Abstract
Purpose
Hypersaline environments are extremely vulnerable and important ecological niches. Because much knowledge has focused on the distribution of heavy metals in these areas, the detailed behavior of key major elements in hypersaline environments has not been elucidated in detail.
This research aims to define the distribution, translocation pathways, and mobility patterns of the major elements in hypersaline sediments and halophytes.
Materials and methods
Samples of Sarcocornia fruticosa plants were collected from evaporation (ES) and crystallization (CA) sites in the Sečovlje Salina area (Republic of Slovenia). The major element contents were measured by digestion in HNO3 then aqua regia and analyzed by ICP-MS for ultra-low detection limits. Rhizo-sediments from EA and CA were processed using sequential extraction analysis to determine the precise fractionation of Al, Ca, Fe, K, Mg, Mn, and Na. To determine the translocation patterns of individual major elements in S. fruticosa, two indices were calculated: bioconcentration (BCF) and translocation factor (TF). Differences and similarities between samples and elements were highlighted using Statistica VII and Grapher 8 statistical software and Ward’s method, respectively.
Results and discussion
The obtained results confirmed that halophyte plants take up large amounts of the essential micronutrient Na due to high salinity, and that macronutrients (Ca, Mg, P, and S) are intensively translocated from the roots to the upper parts of the plant. The overall trend in translocation signature for major elements, distinguished by BCF and TF factor calculations, emphasizes that root tissues accumulate a significant amount of major elements and that accumulation depends on individual major elements. It also showed that the major elements Ca, Mg, Na, P, and S are highly translocated within plants, while the mobility of Al, Fe, and K is limited.
Conclusions
Our results suggest that the major elements are vital macronutrients for halophytes, but their accumulation in the roots and further translocation within the plant depend on individual elements and their dynamics. The translocation pattern of the major elements can be justified as follows: Ca is an essential element for plant growth, maintenance, and membrane integrity; Mg is a specific component of chlorophyll; Na is present because of the hypersaline environment; P is a key component of plant metabolic processes; S represents an important component of enzymes and other key proteins; Al and Fe are preferentially accumulated in roots; and plant leaves are generally undersupplied with K. The presented results are of great importance for the general knowledge and use/application of halophytes in agriculture and biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Estuarine and coastal environments include hypersaline ecosystems, which are more saline than seawater, have widely varying total salinity and ionic composition, and differ in many other aspects, such as temperature, pressure, and nutrient status (Otte et al. 1993; Williams et al. 1994; Sundby et al. 2003; Caetano et al. 2008; Martins et al. 2008; Caçador et al. 2009; Glavaš et al. 2017; Petranich et al. 2017; Kovač et al. 2018). They are also characterized by the appearance of anoxic conditions in sediments and periodic flooding, creating habitats with specific conditions that severely limit their colonization (Otte et al. 1993; Williams et al. 1994; Sousa et al. 2008). Thus, typical communities with distinct species compositions, such as the macrophyte community of salt marshes, are found in these environments. Only a few halophytic species have morphological, anatomical, physiological, and phenological adaptations that allow them to thrive in hypersaline environments. In addition, halophytes influence the biogeochemistry of hypersaline ecosystems through their active and passive cycling of elements (Caetano et al. 2008; Sousa et al. 2008; Caçador et al. 2009).
Because hypersaline environments and their vegetation play an important role in nutrient cycling, any disturbance to biological, chemical, and physical processes can have dramatic effects on the overall well-being of the area (Otte et al. 1993; Williams et al. 1994; Sundby et al. 2003). Of particular concern are the potential effects of pollutants on these sensitive ecosystems, especially heavy metals, whose diverse and widespread use in industry has caused metal content in many estuaries and marine coastal areas to rise well above background geochemical levels. Since the early 1990s, numerous studies have investigated the presence of heavy metals in hypersaline sediments, salt marsh soils, and halophytes (Otte et al. 1993; Salguero and Caçador 2007; Caetano et al. 2008; Sousa et al. 2008; Caçador et al. 2009; Petranich et al. 2017). Because much of this knowledge has focused on the accumulation, mobility, and bioavailability of heavy metals in hypersaline environments, the detailed behavior of major elements in these environments has not been precisely emphasized. Therefore, it is important to define the translocation routes of major elements in hypersaline sediments and halophytes to decipher their signatures in these complex environments.
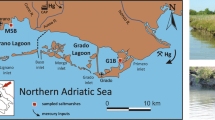
The Sečovlje Salina Nature Park (SSNP), with an area of approximately 700 ha, is located in the southwesternmost part of Slovenia along the border with the Republic of Croatia in the southern part of the municipality of Piran (Fig. 1). The Grande Canal separates the northern part of the park with active salt production, called Lera, from the southern part of the park, Fontanigge. The salt pans of Sečovlje are a part of the nature park. To the north, they border with the St. Bartholomew Canal. To the east, most of the border runs along the former narrow-gauge railroad, while to the south, it is bordered by the Odoric Canal, through which the Dragonja River flows and diverts into the canal years ago. To the west, the dikes in Piran Bay protect the pans.
The area of Lera is divided into crystallization and seawater condensation areas. Lera has typical habitats for animal and plant species, which are limited to salt fields with different salinities, saline channels, and dikes. Fontanigge is located between the Grande Canal and the Dragonja River (Fig. 1). To the west, it borders with a flood dike, and to the east with farmland. Fontanigge has a network of canals that serve as seawater inflows for individual salt fields, outflows for sewage and storm water, and transportation waterways.
These extreme environments of high salinity and aridity provide shelter for rare animal and plant species. The area was designated as a nature park in 1990. In 1993, it became the first Slovenian wetland to be included in the list of internationally important marshes under the auspices of the Ramsar Convention (Kovač et al. 2018). Traditional salt production is practiced in the northern part of SSNP. Sečovlje Salina is one of the few active salinas in the Mediterranean region. Solar salt production involves the traditional manual technique of gathering salt from seawater by passing brine through a series of evaporation ponds (evaporation area) until the salt is harvested in crystallization ponds (crystallization area) (Kovač et al. 2013; Glavaš et al. 2015, 2018).
Thus, SSNP represents a successful model of symbiosis between the traditional use of Salina’s natural resources and the protection of exceptional cultural heritage and biodiversity within the Sečovlje Salina Nature Park. In addition to numerous animals (brine shrimp, Mediterranean killifish, European pond terrapin, and more than 300 birds), salt-resistant and salt-tolerant plants (halophytes) have also successfully inhabited the area. Ivajnšič et al. (2017) identified at least five protected Natura 2000 habitat types: (1) mudflats and sand flats not covered by seawater at low tide; (2) tall rush salt marshes–communities of Juncetalia maritime (association Juncetum maritimi-acuti), (3) Spartina swards: Spartinion maritimae (association Limonio-Spertinetum maritimae); (4) Salicornia and other annuals colonizing mud (Thero-Salicornietea: Suaedo maritime-Salicornietum patulae and Salicornietum emericia; and (5) Mediterranean and thermo-Atlantic halophilous scrubs: Sarcocornetea fruticosi (Puccinellio-Sarcocornetum, Puccinellio-Halimionetum fruticosae, and Limonio angustifoliae-Artemisietum caerulescentis).
Sarcocornia fruticosa is one of the most common species in the examined habitat. S. fruticosa (L.) A. J. Scott belongs to the Family Amaranthaceae. To date, the genus Sarcocornia comprises 28 succulent perennial species that extend in saline environments, such as salt marshes, tidal mud flats, coastal cliffs, inland salt pans, edges of saline lakes, and saline deserts (Davy et al. 2006; Kadereit et al. 2006; Alonso and Crespo 2008; Steffen et al. 2010, 2015; Ventura and Sagi 2013; Custódio et al. 2021). These plants form small bushes (subshrubs and shrubs) that are erected or prostrate, highly branched, and up to 150 cm tall. The genus Sarcocornia is characterized by a simplified morphology with strongly reduced leaves and flowers (Steffen et al. 2015). In the basal part, they have woody stems, with the upper parts being fleshy and joined with opposite leaves (Costa et al. 2014). Individual Sarcocornia flowers are arranged in a horizontal row and are equal in size (Kadereit et al. 2007). Because of their high salinity tolerance and perennial life cycle, they can grow under saline conditions and can be harvested throughout the year under a wide range of salinities (Custódio et al. 2021). The phytoremediation potential of S. fruticosa has been demonstrated in several previous studies (Moreira da Silva et al. 2015; Ben Said et al. 2019).
Sarcocornia species are increasingly used in gourmet cuisine (Barriera et al. 2017), are edible, and, considering their nutritional properties and nutraceuticals, can contribute to sustainable agriculture by producing raw or minimally processed (or ready-to-eat) products (Custódio et al. 2021; Lombardi et al. 2022). They are also important as medicinal plants and as source material for obtaining medicines (Custódio et al. 2021; Al-Azzavi and Flowers 2022). For this reason, it is important to define and highlight the geochemical properties of the major elements that are of great importance for nutrition and medicine (Selinus 2005; Selinus et al. 2015; Barriera et al. 2017; Custódio et al. 2021; Lombardi et al. 2022).
Therefore, the objectives of this study were to (1) determine the distribution of the major elements in the below- and above-ground tissues of S. fruticosa, (2) evaluate the bioaccumulation of the major elements in plants, and (3) identify and evaluate their mobility patterns in these systems.
2 Materials and methods
2.1 Sampling and analyses
Sampling took place in April and June 2020, when samples of the S. fruticosa plant were collected from a wider area of Sečovlje Salina (Figs. 1 and 2) at sampling site EA, located in the evaporation area (Piccia), and at sampling site CA, located in the crystallization area. To obtain a sufficient number of samples, three individuals of S. fruticosa were collected at each sampling point and mixed to form a homogeneous mixed plant sample containing roots, stems, and leaves. All the samples were packed in pre-cleaned plastic bags and immediately transported to the laboratory for subsequent elemental analysis. In the laboratory, rhizo-sediment was carefully and thoroughly removed from the roots using distilled water, Milli-Q water, and an ultrasonic bath. Individual plant samples, such as roots, stems, and leaves, were freeze-dried with liquid nitrogen and ground into a fine powder. The major element contents were measured at Bureau Veritas Mineral Laboratories (Canada) using a 5 g split digested in HNO3 and aqua regia and analyzed by ICP-MS for ultra-low detection limits. Laboratory test quality and objectivity were ensured by using neutral laboratory tests. The accuracy and precision of the analysis were verified using the reference materials STD CDV-1 and STD V16. Precision was greater than 5%.
The preparation of rhizo-sediment samples for elemental analysis was previously reported by Rogan Šmuc et al. (2021). In addition, rhizo-sediment samples from sampling sites EA and CA were also prepared by sequential extraction analysis (Bureau Veritas Commodities Canada Ltd) to determine the precise fractionation of Al, Ca, Fe, K, Mg, Mn, and Na in the rhizo-sediment material. Samples with a particle size less than 0.125 mm and weighing 0.75 g were placed in screw-capped test tubes. All chemicals used were of analytical grade and the leaching procedure (1 → 5 step) began with the weakest to strongest leach:
-
1.
Demineralized water to determine soluble constituents (pH 7.47),
-
2.
1 M ammonium acetate to determine exchangeable cations adsorbed by clay and elements precipitated with carbonates (pH 5.39),
-
3.
0.1 M sodium pyrophosphate to determine the elements adsorbed by organic matter (humic and fulvic compounds) (pH 9.5),
-
4.
Cold 0.1 M hydroxylamine to determine the elements adsorbed by amorphous Mn hydroxide and amorphous Fe hydroxide (pH 2.57), and
-
5.
Hot 0.25 M hydroxylamine to determine the elements in residual, incorporated within Fe and Mn oxide crystal lattices (pH 0.01).
Subsequently, the contents of the analyzed elements in the solutions were measured using a Perkin Elmer 6000 ICP-MS instrument. The QA/QC protocol included a duplicate sample, an aliquot of in-house reference material (STD DS12) to monitor analytical precision and accuracy (within ± 10%), and a reagent blank to fix the background.
Different plant species have different potentials for element accumulation, and the element content is usually higher in the roots than in the aboveground parts (Alloway 2010). Therefore, to identify the significant translocation patterns of the major elements in S. fruticosa, two indices were used: bioconcentration and translocation factors. The capacity of plants to accumulate elements from sediments is estimated using the bioconcentration factor (BCF), which is defined as the ratio of the elemental content in the roots to that in the sediment (Maiti and Jaiswal 2008; Alloway 2010). The capacity of plants to translocate elements from roots to leaves or stems is estimated using the translocation factor (TF), which is defined as the ratio of the element content in leaves or stems to that in roots (Maiti and Jaiswal 2008; Alloway 2010).
To mark the differences between samples and disclose detailed elemental distributions, rotary diagrams were created using Statistica VII and Grapher 8 statistical software. Similarity and dissimilarity between objects were determined by calculating the Euclidean distance, and objects were clustered using the average linkage and Ward’s method (cluster analysis (CA)).
3 Results and discussion
3.1 Basic research area and sediment characteristics
The research area is characterized by a complex hydrological regime with salinity conditions highly impacted by the dynamic natural process of tidal inundation and sea level rise (Ivajnšič et al. 2017) and the salt production processes ongoing throughout the year. This is most evident during the salt production season, when seawater and brine salinity change owing to weather patterns, water movement, operational requirements, and maintenance needs (Sovinc 2005; Sau 2007; Kovač et al. 2013). Due to the resulting water regime, there are large differences in salinity, ranging from seawater concentration (3.5 Bé, i.e., 3.4% S) to hypersaline values (brine can be concentrated up to 20 Bé (20.1% S) (Glavaš 2013; Glavaš et al. 2018). Therefore, monitoring the properties of brine and its impact on the surrounding system (sediment) is difficult because of its very variable salinity.
The grain size distribution, pH, total organic carbon (TOC), total nitrogen (TN), and total sulfur (TS) measured in the Sečovlje sediments were presented in detail in previous studies (Ogorelec et al. 1981; Glavaš 2013; Glavaš et al. 2017; Kovač et al. 2018; Rogan Šmuc et al. 2021).
3.2 Sequential extraction analysis results (rhizo-sediments)
The total content of major elements in the sediments and rhizo-sediments was already interpreted in Rogan Šmuc et al. (2021), when it was pointed out that the content of major elements is influenced by the mineralogical composition of the sediments and geological background of the area. Here, we disclosed the fractionation of major elements in two representative rhizo-sediment samples (from EA and CA sites) (Fig. 3) to evaluate their bioavailability and mobility potential in the studied environment. We did not observe any significant differences between the rhizo-sediments from the two locations or their elemental fractionation.
The major elements extracted in the water-soluble leachate (fraction 1) are relatively labile bound components and are thus potentially bioavailable to the surrounding ecosystems (Dean 2007; Rao et al. 2008). Exchangeable and carbonate fractions (2) included exchangeable cations adsorbed by clay and elements co-precipitated with carbonates. The oxidized fraction (3) consists of elements bound to organic matter and sulfides. The metals associated with the oxidizable fractions generally remain in the sediments for an extended period but can be mobilized by various decomposition processes (Dean 2007; Rao et al. 2008). Reducible fraction (4) is generally associated with amorphous Fe and Mn hydroxides, which are thermodynamically unstable under anoxic conditions. The elements in the residual fraction (5) are incorporated into naturally occurring crystalline Fe and Mn oxide minerals and are therefore stable and highly resistant to various remobilization processes under normal environmental conditions (Dean 2007; Rao et al. 2008).
Na and P were found only in water-soluble fraction 1 (Fig. 3), confirming that Na and P are readily translocated to plant tissues under normal environmental conditions, which is valid because these two macronutrients are essential for all organisms (Reimann and de Caritat 1998). In the rhizo-sediments of Sečovlje, Na originates from salt, and P is loosely adsorbed to organic material, Fe oxides, and/or carbonate minerals (Kovač et al. 2018; Rogan Šmuc et al. 2021).
Extremely high Ca values were found in the exchangeable fraction (2) (Fig. 3), and Ca was dominant among all the elements studied in this fraction. The presence of Ca in the exchangeable phase is due to the mineral composition of the rhizo-sediment, where calcite is the second most abundant mineral (Glavaš et al. 2017; Kovač et al. 2018; Rogan Šmuc et al. 2021). The highest proportion of Mg was also found in the exchangeable fraction (2) (Fig. 3), mainly due the fact that Mg is inorganically incorporated into calcite crystal lattices, e.g., as a substitution ion for Ca2+ (Morse and Bender 1990; Lea et al. 1999, 2000; Bohaty et al. 2012). In addition, similarly high Mg concentrations were obtained in the reducible fraction (4) (Fig. 3), suggesting Mg association at the surfaces of Fe oxyhydroxides, such as the mineral goethite (α-FeOOH) (Rakovan et al. 1999), which is also present in the Sečovlje rhizo-sediment (Rogan Šmuc et al. 2021). Similarly, Mg can specifically adsorb to the surfaces of Fe (hydr)oxides (e.g., ferrihydrite), which have a high ion adsorption capacity and high affinity for binding inorganic ions (Mendez and Hiemstra 2020). Overall, ferrihydrite occurs in almost all natural systems and can be present in the rhizo-sediments of Sečovlje. As ferrihydrite is a nanoparticulate Fe-(hydr)oxide, we could not detect it in previous XRD and SEM–EDS analyses (Rogan Šmuc et al. 2021), mainly/probably because of its size.
Al was found in the oxidizable fraction (3) with the highest values (Fig. 3). Al complexation with organic compounds plays a fundamental role in the dynamics of organic matter, with Al preferentially bound to polysaccharides (Masion et al. 2000; Hernandez-Soriano 2012). The stabilization of organic matter occurs through the formation of insoluble Al-OM complexes, which has already been described as an important pathway for the formation of stable OM soils (Scheel et al. 2007). Glavaš (2013), Glavaš et al. (2017), Kovač et al. (2018), and Rogan Šmuc et al. (2021) reported higher TOC and TN values in sediments from Sečovlje, which can be attributed to the association of organic material with Al and also K (see below). In addition, elevated Al concentrations have been associated with the reducible fraction, where we can associate the presence of Al on the surface areas of Fe oxyhydroxides (Rakovan et al. 1999), indicating goethite (α-FeOOH) from the samples of the Sečovlje rhizo-sediment (Rogan Šmuc et al. 2021).
K is closely related to the oxidizable fraction (3) (Fig. 3) because organic materials significantly increase the initial rapid K adsorption rate and have more accessible adsorption sites for K than the mineral components of sediments/soils (Wang and Huang 2001). A significant amount of K was also found in the exchangeable fraction (2) (Fig. 3), implying that exchangeable K is weakly bound to the outer surfaces and interlayer sites of clay minerals (e.g., smectite, vermiculite, and related mixed-layer minerals) and can be rapidly exchanged by other cations present in the pore solution (Sparks 1987; Binner et al. 2017). K present in the interlayer space of smectite, vermiculite, and related mixed-layer minerals may be slowly available, depending strongly on the layer charge density and the chemistry of the pore solution (Sparks 1987; Binner et al. 2017). The amount of K released from clays depends strongly on the mineralogical composition, in the following order: smectitic > illitic, kaolinitic clay (Binner et al. 2017; Gurav et al. 2019). The sediments of Sečovlje are a mixture of sandy mud, and as a result, we have a relatively high proportion of fine fraction in the sediment, in which clay minerals are naturally present (Rogan Šmuc et al. 2021). Therefore, exchangeable K was most likely derived from smectite minerals detected in the rhizo-sediment samples from Sečovlje (Kovač et al. 2018; Rogan Šmuc et al. 2021).
Fe and S were highest in the reducible (4) and residual (5) fractions (Fig. 3), confirming their definite presence in Sečovlje rhizo-sediments with amorphous Fe oxides, naturally occurring crystalline Fe and Mn oxide minerals (clay and chlorite minerals for Fe), and diagenetic pyrite for Fe and S (Kovač et al. 2018; Rogan Šmuc et al. 2021). A lower proportion of S was also found in fractions 1 and 2 (Fig. 3).
According to the results of the elemental fractionation, the mobility and bioavailability potential of the major elements can be estimated as follows:
-
Na, P, Ca, Mg, and K were the most mobile elements (defined as elements found in fractions 1 and 2) and, therefore, may be the most bioavailable.
-
The dominant association of Al (with organic matter and Fe hydroxides), Fe (with Fe hydroxides, secondary pyrite and clay minerals), and S (with secondary pyrite) under constant, slightly oxygenated conditions demonstrates their low mobility potential.
-
Some Fe is bound to aluminosilicate crystal lattices and is unlikely to be released into surrounding ecosystems under normal environmental conditions.
3.3 Major elements distribution in S. fruticosa
The major elements are present in plant tissues in much higher contents than the trace elements and are therefore referred as “macronutrients”.
The Al, Ca, Fe, K, Mg, Na, P, and S contents in the above- and below-ground tissues of S. fruticosa are summarized in Table 1. There were no obvious or large differences in the content and distribution of the major elements depending on the location and sampling time. The contents of major elements in the aboveground tissues were generally up to two orders of magnitude higher than those in the belowground tissues of the studied plants. However, it should be emphasized that the Na content in the leaves is almost 7.5 times higher than that in the roots. Na, Ca, Mg, P, and S had the highest nutrient contents in plant tissues (especially in the leaves, Table 1). Ahmadi et al. (2022) similarly reported increased levels of Na, Ca, and Mg in the aboveground tissues of S. fruticosa. Conversely, Fe, Al, and K contents were lower in leaves than in roots, which is consistent with Fe and Al data from the studies of Caetano et al. (2008) and Petranich et al. (2017) and with (Selinus 2005; Selinus et al. 2015), who reported that K is less mobile in oxidation zones (surficial environments) and often deficient in plant crops.
These results confirmed the sequential extraction results and the potential mobility and bioavailability of the major elements, as well as the fact that halophyte plants take up large amounts of the essential micronutrient Na due to high salinity (Chaudhary 2019; Ahmadi et al. 2022), and that macronutrients (Ca, Mg, P, and S) are intensively translocated from the roots to the upper parts of the plant (Selinus 2005; Selinus et al. 2015; Ahmadi et al. 2022).
3.4 Evaluation of translocation signature of Al, Ca, Fe, K, Mg, Na, P, and S in plant
Figure 4 shows the BCF values of the major elements studied. K, Na, P, and S had the highest BCF values at both sites studied, followed by the BCF values of Mg and Ca, and Fe and Al. However, minor differences were observed for individual elements within the sampling sites and sampling times. BCF values for K and Na were significantly higher in April at the EA sampling site; conversely, BCF values for S were higher in April and June at the CA sampling site. BCF values for P were significantly higher in April at the EA and at the CA sampling sites, while BCF values for Mg, Fe, and Al were slightly higher in April at the EA sampling site, and the BCF values for Ca were constant. In the Sečovlje area, there was much more rain in April (which also affected the river water regime and produced more dissolved ions and effective uptake) than in summer, which could explain the higher BCF factors for K, Na, P, Mg, Fe, and Al in April. These differences are also highlighted in the BCF rotary diagram (Fig. 5). Multivariate analysis revealed that two major groups clustered according to the time of sampling: April and June (Fig. 6). There was a strong association between all BCF values (EA and CA sampling sites) in April and a slightly outlined association between all BCF values (EA and CA sampling sites) in June. This suggests that there are no significant differences (linkage distance on the tree diagram is only seven (Fig. 6)) between the sampling locations and the calculations of the factor BCF.
The effectiveness of elemental uptake/absorption depends on many factors, such as particle size, sediment pH and redox potential (Eh), salinity, organic matter and clay content, cation exchange capacity, the presence of bacteria-mediated acidification processes, plant species, and developmental stage (Otte et al. 1993; Williams et al. 1994; Sundby et al. 2003; Selinus 2005; Selinus et al. 2015; Caetano et al. 2008; Martins et al. 2008; Caçador et al. 2009). Absorption can be selective for a particular ion and usually occurs at very low concentrations (Selinus 2005; Selinus et al. 2015). Therefore, it is unlikely that the elements absorbed by the roots reflect the trend of element content in the rhizo-sediment. In our case, K, Na, P, and S reflected this trend (BCF values > 1), as indicated by the sequential extraction results. It is very difficult to find exact parallels to the results of sequential extraction analysis for other major elements because the sediment-halophyte translocation system is very complex, and there are many interdependent factors that affect the mobility and bioavailability of elements from the sediment in a very short period (Otte et al. 1993; Caetano et al. 2008; Caçador et al. 2009; Petranich et al. 2017).
In general, the amount of moisture (which also affects the sediment pore waters) and the roots can alter the chemical properties of the sediment by altering redox conditions and consequently increasing or decreasing the availability of elements (such as K, Na, P, and S) (Selinus 2005; Selinus et al. 2015; Caetano et al. 2008). We must also emphasize that Ca, Mg, Al, and Fe could not be translocated from the sediment because they form stable complexes with carbonates (Ca and Mg), organic matter (Al), and oxyhydroxides (Fe) at neutral and alkaline pH values (Almeida et al. 2004; Petranich et al. 2017).
Figure 7 shows TF values with Ca, Mg, Na, P, and S above 1, indicating their intensive positive translocation from the roots to the leaves. Na had the highest TF values, followed by TF values for Ca, Mg, and S, while K, Al, and Fe had TF values < 1 (Fig. 7). This implies that K, A, and Fe accumulated from the rhizo-sediment mostly remained in the roots, as previously reported by (Selinus 2005; Selinus et al. 2015) and Caçador et al. (2009), Petranich et al. (2017), and Ahmadi et al. (2022).
We used a rotary diagram (Fig. 8) to expose the increased values of the TF factor for P (EA), and K (CA) in the April samples and increased values of the TF factor for Ca, Na, Al, Fe (EA), and Mg (CA) in the June samples. Multivariate analysis also revealed two main groups clustered according to differences in the calculated TF values (Fig. 9). The tree diagram clearly shows the TF values for the samples from EA (April) and CA (April and June) as a very similar group (with a linkage distance of only 5 (Fig. 9)), while the TF values for the samples from EA in June are highlighted as an outline. This group consists of extremely high TF values for Na and Ca (Fig. 7), indicating accelerated translocation of Na and Ca from roots to leaves: (1) salinity is higher in June, and thus Na content in plants is higher, and (2) plants require Ca as an essential element for the growth, maintenance, and integrity of membranes (Selinus 2005; Selinus et al. 2015).
The general trend in the translocation signature for major elements (as macronutrients), distinguished by BCF and TF factor calculations, emphasizes that root tissues significantly accumulate a large amount of major elements, whereas accumulation depends on individual major elements, and that major elements represent high translocation within plants for Ca, Mg, Na, P, and S and limited mobility for Al, Fe, and K. This is consistent with Selinus (2005) and Ahmadi et al. (2022): Ca is an essential element for plant growth, maintenance, and membrane integrity; Mg is a specific component of chlorophyll; Na is present due to the hypersaline environment; P is a key component in plant metabolic processes; S is very important as it is a component of enzymes and other key proteins; Al and Fe are preferentially accumulated in roots (Petranich et al. 2017); and plant crops/tops/leaves are generally undersupplied with K.
4 Conclusions
This study was conducted in one of the most important ecological areas on the Adriatic, the Sečovlje Salina Nature Park. It is the first study on the content and extraction of major elements from rhizo-sediments and the distribution of major elements by plants. Our results suggest that the major elements are vital macronutrients for halophytes, but their accumulation in the roots and further translocation within the plant depend on the individual elements and their dynamics, which are closely related to various environmental factors such as sediment pH, sediment moisture, grain size, mineralogical composition of the sediment, and salinity.
Thus, the sampling area in the season without salt production (mid-September to mid-June) is mainly influenced by climatic factors, while in the season with salt production (mid-June to mid-September), it is characterized by unique and complex hydrological, climatic, and anthropogenic features (salt production). Despite the higher salinity in the crystallization (CA) due to the effects of salt production, there are no major differences between the results of the two sampling stations (EA and CA). This is probably due to the complex hydrological system of the study area and the very dynamic, variable and individual element chemistry in the sediments.
The content of Al, Ca, Fe, K, Mg, Na, P, and S in the above- and below-ground tissues of S. fruticosa confirmed the results of sequential extraction for the potential mobility and bioavailability of the major elements, as well as the fact that halophyte plants take up high amounts of the essential micronutrient Na due to high salinity. Macronutrients (Ca, Mg, P, and S) were intensively translocated from the roots to the upper parts of the plants.
The trend in the translocation signature for major elements (macronutrients) defined by BCF and TF factor calculations revealed that root tissues significantly accumulate high levels of major elements, whereas accumulation depends on individual major elements. The major elements Ca, Mg, Na, P, and S show high translocation within plants, whereas the mobility of Al, Fe, and K is limited. The pattern presented can be justified as follows: Ca is an essential element for plant growth, maintenance, and membrane integrity; Mg is a specific component of chlorophyll; Na is present due to the hypersaline environment; P is a key component of plant metabolic processes; S is very important as it is a component of enzymes and other key proteins; Al and Fe are preferentially accumulated in roots; and plant leaves are generally undersupplied with K.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmadi F, Mohammadkhani N, Servati M (2022) Halophytes play important role in phytoremediation of salt-affected soils in the bed of Urmia Lake. Iran Sci Rep 12:1–13. https://doi.org/10.1038/s41598-022-16266-4
Al-Azzawi M, Flowers T (2022) Distribution and potential uses of halophytes within the Gulf Cooperation Council States. Agronomy 12(5):1030. https://doi.org/10.3390/agronomy12051030
Alloway BJ (2010) Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer, New York, pp 11–50
Almeida CMR, Mucha AP, Vasconcelos MTSD (2004) Influence of the sea rush Juncus maritimus on metal concentration and speciation in estuarine sediment colonized by the plant. Environ Sci Technol 38:3112–3118. https://doi.org/10.1021/es049932j
Alonso MÁ, Crespo MB (2008) Taxonomic and nomenclatural notes on South American taxa of Sarcocornia (Chenopodiaceae). Ann Bot Fenn 45:241–254. https://doi.org/10.5735/085.045.0401
Barreira L, Resek E, Rodrigues MJ et al (2017) Halophytes: gourmet food with nutritional health benefits? J Food Compost Anal 59:35–42. https://doi.org/10.1016/j.jfca.2017.02.003
Ben Said O, Moreira da Silva M, Hannier F et al (2019) Using Sarcocornia fruticosa and Saccharomyces cerevisiae to remediate metal contaminated sediments of the Ria Formosa lagoon (SE Portugal). Ecohydrol Hydrobiol 19:588–597. https://doi.org/10.1016/j.ecohyd.2018.10.002
Binner I, Dultz S, Schellhorn M, Schenk MK (2017) Potassium adsorption and release properties of clays in peat-based horticultural substrates for increasing the cultivation safety of plants. Appl Clay Sci 145:28–36. https://doi.org/10.1016/j.clay.2017.05.013
Bohaty SM, Zachos JC, Delaney ML (2012) Foraminiferal Mg/Ca and Mn/Ca ratios across the Eocene-Oligocene transition. Earth Planet Sci Lett 317–318:251–261. https://doi.org/10.1016/j.epsl.2011.11.037
Caçador I, Caetano M, Duarte B, Vale C (2009) Stock and losses of trace metals from salt marsh plants. Mar Environ Res 67:75–82. https://doi.org/10.1016/j.marenvres.2008.11.004
Caetano M, Vale C, Cesário R, Fonseca N (2008) Evidence for preferential depths of metal retention in roots of salt marsh plants. Sci Total Environ 390:466–474. https://doi.org/10.1016/j.scitotenv.2007.10.015
Chaudhary V, Kumar V, Singh K, Kumar R, Kumar V (2019) Pineapple (Ananas cosmosus) product processing: a review. J Pharmacogn Phytochem 8(3):4642–4652
Costa CSB, Vicenti JRM, Morón-Villarreyes JA et al (2014) Extraction and characterization of lipids from Sarcocornia ambigua meal: a halophyte biomass produced with shrimp farm effluent irrigation. An Acad Bras Cienc 86:935–943. https://doi.org/10.1590/0001-3765201420130022
Custódio L, Rodrigues MJ, Pereira CG et al (2021) A review on Sarcocornia species: ethnopharmacology, nutritional properties, phytochemistry, biological activities and propagation. Foods 10:2778. https://doi.org/10.3390/foods10112778
Davy AJ, Bishop GF, Mossman H et al (2006) Biological flora of the British Isles: Sarcocornia perennis (Miller) A.J. Scott J Ecol 94:1035–1048. https://doi.org/10.1111/j.1365-2745.2006.01156.x
Dean JR (2007) Bioavailability, bioaccessibility and mobility of environmental contaminants. Analytical Techniques in the Sciences. John Wiley and Sons Ltd., Chichester. https://doi.org/10.1002/9780470319673
Glavaš N (2013) Composition and transformations of petola and saline mud from Sečovlje Salina. Doctoral dissertation, University of Ljubljana, Ljubljana
Glavaš N, Défarge C, Gautret P et al (2018) The structure and role of the “petola” microbial mat in sea salt production of the Sečovlje (Slovenia). Sci Total Environ 644:1254–1267. https://doi.org/10.1016/j.scitotenv.2018.07.009
Glavaš N, Mourelle ML, Gómez CP et al (2017) The mineralogical, geochemical, and thermophysical characterization of healing saline mud for use in pelotherapy. Appl Clay Sci 135:119–128. https://doi.org/10.1016/j.clay.2016.09.013
Glavaš N, Šmuc NR, Dolenec M, Kovač N (2015) The seasonal heavy metal signature and variations in the microbial mat (petola) of the Sečovlje Salina (northern Adriatic). J Soils Sediments 15:2359–2368. https://doi.org/10.1007/s11368-015-1273-5
Gurav PP, Choudhari PL, Srivastava S (2019) Role of clay minerals in potassium availability of black soils in India. Harit Dhara 2(1):20–22
Hernández-Soriano MC (2012) The role of aluminum-organo complexes in soil organic matter dynamics. In: Hernandez-Soriano MC (ed), Soil health and land use management, InTech, London, 17–32. https://doi.org/10.5772/39117
Ivajnšič D, Lipej L, Škornik I, Kaligarič M (2017) The sea level rise impact on four seashore breeding birds: the key study of Sečovlje Salina Nature Park. Clim Change 140:549–562. https://doi.org/10.1007/s10584-016-1854-3
Kadereit G, Ball P, Beer S et al (2007) A taxonomic nightmare comes true: phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 56:1143–1170. https://doi.org/10.2307/25065909
Kadereit G, Mucina L, Freitag H (2006) Phylogeny of Salicornioideae (Chenopodiaceae): diversification, biogeography, and evolutionary trends in leaf and flower morphology. Taxon 55:617–642. https://doi.org/10.2307/25065639
Kovač N, Glavaš N, Dolenec M et al (2013) Chemical composition of natural sea salt from the Sečovlje Salina (gulf of trieste, northern Adriatic). Acta Chim Slov 60:706–714
Kovač N, Glavaš N, Ramšak T et al (2018) Metal(oid) mobility in a hypersaline salt marsh sediment (Sečovlje Salina, northern Adriatic, Slovenia). Sci Total Environ 644:350–359. https://doi.org/10.1016/j.scitotenv.2018.06.252
Lea DW, Mashiotta TA, Spero HJ (1999) Controls on magnesium and strontium uptake in planktonic foraminifera determined by live culturing. Geochim Cosmochim Acta 63:2369–2379. https://doi.org/10.1016/S0016-7037(99)00197-0
Lea DW, Pak DK, Spero HJ (2000) Climate impact of late quaternary equatorial pacific sea surface temperature variations. Science. 289(5485):1719–1724. https://www.science.org/doi/10.1126/science.289.5485.1719
Lombardi T, Bertacchi A, Pistelli L et al (2022) Biological and agronomic traits of the main halophytes widespread in the Mediterranean region as potential new vegetable crops. Horticulturae 8:195. https://doi.org/10.3390/horticulturae8030195
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess 136:355–370. https://doi.org/10.1007/s10661-007-9691-5
Martins M, Ferreira AM, Vale C (2008) The influence of Sarcocornia fruticosa on retention of PAHs in salt marsh sediments (Sado estuary, Portugal). Chemosphere 71:1599–1606. https://doi.org/10.1016/j.chemosphere.2007.10.054
Masion A, Vilgé-Ritter A, Rose J et al (2000) Coagulation-flocculation of natural organic matter with Al salts: speciation and structure of the aggregates. Environ Sci Technol 34:3242–3246. https://doi.org/10.1021/es9911418
Mendez JC, Hiemstra T (2020) Ternary complex formation of phosphate with Ca and Mg ions binding to ferrihydrite: experiments and mechanisms. ACS Earth Space Chem 4:545–557. https://doi.org/10.1021/acsearthspacechem.9b00320
Moreira Da Silva M, Aníbal J, Duarte D, Chícharo L (2015) Sarcocornia fruticosa and Spartina maritima as heavy metals remediators in Southwestern European salt marsh (Ria Formosa, Portugal). J Environ Prot Ecol 16:1468–1477
Morse JW, Bender ML (1990) Partition coefficients in calcite: examination of factors influencing the validity of experimental results and their application to natural systems. Chem Geol 82:265–277. https://doi.org/10.1016/0009-2541(90)90085-L
Ogorelec B, Mišič M, Šercelj A, Cimerman F et al (1981) Sediment Sečoveljske Soline Geologija 24:179–216
Otte ML, Haarsma MS, Broekman RA, Rozema J (1993) Relation between heavy metal concentrations in salt marsh plants and soil. Environ Pollut 82:13–22. https://doi.org/10.1016/0269-7491(93)90157-J
Petranich E, Acquavita A, Covelli S, Emili A (2017) Potential bioaccumulation of trace metals in halophytes from salt marshes of a northern Adriatic coastal lagoon. J Soils Sediments 17:1986–1998. https://doi.org/10.1007/s11368-016-1545-8
Rakovan J, Becker U, Hochella MF (1999) Aspects of goethite surface microtopography, structure, chemistry, and reactivity. Am Mineral 84:884–894. https://doi.org/10.2138/am-1999-5-623
Rao CRM, Sahuquillo A, Lopez Sanchez JF (2008) A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut 189:291–333. https://doi.org/10.1007/s11270-007-9564-0
Reimann C, de Caritat P (1998) Chemical elements in the environment. Factsheets for the geochemist and environmental scientist. Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-72016-1
Rogan Šmuc N, Kovač N, Hauptman Ž et al (2021) A detailed insight into the detrital and diagenetic mineralogy of metal(oid)s: their origin, distribution and associations within hypersaline sediments. Minerals 11(11):1168. https://doi.org/10.3390/min11111168
Salgueiro N, Caçador I (2007) Short-term sedimentation in Tagus estuary, Portugal: the influence of salt marsh plants. Hydrobiologia 587:185–193. https://doi.org/10.1007/s10750-007-0678-6
Sau D (2007) Pridelava soli v Krajinskem parku Sečoveljske soline. In: Šolar Sv (ed) Mineralne surovine v letu 2006. Geološki zavod Slovenije, Ljubljana, 196−198
Scheel T, Dörfler C, Kalbitz K (2007) Precipitation of dissolved organic matter by aluminum stabilizes carbon in acidic forest soils. Soil Sci Soc Am J 71:64–74. https://doi.org/10.2136/sssaj2006.0111
Selinus O (2005) Essential of medical geology, impacts of the natural environment of public health. Elsevier, AmsterdamSelinus O, Fuge R, Alloway B, Lindh U, Centano JA, Smedley P, Finkelman RB (eds) (2015) Essentials of medical geology. Revised Edition. Springer Netherland, Dordrecht. https://doi.org/10.1007/978-94-007-4375-5
Selinus O, Fuge R, Alloway B et al (eds) (2015) Essentials of medical geology. Revised Edition. Springer Netherland, Dordrecht. https://doi.org/10.1007/978-94-007-4375-5
Sovinc A (2005) Ecological characteristics of the Secovlje salinas. In: Neves R, Petanidou T, Pinto S, Rufino R (eds) ALAS (All About Salt)–salt and salinas in the Mediterranean. Municipality of Figueira da Foz–ALAS, Lisbon, 81–85
Sousa AI, Caçador I, Lillebø AI, Pardal MA (2008) Heavy metal accumulation in Halimione portulacoides: intra- and extra-cellular metal binding sites. Chemosphere 70:850–857. https://doi.org/10.1016/j.chemosphere.2007.07.012
Sparks DL (1987) Potassium dynamics in soils. In: Stewart BA (eds) Springer, New York. Adv Soil Sci 6:1–63. https://doi.org/10.1007/978-1-4612-4682-4_1
Steffen S, Ball P, Mucina L, Kadereit G (2015) Phylogeny, biogeography and ecological diversification of Sarcocornia (Salicornioideae, Amaranthaceae). Ann Bot 115:353–368. https://doi.org/10.1093/aob/mcu260
Steffen S, Mucina L, Kadereit G (2010) Revision of Sarcocornia (Chenopodiaceae) in South Africa, Namibia and Mozambique. Syst Bot 35:390–408. https://doi.org/10.1600/036364410791638379
Sundby B, Vale C, Caetano M, Luther GW (2003) Redox chemistry in the root zone of a salt marsh sediment in the Tagus Estuary, Portugal. Aquat Geochem 9:257–271. https://doi.org/10.1023/B:AQUA.0000022957.42522.9a
Ventura Y, Sagi M (2013) Halophyte crop cultivation: the case for Salicornia and Sarcocornia. Environ Exp Bot 92:144–153. https://doi.org/10.1016/j.envexpbot.2012.07.010
Wang FL, Huang PM (2001) Effects of organic matter on the rate of potassium adsorption by soils. Can J Soil Sci 81:325–330. https://doi.org/10.4141/s00-069
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt marsh environments: a review. Mar Pollut Bull 28:277–290. https://doi.org/10.1016/0025-326X(94)90152-X
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Nives Ogrinc
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kovač, N., Hauptman, Ž., Dolenec, M. et al. Translocation signatures of major elements in halophytes from hypersaline environments: the case study from Sečovlje Salina (Republic of Slovenia). J Soils Sediments 23, 4149–4162 (2023). https://doi.org/10.1007/s11368-023-03654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03654-0