Abstract
Purpose
The effect of thin-layer capping with activated biochar on sediment-to-water flux was investigated. For the first time, the diffusion of both polycyclic aromatic hydrocarbons (PAHs) and arsenic (As) were studied simultaneously. The fate of As was investigated, under successive dysoxic and oxic conditions, in order to assess and discuss potential trade-off effects when using biochar as an active sorbent for capping of multi-contaminated sediments.
Methods
Sediments from the Bureå bay (Sweden), contaminated with PAH and metal elements including As and Hg, were capped with activated biochar and/or bentonite in simple microcosm test systems in the laboratory. The contaminant transport from sediment through the capping to water body was studied by sampling metals in the water phase above the cap over time, or PAH in a heptane layer over water, at regular time intervals.
Results
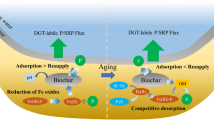
Consistently with the limited previous studies, reductions were observed (e.g., 60–65% for 15-PAH, 100% for chrysene) in sediment-to-water PAH fluxes upon biochar-based thin-layer capping. However, the most important novel finding revealed ambiguous effects of biochar capping on redox-sensitive elements, in particular As. Under the microcosm conditions of the experiment, where pH was affected by the capping material, biochar treatments favoured As mobility under oxic conditions, directly and/or via effects on Fe speciation. While capping limited the diffusion of As under dysoxic conditions, this also favoured greater As mobility under oxic water conditions compared to no capping.
Conclusion
Design and monitoring of capping of PAH contaminated sediments should account for potential negative effects on co-occurring contaminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sediment capping is an increasingly studied remediation strategy for polluted sediments, based on limiting the upwards diffusion of contaminants and isolating them from sediment-dwelling organisms (Ling et al. 1996; Azcue et al. 1998; Mohan et al. 2000). The approach is based on the principle that a layer of any non-contaminated material on top of the contaminated sediment will, according to Ficks law (and in the absence of pore water advection) reduce the sediment-to-water transport of contaminants as the length of the diffusional path increases (Eek et al. 2008; Go et al. 2009). In the past two decades, thin capping with active materials has gained interest, in particular for larger areas where conventional capping requiring large amounts of material and dredging is technically and economically challenging (Ghosh et al. 2011; Patmont et al. 2015).
In thin-layer capping, the active material has the function to sorb contaminants, significantly delaying contaminant breakthrough and decreasing the diffusion rates (Viana et al. 2008). Activated carbon was proven effective at remediating sediments contaminated with hydrophobic organic compounds such as PAH and PCBs (Cornelissen et al. 2011; Patmont et al. 2020), PCDD/F (Cornelissen et al. 2012; Schaanning et al. 2021) and TBT (Eek and Schaanning 2012), as well as mercury (Menzie et al. 2014; Chaudhary et al. 2022). Activated biochar, i.e. pyrolyzed biomass activated at high temperature to increase sorption capacity and affinity (Hagemann et al. 2018), was recently also proven effective for remediating sediments contaminated with PAH (Silvani et al. 2017; Yang et al. 2018; Bianco et al. 2021) or Cu (Zhang et al. 2017), Pb and Cd (Wang et al. 2022) and was suggested to be well suited for sediments contaminated with mercury (Gomez-Eyles et al. 2013). Due to its carbon sequestration effect, the use of biochar as an actively sorbing material in thin capping constitutes a more sustainable approach than many other remediation methods in a life cycle perspective (Sparrevik et al. 2011), but its application is still less documented than that of fossil-based activated carbon, and only for a small number of different contaminants. To our knowledge, no previous study has shown the effect of an active capping strategy based on activated carbon or biochar, on redox-sensitive contaminants such as arsenic (As).
Different types of sorbents are best suited for different families of contaminants (Viana et al. 2008). While activated carbon efficiently limits the diffusion of mercury to the water column, apatite would be a better sorbent for cadmium, chromium and lead, and organoclay would have a good performance for arsenic and methylmercury. But in many sediments, different types of organic and inorganic contaminants coexist (Johnston et al. 2013; Menzie et al. 2014), possibly leading to a trade-off in cap performance (Ye et al. 2017).
The effect of biochar on cocktails of coexisting contaminants with different geochemical behaviours has to date been poorly studied, and to our knowledge, no study has shown the effect of biochar-based capping on arsenic diffusion in sediments, a scenario for which contradictory and complex effects can be expected due to biochar intrinsic properties. For example, biochar alkalinity could mobilise anionic metalloid contaminants such as As or Sb which are typically more soluble under alkaline conditions (Sigg and Stumm 1981). In addition, biochar is rich in functional groups which could shuttle electrons and catalyse redox reactions (Kappler et al. 2014; Do Minh et al. 2020). Nevertheless, the effect of biochar on As mobility is not certain, indeed, experiments where As-contaminated sediments were incubated with various biochars reported both smaller release of As with biochar (Zhang et al. 2020) and higher release of As with biochar (Soares et al. 2022). More research is thus needed on the effect of biochar-based thin capping on sediments contaminated with redox-sensitive metal(loid) contaminants such as Hg, As and Sb.
The purpose of the present study was to investigate if a biochar-based thin capping could be a good solution for such sediments contaminated with a cocktail of contaminants, i.e. PAH, As, Cu, Pb, Zn and Hg, with different geochemical behaviours. This study is the first of its kind in investigating the effectiveness of thin-layer caps with and without biochar on the various contaminants in a complex contamination, especially focusing on the potentially contrasting effects of the caps on organic contaminants (such as PAHs) and redox-sensitive metalloid (such as As).
The following hypotheses were tested: (H1) activated biochar capping can effectively reduce the sediment-to-water diffusion of PAHs and (H2) a trade-off of the capping is the mobilisation of metal(loid) contaminants such as As through modifications in pH, Eh and general biogeochemical conditions at the sediment-cap interface.
These hypotheses were tested in the laboratory using simple microcosms consisting of a series of glass jars filled with sediments from the Bureå bay, which are contaminated with PAHs as well as metal(loid)s such as As and Hg due to past industrial activities. The sediments were capped with bentonite and/or biochar and the diffusion of PAH and metal(loid)s from sediment into the water phase was measured.
2 Materials and methods
2.1 Sediment sampling
The sediment of the estuary of the Skellefteå river (Bureå bay, Sweden) present elevated concentrations of PAH and metal(loid) elements such as As, Cu, Pb, Zn and Hg. The elevated concentrations of metal(loid) elements are the results of drainage of the river through sulfide mineralization in the Skellefteå ore district, and industrial activities, i.e. mainly historic emissions from the copper smelter Rönnskär and activities from a pulp mill in Bureå. The latter was using mercury as conservative during the last century and has led to pollution of mercury and PAH at a local scale in the Bureå bay. The sediment used in this experiment was sampled in November 2020 in the Bureå bay using a Van Veen grabber. A sample of ca 135 L was composed by mixing 15 L samples from nine different locations representing an area of 30 m2 (see SI for details). A subsample of 40 kg was homogenized with a cement mixer before use in the microcosms. The sediment was dark grey in colour, but a thin layer with orange colour, likely due to oxyhydroxide precipitates, had been observed in the field at the surface of the sediment during sampling (Fig. S1). The orange precipitates were mixed with the bulk sample before using the material in laboratory tests. The Bureå sediment contained abundant wood pieces in the centimetre size and wood fibres.
2.2 Capping materials
The materials used for the sediment capping were (1) a commercial activated biochar from Jacobi Carbons (CP1 powdered; Kalmar, Sweden) and (2) a bentonite clay, which is a dry powdered white sodium montmorillonite, saline seal from CETCO and was obtained from FLA Geoprodukter (Lidköping, Sweden). The bentonite material was used as a structuring material to ensure that biochar would stay on the sea bottom. The biochar was produced by pyrolysis of coconut shell biomass using high-temperature steam activation. Steam activation generally requires temperatures > 850 °C (Marsh and Reinoso 2006). The characteristics of these materials have extensively been described in Amstaetter et al. (2012). Briefly, 80% of particles in a size smaller than 74 µm, carbon content was above 90%, and total surface area was 1158 m2 g−1 (pore volume 0.54 cm3 g−1), with 977 m2 g−1 in the 0.35–1.5 nm size range and 61 m2 g−1 in the 1.5–40 nm size range (Table S5).
2.3 Mineralogical and chemical analyses
The organic content of the sediment was estimated by the loss of ignition at 1000 °C on dried sediment. The mineralogical composition of the sediment and capping materials was analysed by X-ray diffraction (XRD). The masses of 16-EPA PAHs in the original sediment (hereafter noted “16-PAH”) or 15 EPA PAHs in the microcosm samples (hereafter noted “15-PAH”) were analysed by chromatography- mass spectrometry (GC–MS). Element concentrations were analysed in HNO3-digested sediment, biochar and bentonite, as well as in the sediment porewater and in the Bureå bay water, respectively by inductively coupled plasma-atomic emission spectrometry (ICP-AES; for major elements) and sector field mass spectrometry (ICP-SFMS; for trace elements). In the water phase of microcosms, major elements were analysed by optical emission spectrometry (ICP-OES) and trace elements by mass spectrometry (ICP-MS). For selected water samples, total mercury was also analysed using the USEPA method 1631 as described in Braaten et al. (2014), which provided a detection limit of 0.1 ng L−1 and a quantification limit of 0.3 ng L−1. Fe(III) and total Fe concentrations were analysed by ferrozine-colorimetry on acid-extracted sediments, following the method from Stookey (1970). pH of biochar and bentonite was measured in a paste (bentonite) or suspension (biochar) with solid:liquid ratio of 1:2.5 in mass, after shaking and settling during 2 h. Details on the analytical methods used are provided in supporting information.
2.4 Microcosm experiment set-up for analysis of PAHs diffusion
Separate glass jars with a diameter of 7.9 cm were prepared for each sediment treatment and each diffusion time tested. One hundred fifty gram of homogenized sediment was placed in each glass jar using a pastry pouch. Caution was taken to avoid air pockets in the sediment or traces of sediment on the walls and to obtain a flat surface. Triplicate glass jars were prepared for a number of samples detailed in Table 1.
Five different treatments were applied to the sediments (Table S6), including a control with untreated sediment (A samples); a control with only bentonite capping (B samples); a control with sediment treated with only biochar mixed into the sediment (C samples); and two recipes of capping with biochar-enriched bentonite, with respectively 0.7 ± 0.1 kg m−2 biochar (D samples, hereafter referred to as “low biochar”) and 1.8 ± 0.1 kg m−2 biochar (E samples, hereafter referred to as “high biochar”). The surface coverage of biochar was chosen to be in the range of dosages tested in previous field-scale sediment capping. For example, the biochar cover applied in a dioxin-contaminated site in the Grenland fjord corresponded to a surface coverage of 0.5–2 kg m−2 (Cornelissen et al. 2012). The amount of bentonite added (2.6 ± 0.4 kg m−2) was chosen so that a paste was obtained, relatively easy to apply as a flat layer. Biochar and bentonite were added as a mixture with NaCl solution (3 g L−1, as a proxy for brackish water). Details about the cap recipes are in supporting information. The cap was applied by taking 50 mL of the paste and applying it above the sediment using a plastic syringe. This resulted in a cap layer of 1.5 cm thickness for all the capped tests (B, D and E).
One hundred fifty milliliter of milliQ water with 3 g L−1 NaCl was then slowly placed above the flat surface of sediment or cap, and 60 mL of heptane was placed above the water (Fig. 1). Heptane was used as an infinite sink for PAHs diffusing from the sediment to the overlying water (Eek et al. 2008). An internal standard, consisting of deuterated naphthalene, acenaphthene, fluorene, phenanthrene, fluoranthene, benz(a)anthracene, benz(a)pyrene and dibenz(ah)anthracene in nonane, was added to the heptane phase prior to placement above water, in order to correct PAH concentrations for eventual losses of heptane during the sampling procedure.
Schematic principle of the experimental tests for diffusion of metal(oid)s (a, b) and PAH (c, d) through the uncapped (a, c) and capped (b, d) sediments. The blue dashed line indicates the theoretical concentration profile of the contaminant in water and capping layer in the microcosm test, assuming steady-state molecular diffusion. Concentration in water in contact with heptane (Cw), concentration in sediment pore water (Cpw_sed), diffusive boundary layer thickness (δe) and cap thickness (hcap) are indicated
The heptane phase was collected after 2, 8, 15, 26, 50, 100, 200 and 386 days of experiment, using methanol-washed Pasteur pipettes and glass test tubes. Then, the volume of heptane was reduced to 0.5 to 1 mL using a rotational vacuum concentrator equipped with a cold trap (®CHRIST, model RVC 2–25 CDplus) before analysis of 15-PAH. PAH concentrations were also analysed in the capping material for a selection of microcosm samples after 100 days and 386 days of diffusion.
2.5 Microcosm experiment set-up for analysis of diffusion of metal(loid) elements
In order to assess the diffusion of metal(loid) elements from the sediment to water, the approach chosen was similar to the microcosm tests for PAH diffusion, but measuring the metal(loid) concentrations directly in the water phase (no heptane or other infinite sink was added) (Fig. 1). In this case, the diffusion regime is not a steady state with infinite sink. Instead, in principle, the flux decreases as concentrations in the water phase increase, until an equilibrium is reached between the porewater in sediment and the water phase above the sediment.
Because oxygen concentrations measured above the sediment at the Bureå site were between 1 and 2 mg L−1 in March 2021, i.e. at the border between dysoxic and oxic conditions (Tyson and Pearson 1991), and because seasonal fluctuations of oxygen concentrations are expected to occur at the site, metal(loid) diffusion fluxes were assessed successively under dysoxic and oxic conditions. For this purpose, subsamples of test sediment (150 g each) were placed in glass jars in glovebags filled with nitrogen. The same five treatments as the ones for assessing the diffusion of PAH (Section 2.4) were applied. The bentonite and biochar powders were equilibrated with N2-atmosphere and the NaCl 3 g L−1 solution was N2-sparged prior to making the capping pastes. One hundred fifty milliliter of N2-sparged milliQ water with NaCl 3 g L−1 was then added slowly to the top with a plastic pipette. The jar samples were stored in zip-lock bags with oxygen consumers (AnaeroGen™, based on ascorbic acid and activated carbon), and additional oxygen consumers were opened in the bags every other day for 2 weeks. The oxygen concentrations measured in the water phase during the first week of experiment were between 0.5 and 0.8 mg L−1 O2. After 10 days of experiment, oxygen concentration was allowed to gradually build up by not adding further oxygen consumers.
The water phase was sampled over time after 3 h to 99 days, by sacrificing a new jar at each time point (Table S6). A plastic syringe was used to extract the water above the sediment, and then water was filtered at 0.45 µm using Whatmann PES filters and stored in plastic bottles with nitric acid at a final concentration of 1 wt%. Every other time point was sampled in triplicates (Table 2). For a given treatment and a given element, the maximum experimental relative error, calculated based on the triplicates obtained, was applied to the other results.
A selection of water samples was also stored in dark glass bottles with 1 wt% hydrochloric acid for Hg analyses. Before sampling, the pH of the water above sediment was measured using a pH paper with a precision of ± 0.5.
The water samples were stored at 4 °C, and the concentrations of metal(loid) elements was analysed. In addition, seven water samples were analysed for total mercury.
2.6 Flux determination from microcosm experiments
Fluxes of PAH and metal(loid) elements were determined based on measured concentrations accumulated in the water or heptane phase over time, considering the following.
In the case of the experiment assessing PAH diffusion fluxes, the heptane layer is an infinite sink for PAHs and the flux (J) from the sediment to water in the microcosm tests can be expressed as follows, in g cm−2 s−1 (Eq. (1)):
with t the diffusion time (s); mheptane the mass of PAH (g) in the heptane phase at time t; and AMi (cm2) the surface area of the sediment water interface in the microcosms.
In the case of the experiment assessing metal(loid) diffusion fluxes, the water phase can be considered an infinite sink only during a first time period T, while it then behaves as a finite sink. Following this consideration, the metal(loid) flux from sediment to water can be expressed as (2):
with Ct the concentration of the element at the time t < T (in g cm−3), and V the volume of water above the sediment in the microcosm (in cm3).
2.7 Theoretical models used for discussing metal(loid) behaviours
In order to discuss the observed behaviours of Mg, As, Fe and Mn in the microcosms, their cumulated concentrations as a function of time in the water phase were compared with a theoretical diffusion model assuming:
-
No sorption on the capping material;
-
The sediment is an infinite source, and the cap material is in equilibrium with the concentration gradient in the pore water throughout the cap;
-
Steady-state molecular diffusion through a diffusive boundary layer (DBL).
This theoretical diffusion model, used for comparison and discussion, is expressed as follows (Jørgensen and Revsbech 1985):
with Jised the diffusive flux (in g cm−2 s−1); Di the diffusion coefficient for a given compound in cm2 s−1, for which values were taken from Yuan-Hui and Gregory (1974); δDBL the diffusive boundary layer in cm, considered to be 0.17 cm, based on previous measurement (0.17 ± 0.02 cm) on similar laboratory tests done following the alabaster method in Eek et al. (2008); Ci pw the concentrations measured in porewater (Table S3); and Ci w the concentration in the water above sediment, in g cm−3. For capped samples, diffusive flux through the cap under the new steady state was approximated by Eq. (4) (Eek et al. 2008),
where the cap thickness (hcap d) was approximated to 1.5 cm; the porosity ε was approximated to 0.5; and the tortuosity τ to ε−1/3 (Boudreau 1997).
Cumulated concentrations in the water above the sediment (Ciw), as well as Jised and Jicap, were calculated following Eqs. (3) and (4) and compared with measured concentrations and fluxes.
3 Results
3.1 Composition of the sediment and capping materials
3.1.1 Composition of the sediment
The water content of the sediment was 69 wt%, and the loss of ignition of the dried sediment was 18 wt%. XRD analyses indicated that the inorganic part of the sediment consisted of a majority of silicate minerals as well as a small but significant amount of pyrite (1 wt% FeS2; Table S1). The sediments contained 240 mg kg−1 of As, 1.7 mg kg−1 Hg and 14.6 mg kg−1 of PAH-16 (on a dry mass basis—Tables S2 and S3). Most of Fe from the sediment was in the reduced form Fe(II) (100% of the extracted Fe, Table S4), as expected from the dark colour and the pyrite content.
3.1.2 Composition of the capping materials
No PAH could be detected in the bentonite material, and only minor concentrations of a few PAHs were detected in the biochar, i.e. naphthalene (0.09 mg kg−1, i.e. 12% of the concentration in the sediment) and phenanthrene, fluoranthene and pyrene (respectively 0.07 mg kg−1, 0.05 mg kg−1 and 0.04 mg kg−1, i.e. < 5% of the concentration in the sediment) (Table S3).
Bentonite contained significant amounts of Fe, Cu, V, Ni, Co and Mn, i.e. higher concentrations than the sediment (Table S2). However, these concentrations were significantly lower in the biochar material. In addition, the concentrations of As, Hg and Pb, which are the most problematic metal(loid) contaminants at the Bureå bay, were negligible in either of the capping materials compared to in the sediment (Table S2).
The bentonite material contained 124 mg kg−1 of 0.5 M HCl-extractible Fe, from which 89% was in the oxidized Fe(III) form (Table S4). Biochar contained 36 mg kg−1 Fe, from which 89% was in the reduced form Fe(II) (Table S4).
The paste pH of bentonite was 9.3, and the paste pH of biochar was 10.0.
3.2 Diffusive flux of PAHs
3.2.1 Diffusion of PAHs from uncovered sediments
PAH mass diffused from uncovered sediment to water and trapped in heptane, increased as a function of time, following a linear trend (Fig. 2). The linear increase of PAH concentrations in heptane is in accordance with diffusion-controlled processes where the PAH porewater concentration would be controlled by equilibrium with an infinite solid source (i.e. where Ciw is 0 in Eq. (3); Eek et al. 2008).
Sediment-to-water transport (g/m2) of total 15-PAH (a) and acenaphtylene (b), fluoranthene (c) and chrysene (d) (as example PAHs of varying hydrophobicity), as a function of diffusion time, for the five treatments tested. Error bars are standard deviations on triplicate analyses and were obtained only for a number of samples detailed in Table 1. Dashed lines indicate a linear regression of PAHs from uncapped sediment (A) and after break through the capped tests B, D and E
3.2.2 Effect of capping on PAH diffusion
The diffusion flux of 15-PAHs, i.e. the slope of diffused mass as a function of time, was reduced by 56% in the case of bentonite-capped sediment compared to uncapped and further reduced by 65%, in case of addition of biochar in the capping (Fig. 2, Table 3). This flux reduction after capping is due to the addition of the cap thickness to the diffusive boundary layer, as well as increase of tortuosity and decrease in porosity (Eq. (4)).
In addition, PAHs broke through the cap at least 50 or 150 days later, respectively in the case of sediment covered with a low-biochar- or high-biochar-enriched cap, compared to a cap with only bentonite (Fig. 2). This breakthrough delay is likely due to PAH sorption on biochar, which is consistent with higher PAH concentrations measured in the biochar-enriched cap compared to bentonite cap after 100 and 386 days of experiment (Fig. S5). For example, 15-PAH measured in the high-biochar cover was 94% higher after 100 days and 64% higher after 386 days than that measured in the bentonite cover.
The effect on reduction in flux was variable among the various PAH compounds, with generally stronger reductions in flux for PAHs with higher log Kow (correlation coefficient of 0.8 or R2 = 0.6; Fig. S7). For example, the reduction in flux for a cap with low biochar (D samples) was 39% for acenaphtylene (log Kow = 3.8 (Smedes 2019)), 95% for fluoranthene (log Kow = 5.3) and 100% for chrysene (log Kow = 6; Table 3).
3.3 Diffusive flux of metal(loid) elements
3.3.1 Diffusion of redox-inactive elements
As an example of a redox-inactive element that does not significantly sorb to biochar, Mg behaviour was consistent with pure diffusion from sediment to water in a closed system, i.e. where water is a finite sink (Figs. 3 and S13). Indeed, concentration of Mg in water above uncapped sediment increased fast at start, then slower with time, converging to equal porewater concentration. Similar behaviours were observed for Ca and Si (Fig. S7).
The concentration of Mg increased significantly more slowly above capped sediment than above uncapped sediment (Fig. 3). The fact that capping limited Mg diffusion is qualitatively consistent with the diffusion model showing a smaller slope of diffused mass as a function of time (Fig. S13).
Biochar addition to the bentonite cap did not significantly limit Mg diffusion, suggesting that Mg concentrations were not affected by sorption to biochar (Fig. 3).
3.3.2 Evolution of pH and Fe, S and O2 concentrations above the sediments
In the experiment presented, the conditions were initially dysoxic, with less than 1 mg L−1 O2 in the water above sediment, which corresponds to the lower values of concentrations measured in the field (1–2 mg L−1).
During the first week, Fe concentrations built up above the uncapped sediment, in consistence with a diffusion process (Fig. S13). In contrast, Fe concentration dropped between 7 and 16 days. Concerning S concentration above uncapped sediments, they increased up to 8 µg L−1 during the first 7 days, then faster. However, in the case of covered sediment, after the first 10 days (i.e. under oxic conditions in water), Fe and S concentrations did not increase.
In addition, capping led to higher pH values, especially right after application of the cap (Fig. S9). This is explained by the high pH measured in biochar (pH 10) and in bentonite (pH 9).
3.3.3 Effect of capping on the diffusion of Arsenic
As diffusion from the uncapped sediment followed a pattern similar to that observed for Fe, with concentration increasing under dysoxic conditions and dropping under subsequent oxic water conditions (after day 10). All the caps tested limited As diffusion under dysoxic conditions, i.e. during the first week, with an almost 100% reduction in diffusion rates (Figs. 3 and S12, Table 4). However, when the water became oxic, As concentrations continued to increase above the capped sediment (Fig. 3), following a pattern consistent with diffusion through the cap for at least the first 32 days (Fig. S13). As a result, As concentrations eventually increased to higher values above the capped sediments compared to above the uncapped ones. The negative effect of capping under oxic water conditions was stronger in the case of biochar-enriched coverings (Fig. 3).
4 Discussion
4.1 Capping with biochar-enriched thin layer is effective for limiting PAH diffusion
This study shows that a thin cap with bentonite and biochar can significantly reduce the flux of PAHs diffusing to water, and to a greater extent than capping with bentonite alone, confirming the working hypothesis H1. Capping with bentonite alone also led to a significant flux reduction for most of PAH compounds (Fig. 2, Table 4). This positive effect of bentonite capping can for a large part be explained by the increase of diffusion boundary layer thickness (Eek et al. 2008). Bentonite can also sorb PAHs to a small extent (Cornejo et al. 2015; Fig. S6), delaying slightly the breakthrough. The observed bentonite effect on sediment-to-water PAH fluxes is also consistent with a previous study which showed that capping with clay and activated carbon was more effective at limiting PAH diffusion (and PAH porewater) than activated carbon alone (Cornelissen et al. 2011).
The effectiveness of biochar for PAH remediation in sediments was already shown in several studies (Bianco et al. 2021 and references therein). The reduction in PAH diffusion flux will depend on PAH concentrations in sediment, sediment composition more generally, and remediation time, biochar dosage and manufacture, pH, etc. (Bianco et al. 2021). For comparison, while under the conditions of this experiment, the biochar capping reduced 15-PAH fluxes by 60 to 65% through a cover of 0.7 to 1.8 kg.m−2 of biochar after 386 days (Table 3), in situ thin capping with 5 kg m−2 of activated carbon reduced PAH fluxes from sediments in the Trondheim Harbour by 50 to 90% after 1 year of remediation (Cornelissen et al. 2011). As another example, in estuary sediments contaminated with 8 mg kg−1 16-PAH, Gomez-Eyles and Ghosh (2018) showed that addition of 5 wt% biochar to sediments led to a removal of 98% in porewater after 28 days.
In addition, our results show variable flux reductions depending on PAH compounds. We hypothesise based on a significant correlation between reductions in diffusion fluxes and log Kow (Fig. S7) that the main driver of this variation is the compound’s hydrophobicity. This would be consistent with increasing effectiveness of biochar covers with increasing PAH hydrophobicity as observed by Silvani et al. (2017). These authors explained this behaviour by suggesting that more hydrophobic compounds would be less soluble in the porewater and more adsorbed onto the hydrophobic aromatic functional groups of the biochar sorbent.
4.2 Effect of the capping on redox conditions and Fe (oxyhydr)oxides precipitates
The Fe concentration changes (Fig. 3) are interpreted to reflect the evolution of oxygen concentration in the water above sediment. Fe concentrations building up above uncapped sediments were likely in the form of Fe(II), while the drop in Fe concentration between 7 and 16 days suggests oxidation of Fe(II) to Fe(III) and subsequent precipitation, most likely as Fe (oxyhydr)oxides. Consistently, an orange precipitate was observed at the surface of uncapped samples (Fig. S10).
In addition, concentrations of sulphur (S) are interpreted to reflect the redox conditions deeper in the sediment. Indeed, the sediment contains pyrite (Table S1) which generates Fe(II) and sulphate ions upon oxidation. While Fe(II) quickly oxidizes into insoluble Fe(III), sulphate ions are generally more soluble. The fact that S concentrations increased steadily above the uncapped sediment (A and C), instead of reaching a maximum, and far above the concentration of 50 µg L−1 that would be consistent with a pure diffusion process (Fig. S13), suggests that pyrite in the uncapped sediment oxidized when the atmosphere became oxic and oxygen diffused through the water layer to the sediment. However, the fact that Fe and S concentrations did not increase after day 10 in the case of covered sediment suggests that pyrite did not oxidize even when water above the sediment was under oxic conditions, probably because the cover limited the diffusion of oxygen downwards towards the sediment. Therefore, the cap may have favoured anoxic or/and reducing conditions. Ndungu et al. (2016) also observed anoxic conditions in capped sediments in boxcosms, with nearly 0% O2 saturation below 1 cm. In addition, Bessinger et al. (2012) previously predicted, using a 1-D biogeochemical transport model, that covering estuarine sediments with 1 m sand would lead to sulphate-reducing conditions, preventing the precipitation of Fe (oxyhydr)oxides. These authors emphasised the importance of thickness dimensioning for determining effects on redox processes. In our case, the capping is thin (1.5 cm) but the fine material used (biochar and bentonite) led to oxygen diffusion limitation.
4.3 Trade-off effects of biochar capping on arsenic as a redox-sensitive element
Biochar-enriched thin capping not only limited the diffusion of metal(loid) elements but also changed the pH and redox conditions of the experimental system. The most important finding of our study is that capping may result in negative trade-off effects on the transport of redox-sensitive metal(loid) elements like As, as stated in the working hypothesis H2. Even though capping was effective for limiting As diffusion under dysoxic conditions, it led to reducing conditions closer to the surface and higher As mobility under oxic water conditions. Several studies indicate that biochar amendments can result in increasing As mobility from other types of environments like paddy soils (Wang et al. 2017; Qiao et al. 2018) or mine tailing sediments (Chen et al. 2016).
One interpretation is that the higher As flux is due to lower amounts of Fe(III)-(oxyhydr)oxide available for As sorption at the surface of the capped samples compared to uncapped ones. As discussed above (4.2), more Fe diffused to the water and precipitated above the uncapped sediment compared to capped sediment. The correlation between As and Fe concentrations above uncapped sediment suggests As co-precipitation with or sorption on Fe (oxyhydr)oxides formed at the surface (Fig. S10). Such processes are common in the environment due to high affinity of As for Fe (oxyhydr)oxides (Stollenwerk 2003). Bessinger et al. (2012) also predicted that covering estuarine sediments with 1 m sand would hinder the precipitation of Fe (oxyhydr)oxides and thus lower the mineral surface availability for As sorption. In the case of our experiments where the surface layer of Fe (oxyhydr)oxides was mixed with the sediment prior to capping, electron shuttling effects of biochar could also have to some extent stimulated the reduction and dissolution of Fe (oxyhydr)oxides in contact with biochar, releasing sorbed As (Kappler et al. 2014; Chen et al. 2016) and resulting in the higher As flux observed in test C than in test A.
Another explanation for the greater As diffusion with capping under the conditions of this experiment is that porewater pH increased locally due to high alkalinity of the capping material (Fig. S9; Mohan et al. 2007). Indeed, arsenate sorption on Fe (oxyhrdr)oxides is generally weaker at higher pH (Sigg and Stumm 1981; Stollenwerk 2003).
Under field conditions, the system would not be closed; therefore, pH in the shallow cover would be buffered by sea water, and pH effects on metal(loid) mobility would likely be less pronounced. However, over time, the old As-enriched Fe (oxyhydr)oxides layer could get reduced after burial under a capping layer. The consequence of this could be that As sorbed on Fe-(oxyhydr)oxides is remobilized, leading to an increase in As concentrations in porewater in the top sediment layer. In addition, if the conditions were reducing enough for As(V) to be reduced to As(III), As could become more toxic (He et al. 2009). The effect of increased As concentration in the porewater (CAs_pw) could be abated by increasing the thickness of the cap (hcapd) (Eq. (4)).
Even though capping could enhance As migration to the water, it could also lower the As concentration at the seafloor surface where benthic organisms live, at least for a time. Indeed, in the field, the surface of the sediment is naturally covered by a layer of Fe (oxyhydr)oxides (Fig. S1). This layer of Fe (oxyhydr)oxides provides a natural sorption trap for As, concentrating As at the surface where benthic organisms live. Capping, by limiting Fe and As diffusion and hindering precipitation of Fe (oxyhydr)oxides, could decrease the amount of As at the seafloor and protect organisms from being exposed to older As-enriched Fe (oxyhydr)oxides layers. The positive effect of the cap on diffusion limitation and on limiting the abundance of As-rich Fe (oxyhydr)oxydes at the surface, relative to the negative on As mobility and relative to eventual natural deposition of exogenous Fe-rich minerals, should be further studied.
In addition to As, trade-off effects of thin-layer capping could exist for other redox-sensitive elements. For example, Hg methylation could be favoured under the more reducing and sulphate-rich conditions induced by capping. Capping with activated biochar has been observed to increase Hg methylation (Sørmo et al. 2022). However, this methylation was offset by stronger sorption of methyl-Hg by the biochar. In the present study though, total Hg fluxes were reduced by capping (Fig. S14).
Microbiological and algal activity, as well as biodegradation, vary with seasons, and microbial compositions and benthic organisms are likely to be influenced by the addition of biochar (Zhang et al. 2020; Yang et al. 2021), increasing the complexity of the biogeochemical effects of a biochar-enriched thin cap compared to the experimental system presented.
5 Conclusions
This study is one of the firsts to report that a biochar-amended thin capping strongly reduces upwards diffusion of PAHs and thus would have a positive effect on PAH contamination levels in the sediment/sea interface where benthic organisms are living. However, the capping could lead (locally) to higher pH and reducing conditions at the sediment/capping interface, inducing the mobilisation of redox-sensitive contaminants such as arsenic, or potentially methylation of mercury. This study demonstrates the need for (site-specific) in situ investigation of As and Hg speciation to reveal the net effects a biochar-based capping would have on a site with mixed contamination.
Data availability
Raw data produced for this study will be made available upon request.
References
Amstaetter K, Eek E, Cornelissen G (2012) Sorption of PAHs and PCBs to activated carbon: coal versus biomass-based quality. Chemosphere 87:573–578. https://doi.org/10.1016/j.chemosphere.2012.01.007
Azcue JM, Zeman AJ, Förstner U (1998) International review of application of subaqueous capping techniques for remediation of contaminated sediments. In: Seco e Pinto PS (ed) Environmental Geotechnics. Balkema, Rotterdam, pp 537–542
Bessinger BA, Vlassopoulos D, Serrano S, O’Day PA (2012) Reactive transport modeling of subaqueous sediment caps and implications for the long-term fate of arsenic, mercury, and methylmercury. Aquat Geochem 18(4):297–326
Bianco F, Race M, Papirio S, Oleszczuk P, Esposito G (2021) The addition of biochar as a sustainable strategy for the remediation of PAH–contaminated sediments. Chemosphere 263:128274
Boudreau BP (1997) Diagenetic models and their implementation. Springer, Berlin, p 414
Braaten HFV, de Wit HA, Harman C, Hageström U, Larssen T (2014) Effects of sample preservation and storage on mercury speciation in natural stream water. Int J Environ Anal Chem 94(4):381–384
Chaudhary DK, Bailon MX, Kim H, Reible D, Hong Y (2022) Evaluation of sediment capping effectiveness in Hyeongsan River for in-situ management of total mercury and methylmercury contamination. J Soils Sediments 22(9):2578–2591
Chen Z, Wang Y, Xia D, Jiang X, Fu D, Shen L, Wang H, Li QB (2016) Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition. J Hazard Mater 311:20–29
Cornejo J, Bruna González F, Real Ojeda M, Guzmán Carrizosa I, Celis R (2015) Retention of PAHs in a Mediterranean soil after different amendments. 18th International Symposium on Environmental Pollution and its Impact on Life in the Mediterranean Region. Book of abstracts p. 195
Cornelissen G, Amstaetter K, Hauge A, Schaanning M, Beylich B, Gunnarsson J, Breedveld G, Oen A, Eek E (2012) Large-scale field study on thin-layer capping of marine PCDD/F contaminated sediments in Grenlandfjords, Norway: physicochemical effects. Environ Sci Technol 46:12030–12037
Cornelissen G, Kruså M, Breedveld G, Eek E, Oen A, Arp HP, Raymond C, Samuelsson G, Hedman J, Stokland Ø, Gunnarsson J (2011) Remediation of contaminated marine sediment using thin-layer capping with activated carbon—a field experiment in Trondheim Harbor, Norway. Environ Sci Technol 45:6110–6116
Do Minh T, Song J, Deb A, Cha L, Srivastava V, Sillanpää M (2020) Biochar based catalysts for the abatement of emerging pollutants: a review. Chem Eng J 394:124856
Eek E, Cornelissen G, Kibsgaard A, Breedveld GD (2008) Diffusion of PAH and PCB from contaminated sediments with and without mineral capping; measurement and modelling. Chemosphere 71(9):1629–1638
Eek E, Schaanning M (2012) Opticap Report [cited 2014 April]. Available from: http://www.ngi.no/upload/Prosjektweb/opticap/Opticap%20sluttrapport.pd
Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA (2011) In-situ sorbent amendments: a new direction in contaminated sediment management. Environ Sci Technol 45(4):1163–1168
Go J, Lampert DJ, Stegemann JA, Reible DD (2009) Predicting contaminant fate and transport in sediment caps: mathematical modelling approaches. Appl Geochem 24(7):1347–1353. https://doi.org/10.1016/j.apgeochem.2009.04.025
Gomez-Eyles JL, Ghosh U (2018) Enhanced biochars can match activated carbon performance in sediments with high native bioavailability and low final porewater PCB concentrations. Chemosphere 203:179–187
Gomez-Eyles JL, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U (2013) Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environ Sci Technol 47:13721–13729
Hagemann N, Spokas K, Schmidt HP, Kägi R, Böhler MA, Bucheli TD (2018) Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABC s. Water 10(2):182
He W, Megharaj M, Naidu R (2009) Toxicity of tri- and penta-valent arsenic, alone and in combination, to the cladoceran Daphnia carinata: the influence of microbial transformation in natural waters. Environ Geochem Health 31:133–141
Johnston R, Kirtay V, Chadwick D, Rosen G, Guerrero J, Collins J, Ortega C, Webb R, May R, Germano J, Browning D, Beaver E, Wicklein M, Pittz J, Leisle DE, Doyle L, Hsu L (2013) Installing an activated carbon sediment amendment at the Puget Sound Naval Shipyard and Intermediate Maintenance Facility, Bremerton, WA. In Proceedings of the Seventh International Conference on Remediation of Contaminated Sediments (pp 4–7)
Jørgensen BB, Revsbech NP (1985) Diffusive boundary layers and the oxygen uptake of sediments and detritus. Limnol Oceanogr 30:111–122
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an electron shuttle between bacteria and Fe (III) minerals. Environ Sci Technol Lett 1(8):339–344
Ling HI, Leshchinsky D, Gilbert PA, Palermo MR (1996) In-situ capping of contaminated submarine sediments: geotechnical considerations. In: Kamon M (ed) Environ Geotech. Balkema, Rotterdam, pp 575–580
Marsh H, Reinoso FR (2006) Chapter 5 activation processes (thermal or physical). In Marsh H, Reinoso FR (eds) Activated carbon, 1st edn. Elsevier Science Books, pp 243–321
Menzie C, Amos B, Kane-Driscoll S, Ghosh U, Gilmour C (2014) Evaluating the efficacy of a low-impact delivery system for in-situ treatment of sediments contaminated with methylmercury and other hydrophobic chemicals. ESTCP Environmental Restoration Project ER-200835. [cited 2014 November]. Available from: http://www.serdp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/ER-200835
Mohan D, Pittman CU, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gómez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310:57–73
Mohan RK, Brown MP, Barnes CR (2000) Design criteria and theoretical basis for capping contaminated marine sediments. Appl Ocean Res 22:85–93
Ndungu K, Schaanning M, Braaten HFV (2016) Effects of organic matter addition on methylmercury formation in capped and uncapped marine sediments. Water Res 103:401–407
Patmont CR, Ghosh U, LaRosa P, Menzie CA, Luthy RG, Greenberg MS, Cornelissen G, Eek G, Collins J, Hull J, Hjartland T, Glaza E, Beiler J, Quadrini J (2015) In situ sediment treatment using activated carbon: a demonstrated sediment cleanup technology. Integr Environ Assess 11(2):195–207
Patmont E, Jalalizadeh M, Bokare M, Needham T, Vance J, Greene R, Cargill J, Ghosh U (2020) Full-scale application of activated carbon to reduce pollutant bioavailability in a 5-acre lake. J Environ Eng 146(5):04020024
Qiao JT, Li XM, Li FB (2018) Roles of different active metal-reducing bacteria in arsenic release from arsenic-contaminated paddy soil amended with biochar. J Hazard Mater 344:958–967
Schaanning MT, Beylich B, Gunnarsson JS, Eek E (2021) Long-term effects of thin layer capping in the Grenland fjords, Norway: reduced uptake of dioxins in passive samplers and sediment-dwelling organisms. Chemosphere 264:128544
Sigg L, Stumm W (1981) The interaction of anions and weak acids with the hydrous goethite (α-FeOOH) surface. Colloids Surf 2(2):101–117
Silvani L, Di Palma PR, Riccardi C, Eek E, Hale SE, Viotti P, Papini MP (2017) Use of biochar as alternative sorbent for the active capping of oil contaminated sediments. J Environ Chem Eng 5(5):5241–5249
Smedes F (2019) SSP silicone–, lipid–and SPMD–water partition coefficients of seventy hydrophobic organic contaminants and evaluation of the water concentration calculator for SPMD. Chemosphere 223:748–757
Soares MB, Dos Santos FH, Alleoni LRF (2022) Temporal changes in arsenic and lead pools in a contaminated sediment amended with biochar pyrolyzed at different temperatures. Chemosphere 287:132102
Sørmo E, Silvani L, Braaten HFV, Bryntesen T, Eek E, Cornelissen G (2022) Formation and availability of methylmercury in mercury-contaminated sediment: effects of activated carbon and biochar amendments. J Soils Sediments 22:1041–1053
Sparrevik M, Saloranta T, Cornelissen G, Eek E, Fet AM, Breedveld GD, Linkov I (2011) Use of life cycle assessments to evaluate the environmental footprint of contaminated sediment remediation.
Stollenwerk KG (2003) Geochemical processes controlling transport of arsenic in groundwater: a review of adsorption. Arsenic in ground water: Geochemistry and occurrence, 67–100
Stookey LL (1970) Ferrozine-a new spectrophotometric reagent for iron. Anal Chem 42:779–781
Tyson RV, Pearson TH (1991) Modern and ancient continental shelf anoxia: an overview. Geol Soc Lond Spec Publ 58(1):1–24
Viana PZ, Yin K, Rockne KJ (2008) Modeling active capping efficacy. 1. Metal and organometal contaminated sediment remediation. Environ Sci Technol 42(23):8922–8929
Wang N, Xue XM, Juhasz AL, Chang ZZ, Li HB (2017) Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ Pollut 220:514–522
Wang Z, Song S, Wang H, Yang W, Han J, Chen H (2022) Feasibility of remediation of heavy-metal-contaminated marine dredged sediments by active capping with Enteromorpha Biochar. Int J Environ Res Public Health 19(9):494
Yang X, Chen Z, Wu Q, Xu M (2018) Enhanced phenanthrene degradation in river sediments using a combination of biochar and nitrate. Sci Total Environ 619:600–605
Yang Y, Ye S, Zhang C, Zeng G, Tan X, Song B, Zhang P, Yang H, Li M, Chen Q (2021) Application of biochar for the remediation of polluted sediments. J Hazard Mater 404:124052
Ye S, Zeng G, Wu H, Zhang C, Liang J, Dai J, Liu Z, Xiong W, Wan J, Xu P, Cheng M (2017) Co-occurrence and interactions of pollutants, and their impacts on soil remediation—a review. Crit Rev Environ Sci Technol 47(16):1528–1553
Yuan-Hui L, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38(5):703–714
Zhang S, Tian K, Jiang SF, Jiang H (2017) Preventing the release of Cu2+ and 4-CP from contaminated sediments by employing a biochar capping treatment. Ind Eng Chem Res 56(27):7730–7738
Zhang W, Tan X, Gu Y, Liu S, Liu Y, Hu X, Li J, Zhou S, Liu S, He Y (2020) Rice waste biochars produced at different pyrolysis temperatures for arsenic and cadmium abatement and detoxification in sediment. Chemosphere 250:126268
Acknowledgements
Valentina Zivanovic, at the isotope laboratory of the Norwegian University of Life Sciences (NMBU), is acknowledged for analysing the chemistry of the water phase with ICP-OES and ICP-MS; Ove Bergersen is thanked for help with Fe(II)/Fe(III) analysis at the Norwegian Institute of Bioeconomy Research (NIBIO); and Horst Rottler is acknowledged for help with PAH analyses at Ökometric (Bayreuth, Germany) and for providing the deuterium standard. Tina Bryntesen is acknowledged for analysing the total mercury in 7 samples at the Norwegian Institute for Water Research (NIVA). Lorenza Sardisco at xrayminerals, UK, is acknowledged for doing the XRD analysis and Rietveld refinement. Nicolas Estoppey is thanked for his help in the laboratory at the end of the experiment. Christer Svenson, at Skellefteå Municipality, is acknowledged for participation to funding acquisition and to the project completion.
Funding
Open access funding provided by Norwegian Geotechnical Institute The authors received funding from the Geological Survey of Sweden (SGU) as part of the government assignment for polluted sediment (RUFS-Regeringsuppdraget för förorenade sediment), to test technologies for sediment remediation and the Research Council of Norway via internal funds at the Norwegian Geotechnical Institute for the research projects GBV20200775 and GBV20210140.
Author information
Authors and Affiliations
Contributions
GC: funding acquisition, conceptualization, methodology, writing—original draft, writing—review and editing. GD-A: conceptualization, methodology, investigation, formal analysis, writing—original draft, writing—review and editing. EE: conceptualization, methodology, writing—review and editing. ES: conceptualization, methodology, writing—review and editing. CBH: methodology, investigation, formal analysis. MVT: investigation. CM: funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Victor Magar
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dublet-Adli, G., Cornelissen, G., Eek, E. et al. A trade-off in activated biochar capping of complex sediment contamination: reduced PAH transport at the cost of potential As mobilisation. J Soils Sediments 24, 497–508 (2024). https://doi.org/10.1007/s11368-023-03666-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03666-w