Abstract
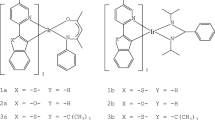
The structural and optical properties of five ruthenium complexes, recently synthesized for their photooxidative and photophysical properties, have been studied by means of density functional theory (DFT) and time-dependent DFT (TD-DFT). The structures of [Ru(bpy)2(BiimH2)]2+ (bpy = 2,2’-bipyridine; BiimH2 = 2,2’-biimidazole) 1, [Ru(bpy)2(TMBiimH2)]2+ (TM BiimH2 = 4,5,4’,5’-tetramethyl-2,2’-biimidazole) 5, [Ru(bpy)2(L1H2)]2+ (L1H2 = 4,5-dimethyl-2(N,N-diacetyl)(carboximidamide-1H-imidazole)) 6, [Ru(bpy)2(L2H2)]2+ (L2H2 = N1,N1,N2,N2-tetrakis(acetyl)ethanediimidamide) 7 and [Ru(phen)2(TMBiimH2)]2+ (phen = 1,10’-phenanthroline) 8 have been fully optimized in the electronic ground state as well as in the lowest triplet T1 excited state. The theoretical absorption spectra of the five complexes that compare rather well with the experimental spectra have been analyzed on the basis of TD-DFT calculations without and with spin-orbit coupling (SOC). The deprotonated form [Ru(bpy)2(L2H)]+7d contributes mostly to the experimental absorption spectrum of complex 7. The spectra of all molecules are characterized by the presence of low-lying metal-to-ligand charge transfer (MLCT) excited states between 500 and 400 nm, ligand-centered (LC) excited states on the biimidazole-like ligands between 350 and 300 nm and on the bpy ligands between 300 and 250 nm. The theoretical emission wavelengths deduced from the lowest triplet T1 properties calculated at 661 nm (1), 690 nm (5) and 660 nm (8) reproduce the experimental emission spectra of these molecules characterized by a maximum at 638 nm (1), 646 nm (5) and 652 nm (8). In contrast the low theoretical emission wavelengths (>1000 nm) obtained for complexes 6, 7 and 7d favorable to non-radiative decays explain the low intensity of the experimental emission spectra of these two complexes. The SOC is of little effect in this class of molecules where metal-centered (MC) excited states do not perturb the lowest part of the absorption spectra leading to negligible splitting of low-lying triplet states.
Similar content being viewed by others
References
R. J. Sundberg, R. B. Martin, Interactions of Histidine and other Imidazole Derivatives with Transition Metal Ions in Chemical and Biological Systems, Chem. Rev., 1974, 74, 471–517.
F. Gao, H. Chao, L. N. Ji, DNA Binding, Photocleavage, and Topoisomerase Inhibition of Functionalized Ruthenium(ii) Polypyridine Complexes, Chem. Biodiversity, 2008, 5, 1962–1979.
M.-J. Han, L.-H. Gao, Y.-Y. Hu, K.-Z. Wang, Ruthenium(ii) Complex of Hbopip: Synthesis, Characterization, pH-Induced Luminescence “Off-On-Off” Switch, and Avid Binding to DNA, J. Phys. Chem. B, 2006, 110, 2364–2371.
S.-H. Fang, A.-G. Zhang, C.-C. Ju, L.-H. Gao, K.-Z. Wang, A Triphenylamine-Grafted Imidazo[4,5-f][1,10]phenanthroline Ruthenium(ii) Complex: Acid-Base and Photoelectric Properties, Inorg. Chem., 2010, 49, 3752–3763.
X. Zhu, W. Lu, Y. Zhang, A. Reed, B. Newton, Z. Fan, H. Yu, P.-C. Ray, R. Gao, Imidazole-Modified Porphyrin as a pH-Responsive Sensitizer for Cancer Photodynamic Therapy, Chem. Commun., 2011, 47, 10311–10313.
A. J. Hallett, N. White, W. Wu, X. Cui, P. N. Horton, S. J. Coles, J. Zhao, S. J. A. Pope, Enhanced Photooxidation Sensitizers: The first Examples of Cyclometalated Pyrene Complexes of Iridium(iii), Chem. Commun., 2012, 48, 10838–10840.
W. Wu, S. Ji, W. Wu, J. Shao, H. Guo, T. D. James, J. Zhao, Ruthenium(ii) Polyimine-Coumarin Dyad with Non-emissive 3IL Excited State as Sensitizer for Triplet-Triplet Annihilation Based Upconversion, Angew. Chem., Int. Ed., 2011, 50, 8283–8286.
H.-J. Mo, Y. Shen, B.-H. Ye, Selective Recognition of Cyanide Anion via Formation of Multipoint NH and Phenyl CH Hydrogen Bonding with Acyclic Ruthenium Bipyridine Imidazole Receptors in Water, Inorg. Chem., 2012, 51, 7174–7184.
C. Sheu, P. Kang, S. Khan, C. S. Foote, Low-Temperature Photosensitized Oxidation of a Guanosine Derivative and Formation of an Imidazole Ring-Opened Product, J. Am. Chem. Soc., 2002, 124, 3905–3913.
M. Davies, Reactive Species Formed on Proteins Exposed to Singlet Oxygen, Photochem. Photobiol. Sci., 2004, 3, 17–25.
Y. Cui, H. J. Mo, J. C. Chen, Y. L. Niu, Y. R. Zhong, K. C. Zheng, B. H. Ye, Anion-Selective Interaction and Colorimeter by an Optical Metalloreceptor Based on Ruthenium(ii) 2,2’-Biimidazole: Hydrogen Bonding and Proton Transfer, Inorg. Chem., 2007, 46, 6427–6436.
Y. Cui, Y. L. Niu, M. L. Cao, K. Wang, H. J. Mo, Y. R. Zhong, B. H. Ye, Ruthenium(ii) 2,2’-Bibenzimidazole Complex as a Second-Sphere Receptor for Anions Interaction and Colorimeter, Inorg. Chem., 2008, 47, 5616–5624.
H. J. Mo, Y. L. Niu, M. Zhang, Z. P. Qiao, B. H. Ye, PhotophysicalElectrochemical and Anion Ssensing Properties of Ru(ii) Bipyridine Complexes with 2,2’-biimidazole-like Ligand, Dalton Trans., 2011, 40, 8218–8225.
H. J. Mo, J. J. Wu, Z. P. Qiao, B. H. Ye, Interaction Between Biimidazole Complexes of Ruthenium and Acetate: Hydrogen Bonding and Proton transfer, Dalton Trans., 2012, 41, 7026–7036.
Z.-Z. Li, Y. L. Niu, H. Y. Zhou, H. Y. Chao, B. H. Ye, Visible-Light-Induced Photooxidation of Ruthenium(ii) Complex with 2,2’-Biimidazole-like Ligand by Singlet Oxygen, Inorg. Chem., 2013, 52, 10087–10095.
A. D. Becke, Density Functional Thermochemistry. III. The role of Exact Exchange, J. Chem. Phys., 1993, 98, 5648–5652.
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, Ab initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields, J. Phys. Chem., 1994, 98, 11623–11627.
E. van Lenthe, E. J. Baerends, Optimized Slater-type Basis Sets for the Elements 1-118, J. Comput. Chem., 2003, 24, 1142–1156.
E. van Lenthe, R. van Leeuwen, E. J. Baerends, J. G. Snijders, Relativistic Regular Two-Component Hamiltonians, Int. J. Quantum Chem., 1996, 57, 281–293.
A. Klamt, G. Schüürmann, COSMO: a New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and its Gradient, J. Chem. Soc., Perkin Trans.1, 1993, 2, 799–805.
A. Klamt, Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena, J. Phys. Chem., 1995, 99, 2224–2235.
A. Klamt, V. Jones, Treatment of the Outlying Charge in Continuum Solvation Models, J. Chem. Phys., 1996, 105, 9972–9981.
A. Rosa, E. J. Baerends, S. J. A. van Gisbergen, E. van Lenthe, J. A. Groeneveld, J. G. Snijders, Electronic Spectra of M(CO)6 (M = Cr, Mo, W) Revisited by Relativistic TDDFT Approach, J. Am. Chem. Soc., 1999, 121, 10356–10365.
C. Pye, T. Ziegler, An Implementation of the Conductor-Like Screening Model of Solvation within the Amsterdam Density Functional Package, Theor. Chem. Acc., 1999, 101, 396–408.
E. Runge, E. K. U. Gross, Density-Functional Theory for Time-Dependent Systems, Phys. Rev. Lett., 1984, 52, 997–1000.
M. Petersilka, U. J. Gossmann, E. K. U. Gross, Excitation Energies from Time-Dependent Density-Functional Theory, Phys. Rev. Lett., 1996, 76, 1212–1215.
M. J. Peach, D. J. Tozer, Overcoming Low Orbital Overlap and Triplet Instability Problems in TDDFT, J. Phys. Chem. A, 2012, 116, 9783–9789.
ADF, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, 2013, https://www.scm.com/Downloads/2013.
M. Chergui, Ultrafast Photophysics of Transition Metal Complexes, Acc. Chem. Res., 2015, 48, 801–808.
C. Gourlaouen, C. Daniel, Spin-Orbit Effects in Square-Planar Pt(ii) Complexes with Bidentate and Terdentate Ligands: Theoretical Absorption/Emission Spectroscopy, Dalton Trans., 2014, 43, 17806–17819.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Cartesian coordinates of optimized geometries in the S0 and T1 electronic states and associated DFT structures - TD-DFT excited states and transition energies - the structure of complex 7b, and the TD-DFT spectrum of complex 1 with and without SOC-Optimized structures of the lowest T1 electronic states.
Rights and permissions
About this article
Cite this article
Xia, SH., Fang, WH., Cuia, G. et al. A theoretical study of ruthenium complexes with 2,2′-biimidazole-like ligands: structural, optical and emissive properties. Photochem Photobiol Sci 15, 1138–1147 (2016). https://doi.org/10.1039/c6pp00148c
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00148c