Chem Rev, 1996, 96: 759–834
Vlček Jr. A. Mechanistic roles of metal-to-ligand charge-transfer excited states in organometallic photochemistry. Coord Chem Rev, 1998, 177: 219–256
Demadis K D, Hartshorn C M, Meyer T J. The localized-to-delocalized transition in mixed-valence chemistry. Chem Rev, 2001, 101: 2655–2686
Demas J N, DeGraff B A. Applications of luminescent transition platinum group metal complexes to sensor technology and molecular probes. Coord Chem Rev, 2001, 211: 317–351
Wand Y, Herron N, Grushin V V, LeCloux D D, Petrov V A. Highly efficient electroluminescent materials based on fluorinated organo-metallic iridium compounds. Appl Phys Lett, 2001, 79: 449–451
Lo K K W, Chung C K, Lee T K M, Lui L H, Tsang K H K, Zhu N Y. New luminescent cyclometalated iridium(III) diimine complexes as biological labeling reagents. Inorg Chem, 2003, 42: 6886–6897
Silaware N D, Goldman A S, Ritter R, Tyler D R. Reduction of carbon dioxide and other substrates using photochemical reactions of the decacarbonylditungstate(2-) complex. Inorg Chem, 1989, 28:1231–1236
Djurovich P I, Thompson M E. Bis-cyclometalated Ir(III) complexes as efficient singlet oxygen sensitizers. J Am Chem Soc, 2001, 124:14828–14829
Adachi C, Baldo M A, Forrest S R, Thompson M E. Highly efficient electroluminescent materials based on fluorinated organometallic iridium compounds. Appl Phys Lett, 2000, 77: 904–906
Paris J P, Brandt W W. Charge rransfer luminescence of a ruthenium( II) chlate. J Am Chem Soc, 1959, 81: 5001–5002
Yersin H, Braun D. Localization in excited states of molecules. Application to [Ru(bpy)3]2+. Coord Chem Rev, 1991, 111: 39–46
Fantacci S, De Angelis F, Selloni A. Absorption spectrum and solvatochromism of the [Ru(4,4′-COOH-2,2′-bpy)2(NCS)2] molecular dye by time dependent density functional theory. J Am Chem Soc, 2003, 125: 4381–4387
Erkkila K E, Odom D T, Barton J K. Recognition and reaction of metallointercalators with DNA. Chem Rev, 1999, 99: 2777–2796
Nazeeruddin M K, Kay A, Rodicio I, Humphry-Baker R, Müeller E, Liska P, Vlachopoulos N, Graetzel M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc, 1993, 115: 6382–6390
Nazeeruddin M K, Pėchy P, Renouard T, Nazeeruddin S M, Humphry-Baker R, Comte P, Liska P, Cevey L, Costa E, Shklover V, Spiccia L, Deacon G B, Bignozzi C A, Grätzel M. Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J Am Chem Soc, 2001, 123: 1613–1624
Tung Y L, Chen L S, Chi Y, Chou P T, Cheng Y M, Li E Y, Lee G H, Shu C F, Wu F I, Carty A J. Orange and red organic light-emitting devices employing neutral Ru(II) emitters: Rational design and prospects for color tuning. Adv Funct Mater, 2006, 16:1615–1626
Tung Y L, Wu P C, Liu C S, Chi Y, Yu J K, Hu Y H, Chou P T, Peng S M, Lee G H, Tao Y, Carty A J, Shu C F, Wu F I. Highly efficient red phosphorescent osmium(II) complexes for OLED applications. Organometallics, 2004, 23: 3745–3748
Chou P T, Y. Osmium-and ruthenium-based phosphorescent materials: Design, photophysics, and utilization in OLED fabrication. Eur J Inorg Chem, 2006, 3319–3332
Zhen H, Jiang C, Yang W, Jiang J, Huang F, Cao Y. Synthesis and properties of electrophosphorescent chelating polymers with iridium complexes in the conjugated backbone. Chem Eur J, 2005, 11:5007–5016
Wu P C, Yu J K, Song Y H, Chi Y, Chou P T, Peng S M, Lee G H. Synthesis and characterization of metal complexes possessing the 5-(2-pyridyl) pyrazolate ligands: The observation of remarkable osmium-induced blue phosphorescence in solution at room temperature. Organometallics, 2003, 22: 4938–4946
Li S W, Cheng Y M, Yeh Y S, Hsu C C, Chou P T, Peng S M, Lee G H, Tung Y L, Wu P C, Chi Y, Wu F I, Shu C F. Interplay between intra-and interligand charge transfer with variation of the axial N-heterocyclic ligand in osmium(II) pyridylpyrazolate complexes: Extensive color tuning by phosphorescent solvatochromism. Chem Eur J, 2005, 11: 6347–6357
Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, von Zelewsky A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord Chem Rev, 1988, 84: 85–277
Runge E, Gross E K U. Density-functional theory for time-dependent systems. Phys Rev Lett, 1984, 52: 997–1000
Becke A D. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys, 1993, 98: 5648–5652
Stanton J F, Gauss J, Ishikawa N, Head-Gordon M. A comparison of single reference methods for characterizing stationary points of excited state potential energy surfaces. J Chem Phys, 1995, 103:4160–4174
Matsuzawa N N, Ishitani A. Time-dependent density functional theory calculations of photoabsorption spectra in the vacuum ultraviolet region. J Phys Chem A, 2001, 105: 4953–4962
Barone V, Cossi M. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys, 1997, 107: 3210–3221
Liu T, Xia B H, Zhou X, Zhang H X, Pan Q J, Gao J S. Theoretical studies on structures and spectroscopic properties of bis-cyclo-metalated iridium complexes. Organometallics, 2007, 26: 143–149
Monat J E, Rodriguez J H, McCusker J K. Ground-and excited-state electronic structures of the solar cell sensitizer bis(4,4′-dicarboxylato-2,2′-bipyridine)bis(isothiocyanato)ruthenium(II). J Phys Chem A, 2002, 106: 7399–7406
De Angelis F, Fantacci S, Sgamellotti A. An integrated computational tool for the study of the optical properties of nanoscale devices: Application to solar cells and molecular wires. Theor Chem Acc, 2007, 117: 1093–1104
Hay P J, Wadt W R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J Chem Phys, 1985, 82: 299–310
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03, Revision C02. Wallingford CT: Gaussian Inc, 2004
Zalis S, Farrell I R, Vlček Jr. A. The involvement of metal-to-CO charge transfer and ligand-field excited states in the spectroscopy and photochemistry of mixed-ligand metal carbonyls. A theoretical and spectroscopic study of [W(CO)4(1,2-ethylenediamine)] and [W(CO)4(N,N′-bis-alkyl-1,4-diazabutadiene)]. J Am Chem Soc, 2003, 125:4580–4592
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20573042, 20703015, and 20333050)
Rights and permissions
About this article
Cite this article
Liu, T., Zhou, X., Bai, F. et al. Theoretical studies on Ru(fppz)2(CO)L (L = N-heterocyclic ligand): Electronic structure, absorption, phosphorescence, and solvatochromism. Sci. China Ser. B-Chem. 51, 1211–1220 (2008). https://doi.org/10.1007/s11426-008-0131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-008-0131-3