Abstract
Ophiorrhiza plants (Family Rubiaceae) are known to produce diverse monoterpenoid indole alkaloids including camptothecin with potent antitumor activity. This review contains a summary of recent chemical studies reported over the past 10 years regarding alkaloids (monoterpenoid indole and tetrahydroisoquinoline alkaloids, and cyclopeptide) in Ophiorrhiza plants. In addition, the alkaloid biosynthetic pathways based on their reported structures were proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ophiorrhiza plants belong to the Rubiaceae family and are widely distributed in tropical and subtropical Asia, Australia, New Guinea, and the Pacific Islands. Some of Ophiorrhiza plants have been used traditionally to treat snakebites, ulcers, skin disorders, etc. [1, 2]. Ophiorrhiza species are known to produce diverse monoterpenoid indole alkaloids including camptothecin with potent antitumor activity [1,2,3,4,5]. Camptothecin biosynthesis has been investigated at the genetic level, and its biotechnological production continues to attract research interest [6,7,8,9,10]. This review contains a summary of recent chemical studies on alkaloids isolated from Ophiorrhiza plants over the past 10 years. In addition, the biosynthetic pathways for some isolated monoterpenoid indole and tetrahydroisoquinoline alkaloids based on their reported structures were proposed.
Monoterpenoid indole alkaloid glycosides from Ophiorrhiza trichocarpon
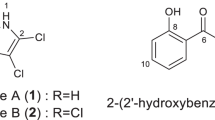
In 2013, ophiorrhisides A–F (1–6), β-carboline-type monoterpenoid indole alkaloid glycosides, were isolated from Ophiorrhiza trichocarpon collected in Thailand, together with four known alkaloid glycosides [dolichantoside (7), 5-carboxystrictosidine (8), lyaloside (9), and 3,4,5,6-tetradehydrodolichantoside (10)] (Fig. 1) [11]. Ophiorrhisides A (1) and B (2) both possess a lactam moiety on the C ring and a disaccharide residue. The stereochemistry at C-3 of 1 was concluded to be S form, deduced from biogenetic considerations and comparison of its electronic circular dichroism (ECD) spectrum with that of a chiral model compound possessing a 1,2,3,4-tetrahydro-β-carbolin-3-one skeleton [12]. Ophiorrhiside C (3) with an E-ferulate residue on the sugar portion and ophiorrhiside D (4) with a fully substituted tetrahydropyran ring are analogs of 3,4,5,6-tetradehydrodolichantoside (10), which was also isolated from the same plant. Ophiorrhisides E (5) and F (6) each have an impressive C ring. Thus, the former has an N-methylpyridone ring. The latter has a highly oxidized C ring with a 1,2-dicarbonyl function at C-5 and C-6, and a double bond belonging to enamine between C-3 and C-14.
A cyclopeptide, monoterpenoid indole alkaloid glycosides, and monoterpenoid tetrahydroisoquinoline alkaloids from Ophiorrhiza nutans
In 2017, ophiorrhisine A (11), a cyclopeptide, and 7’,10-dide-O-methylcephaeline (12), a monoterpenoid tetrahydroisoquinoline alkaloid, were isolated from Ophiorrhiza nutans collected in Thailand, together with two known monoterpenoid indole alkaloid glycosides [5-carboxystrictosidine (8) and lyaloside (9)] and four known tetrahydroisoquinoline alkaloids [demethylalangiside (13), alangiside (14), isoalangiside (15), and 10-O-demethylprotoemetine (16)] (Fig. 2) [13]. Among these, 5-carboxystrictosidine (8), demethylalangiside (13), and alangiside (14) were isolated as main alkaloids. This is the first example of monoterpenoid tetrahydroisoquinoline alkaloids isolated from the genus Ophiorrhiza, and the second example of monoterpenoid indole alkaloids co-existing with monoterpenoid tetrahydroisoquinoline alkaloids in the same plant species.
Ophiorrhisine A (11) is a cyclic tetrapeptide having a 14-membered paracyclophane ring. The characteristic functionalities of 11 include a carboxylate group at C-10 and an N,N,N-trimethylated tyrosine residue in the side chain. The structure and absolute configuration of 11 were determined by spectroscopic analyses and asymmetric total synthesis involving an intramolecular aromatic nucleophilic substitution reaction (SNAr) of linear tripeptide 17 to construct a 14-membered paracyclophane ring (Fig. 3) [14]. No cytotoxic activity was observed for naturally occurring 11 with an ionic character against cancer cells. On the other hand, some 14-membered cyclophane derivatives without ionic character, such as primary amine derivative 18, N,N-dibenzyl derivative 19, N,N-dimethyl derivative 20, phenylalanine derivative 21, and tryptophan derivative 22, showed cytotoxicity against various types of human cancer cell lines (A549, HT29, HCT116) with IC50 values in the range of 2.9–11.6 µM (Fig. 4).
Monoterpenoid indole alkaloid glycosides from Ophiorrhiza japonica
The isolation of several alkaloids from Ophiorrhiza japonica has been reported. In 2018, ophiorrhines A (23) and B (24), immunosuppressive monoterpenoid indole alkaloid glycosides, were isolated from O. japonica, a folk herbal medicine collected in China (Fig. 5) [15]. The structures of 23 and 24 were elucidated by spectroscopic analyses and single-crystal X-ray diffraction. Both 23 and 24 possess a novel spirocyclic ring system and bridged carbon ring system. The major alkaloid isolated from the plant is 5-oxodolichantoside (25). The same group isolated ophiorrhines F (26) and G (27) from O. japonica in 2022, and proposed a biosynthetic pathway for ophiorrhines A (23) and B (24) via 26 and 27 as described (vide infra) [16]. The structures of 26 and 27 were elucidated by spectroscopic methods, ECD, and calculated NMR with DP4 + analysis. Then, the inhibitory activity against lipopolysaccharide (LPS)-induced B cell proliferation was observed with ophiorrhines A (23), B (24), F (26), and G (27); the IC50 value of 26 was 0.38 μM. Furthermore, ophiorrhine B (24) also showed potent inhibitory activity against concanavalin A (Con A)-induced T cell proliferation, with an IC50 value of 13.34 μM. In addition, compounds 23 and 24 showed no cytotoxic activity against five human cancer cell lines HL-60, A549, SMMC-7721, SW480, and MCF-7.
Alkaloids from Ophiorrhiza cantoniensis
In 2021, ophiorrhines C–E (28–30) were isolated from Ophiorrhiza cantoniensis collected in China, together with one known alkaloid Δ1’,2’-deoxytubulosine (31) (Fig. 6) [17]. The structures of 28–30 and their absolute configurations were elucidated by spectroscopic methods, ECD, and calculated NMR with DP4 + analysis. The relative configuration of 28 at C-16 was assigned by gauge-independent atomic orbital (GIAO) 13C NMR calculations and DP4 + analysis. Immunosuppressive activity assays demonstrated the inhibitory activity of compounds 28 and 29 against Con A-induced T cell proliferation with IC50 values of 23.6 and 17.9 μM, respectively, and the inhibitory activity of 28 against LPS-induced B cell proliferation with an IC50 value of 8.7 μM. Vincoside lactam (32) was also isolated from O. cantoniensis [18].
Alkaloids from other Ophiorrhiza plants
Camptothecin (33) is a well-known alkaloid with potent antitumor activity (Fig. 7). It was first isolated from Camptotheca acuminata (Nyssaceae) in 1966 and after that, isolated from several Ophiorrhiza plants. In 2016, 33 was isolated from O. shendurunii collected in South India [19]. Compound 33 was also detected in O. mungos var. angustifolia collected in India [20], field-grown plants of O. pectinata [21], and O. cantoniensis cultivated by hydroponics [22]. Searches for camptothecin-producing Ophiorrhiza species in India using quantification of 33 using HPTLC-densitometry have been reported [23, 24].
Vincoside lactam (vincosamide) (32) and 5-carboxystrictosidine (8) were isolated from O. baviensis collected in Vietnam [25]; the inhibitory effect on NO production in LPS-stimulated RAW264.7 cells of 8 was found. Harmaline (34) was isolated from O. nicobarica, a traditional herb collected in India, and was shown to have anti-herpes simplex virus type 2 (HSV-2) activity [26] and anti-HSV-1 activity [27] in biological evaluations.
Plausible biosynthetic pathways for monoterpenoid indole and tetrahydroisoquinoline alkaloids in Ophiorrhiza plants
Roughly 50 alkaloids have been isolated from various Ophiorrhiza plants. Plausible biosynthetic pathways for some of the isolated monoterpenoid indole alkaloids based on their reported structures are summarized in Fig. 8 [28]. The condensation of tryptamine with secologanin produces strictosidine (35), a common intermediate of monoterpenoid indole alkaloids. Strictosidine 35 itself has never been isolated from Ophiorrhiza plants, but strictosidinic acid (36), a carboxylic acid congener, has been isolated from O. filistipula [29]. Thus, compound 35 is utilized in this biosynthetic pathway. 5-Carboxystrictosidine (8) having a carboxyl group at C-5 would be formed from the reaction of secologanin and tryptophan instead of tryptamine. Alkaloids such as ophiorrhisides A–F (1–6), lyaloside (9), 3,4,5,6-tetradehydrodolichantoside (10), and ophiorrhine C (28) would be derived from strictosidine (35) or its congeners dolichantoside (7), 36, and palicoside (37) [30, 31] without additional ring formation. Lactam formation between N-4 and the methyl ester group of strictosidine (35) is considered to give strictosamide (38) [32, 33]. Pumiloside (39) [32,33,34] and 3S-deoxypumiloside (40) [35] possess both the 6–6-5 (ABC)-ring system like camptothecin (33) and the DE-ring moiety as strictosamide (38). In 2015, camptothecoside (41), which has the same ABCD-ring system as 33 and the E ring acetal glucoside moiety as 38, was isolated from Camptotheca acuminata [36]. Thus, camptothecin (33) would be derived from strictosamide (38) via the formation of pumiloside (39), 3S-deoxypumiloside (40), and camptothecoside (41) followed by structural conversion of the E ring [5]. Ophiorrhine D (29) with a seven-membered azepane ring would be formed from 35 via epoxidation of the C-18–C-19 double bond followed by nucleophilic addition of N-4 to C-18. Cleavage of the glucose unit in 35, 7, or 37 would give aldehyde intermediate 42, from which alkaloids 43–46 and related compounds might be produced. (1) Ophiorrhizine (43) [37] would be formed via cyclization of N-4 and both carbons at C-21 and C-17. (2) Normalindine (44) [29] would be derived by bond formation between N-4 and C-19 and incorporation of the third nitrogen atom. (3) Dihydrocycloakagerine (45) [38] would be produced via cyclization of N-1 and C-17 and formation of an ether linkage between C-17 and C-21 to form a hemiaminal ether moiety. (4) 3,14-Dihydrodecussine (mostueine) (46) [38] would be derived by bond formation between N-1 and C-19 and incorporation of the third nitrogen atom. The biosynthetic pathway for ophiorrhines A (23) and B (24) via ophiorrhines F (26) and G (27) was proposed by Feng and Liu et al. in 2021 [16]. Thus, the condensation at the C-2 position of tryptamine and secologanin (or secoxyloganin derivative) would yield ophiorrhines F (26) and G (27), respectively, which would then be metabolized into ophiorrhines A (23) and B (24), respectively, via an intramolecular [4 + 2] Diels–Alder cycloaddition of hypothetical intermediate 47.
The proposed biosynthetic pathways for monoterpenoid tetrahydroisoquinoline alkaloids in Ophiorrhiza plants are shown in Fig. 9. The condensation of dopamine with secologanin is considered to produce deacetylisoipecoside (48) with H-1α and deacetylipecoside (49) with H-1β, although neither 48 nor 49 has been isolated from Ophiorrhiza plants to date. Isoalangiside (15) having H-1α and demethylalangiside (13) and alangiside (14) having H-1β would be formed from 48 and 49, respectively, via lactam formation between N-2 and the methyl ester group. On the other hand, the hydrolysis of the glucose unit in 48 and piperidine ring formation in the resulting aldehyde intermediate 50, followed by a sequence of reactions, would give 10-O-demethylprotoemetine (16). The reaction of 16 or its analogs with a second dopamine would yield 7’,10-dide-O-methylcephaeline (12), whereas the reaction of 16 with tryptamine would lead to the formation of ophiorrhine E (30) via Δ1’,2’-deoxytubulosine (31).
Conclusion
This review contains a summary of chemical studies reported over the past 10 years regarding the alkaloidal constituents of Ophiorrhiza plants. A number of alkaloids having unique chemical structures have been isolated, including monoterpenoid indole alkaloid glycosides, monoterpenoid tetrahydroisoquinoline alkaloids, and a cyclopeptide. Among them, some (including synthetic analogs) have demonstrated useful biological activities. The second half of this review discussed the plausible biosynthetic pathways for the isolated monoterpenoid indole and tetrahydroisoquinoline alkaloids based on their reported structures. Their diverse chemical structures would be derived from common intermediates obtained by the condensation of secologanin with tryptamine (tryptophan) or dopamine. It is highly anticipated that the candidate biosynthetic intermediates of the related alkaloids and novel alkaloids having unique skeletons and biological activities would be discovered from Ophiorrhiza plants in the future.
References
Krishnakumar G, Dintu KP, Varghese SC, Nair DS, Gopinath G, Rameshkumar KB, Satheeshkumar K, Krishnan PN (2020) Ophiorrhiza, a promising herbaceous source of the anticancer compound camptothecin. Plant Sci Today 7:240–250
Taher M, Shaari SS, Susanti D, Arbain D, Zakaria ZA (2020) Genus Ophiorrhiza: a review of its distribution, traditional uses, phytochemistry, biological activities and propagation. Molecules 25:2611
Johnson AJ, Rajan R, Baby S (2018) Secondary metabolites from Ophiorrhiza. Nat Prod J 8:248–267
Arbain D (2012) Inventory, constituents and conservation of biologically important Sumatran plants. Nat Prod Commun 7:799–806
Kitajima M (2007) Chemical studies on monoterpenoid indole alkaloids from medicinal plant resources, Gelsemium and Ophiorrhiza. J Nat Med 61:14–23
Rai A, Hirakawa H, Nakabayashi R, Kikuchi S, Hayashi K, Rai M, Tsugawa H, Nakaya T, Mori T, Nagasaki H, Fukushi R, Kusuya Y, Takahashi H, Uchiyama H, Toyoda A, Hikosaka S, Goto E, Saito K, Yamazaki M (2021) Chromosome-level genome assembly of Ophiorrhiza pumila reveals the evolution of camptothecin biosynthesis. Nat Commun 12:405
Kang M, Fu R, Zhang P, Lou S, Yang X, Chen Y, Ma T, Zhang Y, Xi Z, Liu J (2021) A chromosome-level Camptotheca acuminata genome assembly provides insights into the evolutionary origin of camptothecin biosynthesis. Nat Commun 12:3531
Yang M, Wang Q, Liu Y, Hao X, Wang C, Liang Y, Chen J, Xiao Y, Kai G (2021) Divergent camptothecin biosynthetic pathway in Ophiorrhiza pumila. BMC Biol 19:122
Swamy MK, Nath S, Paul S, Jha NK, Purushotham B, Rohit KC, Dey A (2021) Biotechnology of camptothecin production in Nothapodytes nimmoniana, Ophiorrhiza sp. and Camptotheca acuminata. Appl Microbiol Biotechnol 105:9089–9102
Kai G, Wu C, Gen L, Zhang L, Cui L, Ni X (2015) Biosynthesis and biotechnological production of anti-cancer drug camptothecin. Phytochem Rev 14:525–539
Kitajima M, Ohara S, Kogure N, Santiarworn D, Takayama H (2013) β-Carboline-type indole alkaloid glycosides from Ophiorrhiza trichocarpon. Tetrahedron 69:9451–9456
Onozawa T, Kogure N, Takayama H, Kitajima M (2021) Elucidation of absolute configuration of ophiorrhiside A by comparison of ECD spectra with that of model chiral compound having a 1,2,3,4-tetrahydro-β-carbolin-3-on skeleton. Heterocycles 102:35–43
Onozawa T, Kitajima M, Kogure N, Peerakam N, Santiarworn D, Takayama H (2017) A cyclopeptide and a tetrahydroisoquinoline alkaloid from Ophiorrhiza nutans. J Nat Prod 80:2156–2160
Onozawa T, Kitajima M, Kogure N, Takayama H (2018) Asymmetric total synthesis and evaluation of antitumor activity of ophiorrhisine A and its derivatives. J Org Chem 83:15312–15322
Feng T, Duan K, He S, Wu B, Zheng Y, Ai H, Li Z, He J, Zuo J, Liu J (2018) Ophiorrhines A and B, two immunosuppressive monoterpenoid indole alkaloids from Ophiorrhiza japonica. Org Lett 20:7926–7928
Shi B, Ai H, Duan K, Feng T, Liu J (2022) Ophiorrhines F and G, key biogenetic intermediates of ophiorrhine alkaloids from Ophiorrhiza japonica and their immunosuppressant activities. J Nat Prod 85:453–457
Xie W, Yang H, Li Z, Feng T, Liu J (2021) Indole alkaloids from Ophiorrhiza cantoniensis with immunosuppressive activity. Fitoterapia 148:104777
Li W, Song Q, Xiang W, Wang Y (2014) Study on chemical constituents and antibacterial activity of Ophiorrhiza cantoniensis hance. Nat Prod Res Dev 26:683–686
Rajan R, Venkataraman R, Baby S (2016) A new lupane-type triterpenoid fatty acid ester and other isolates from Ophiorrhiza shendurunii. Nat Prod Res 30:2197–2203
Kumar GK, Fayad AM, Nair AJ (2018) Ophiorrhiza mungos var. angustifolia - estimation of camptothecin and pharmacological screening. Plant Sci Today 5:113–120
Lekshmi GM, Gangaprasad A (2019) Phytochemical analysis of Ophiorrhiza pectinata ARN. (Rubiaceae) a potential anticancer plant. J Pharmacog Phytochem 8:2313–2315
Cheng X, Liu Z, Deng R, Li Z, Jiang X, Zheng S (2013) Identification of camptothecin, hyperin and other components in Ophiorrhiza hydroponic system. Guangdong Agric Sci 40:94–97
Rajan R, Varghese SC, Kurup R, Gopalakrishnan R, Venkataraman R, Satheeshkumar K, Baby S (2013) Search for camptothecin-yielding Ophiorrhiza species from southern Western Ghats in India: A HPTLC-densitometry study. Indust Crops Prod 43:472–476
Rajan R, Varghese SC, Kurup R, Gopalakrishnan R, Venkataraman R, Krishnan Satheeshkumar K, Baby S (2016) HPTLC-based quantification of camptothecin in Ophiorrhiza species of the southern Western Ghats in India. Cogent Chem 2:1275408
Viet Cuong LC, Anh LT, Huu Dat TT, Anh TTP, Lien LQ, Kim YH, Tuan Anh HL (2021) Cytotoxic and anti-inflammatory activities of secondary metabolites from Ophiorrhiza baviensis growing in Thua Thien Hue. Vietnam Nat Prod Res 35:4218–4224
Bag P, Ojha D, Mukherjee H, Halder UC, Mondal S, Chandra NS, Nandi S, Sharon A, Sarkar MC, Chakrabarti S, Chattopadhyay D (2013) An indole alkaloid from a tribal folklore inhibits immediate early event in HSV-2 infected cells with therapeutic efficacy in vaginally infected mice. PLoS ONE 8:e77937
Bag P, Ojha D, Mukherjee H, Halder UC, Mondal S, Biswas A, Sharon A, Kaer LV, Chakrabarty S, Das G, Mitra D, Chattopadhyay D (2014) A dihydro-pyrido-indole potently inhibits HSV-1 infection by interfering the viral immediate early transcriptional events. Antiviral Res 105:126–134
Pu X, Zhang C, Zhu L, Li Q, Huang Q, Zhang L, Luo Y (2019) Possible clues for camptothecin biosynthesis from the metabolites in camptothecin-producing plants. Fitoterapia 134:113–128
Arbain D, Putra DP, Sargent MV (1993) The alkaloids of Ophiorrhiza filistipula. Aust J Chem 46:977–985
Nonato MG, Truscott RJW, Carver JA, Hemling ME, Garson MJ (1995) Glucoindole alkaloids from Ophiorrhiza acuminata. Planta Med 61:278–280
Dachriyanus AD, Putra DP, Sargent MV, Susila R, Wahyuni FS (2000) Indole alkaloids from two species of Ophiorrhiza. Aust J Chem 53:221–224
Kitajima M, Fujii N, Yoshino F, Sudo H, Saito K, Aimi N, Takayama H (2005) Camptothecins and two new monoterpene glucosides from Ophiorrhiza liukiuensis. Chem Pharm Bull 53:1355–1358
Kitajima M, Nakamura M, Takayama H, Saito K, Stöckigt J, Aimi N (1997) Constituents of regenerated plants of Ophiorrhiza pumila; formation of a new glycocamptothecin and predominant formation of (3R)-deoxypumiloside over (3S)-congener. Tetrahedron Lett 38:8997–9000
Aimi N, Nishimura M, Miwa A, Hoshino H, Sakai S, Haginiwa J (1989) Pumiloside and deoxypumiloside; plausible intermediates of camptothecin biosynthesis. Tetrahedron Lett 30:4991–4994
Kitajima M, Masumoto S, Takayama H, Aimi N (1997) Isolation and partial synthesis of 3(R)- and 3(S)-deoxypumilosides; structural revision of the key metabolites from the camptothecin producing plant, Ophiorrhiza pumila. Tetrahedron Lett 38:4255–4258
Wang P, Luo J, Wang X, Fan B, Kong L (2015) New indole glucosides as biosynthetic intermediates of camptothecin from the fruits of Camptotheca acuminata. Fitoterapia 103:1–8
Arbain D, Byrne LT, Putra DP, Sargent MV, Skelton BW, White AH (1992) Ophiorrhizine, a new quaternary indole alkaloid related to cinchonamine, from Ophiorrhiza major Ridl. J Chem Soc Perkin Trans 1:663–664
Arbain D, Lajis NH, Putra DP, Sargent MV, Skelton BW, White AH (1993) The alkaloids of Ophiorrhiza cf. ferruginea. Aust J Chem 46:969–976
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitajima, M. Recent studies on chemical constituents of Ophiorrhiza plants. J Nat Med 76, 748–755 (2022). https://doi.org/10.1007/s11418-022-01640-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01640-3