Abstract
Purpose
The aim of this work was to determine the characteristics of SOM decomposition under forest vegetation and to investigate the influence of soil mineralogy on SOM turnover.
Methods
Thirteen Hungarian forest topsoil samples amended with maize residues were incubated at 20 °C for 163 days. The CO2 evolved was measured and the fast and slow decomposition rate constants (k1 and k2, respectively) of SOM were quantified using a first-order two pools model. Linear regression analysis was applied between the quantity of total mineralized carbon (TMC), k1 and k2 values and the mineralogical parameters of the soils.
Results
The illite (R2 = 0.797, p < 0.001) and non-swelling clay mineral (R2 = 0.767, p < 0.001) content and the dithionite–citrate–bicarbonate-extractable Al (AlDCB, R2 = 0.708, p < 0.001) and ammonium-oxalate-extractable Al concentration (AlOX, R2 = 0.627, p < 0.01) reduced the TMC to the greatest extent. The AlDCB (R2 = 0.681, p < 0.001), AlOX (R2 = 0.583, p < 0.01) and illite (R2 = 0.545, p < 0.01) contents had strong negative relationship with the k1 value. The k2 value was only affected by the non-swelling clay mineral (R2 = 0.467, p < 0.05) and illite (R2 = 0.574, p < 0.01) contents.
Conclusion
These results confirm that the mineral composition of the soil, including the Al oxide, non-swelling clay mineral and illite contents, may significantly inhibit the decomposition of SOM, showing that illite minerals may provide binding surfaces for SOM over a longer timescale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soil organic matter (SOM) regulates many soil processes, but its significance is not restricted to the soil ecosystem, as it also plays an important role in the global carbon cycle. However, SOM is not a single homogeneous pool; it can be divided into carbon pools with different decomposition kinetics: pools with fast turnover and others with slow turnover. Therefore, the residence time of SOM is very diverse, ranging from a few hours, weeks or months to as much as 10,000 years (Trumbore 2000; Kuzyakov 2006; Schmidt et al. 2011; Fekete et al. 2021) depending on many factors. The main mechanisms, which provides the resistance of organic matter — even over a longer timescale — against microbial decomposition are chemical and physical stabilization processes of SOM (Angst et al. 2021). The chemical protection of SOM adsorption via forming stable complexes of organic molecules on the surface of minerals is one of the most important mechanism responsible for the long-term stabilization of SOM (Mikutta et al. 2006; von Lützow et al. 2008; Kleber et al. 2021). The physical protection of SOM by aggregates is another important SOM stabilization process (Six et al. 2002; Angst et al. 2017; Guidi et al. 2021). Therefore, the silt and clay content of the soil are considered main factors for SOM stabilization (Hassink 1997; von Lützow et al. 2008; Wiesmeier et al. 2019). It is widely accepted that sand-associated SOM has a faster turnover rate, whereas silt and clay-associated SOM has a slower turnover rate, thus representing a more stabilized carbon pool (Trumbore et al. 1996; Saviozzi et al. 2014; Kögel-Knabner and Amelung 2021).
Also, the type of mineral phase plays a crucial role in the stabilization of SOM, often having a more significant effect than the amount of fine particles (Saggar et al. 1996; Bruun et al. 2010; Rasmussen et al. 2018). Clay-sized minerals (< 2 µm), oxides with R2O3 structure (primarily Fe and Al oxides with 5 − 100 nm), short-range order Fe-oxides (3 − 10 nm) and amorphous Al-oxides (< 3 nm) provide the most suitable surfaces for the effective binding of SOM (Kögel-Knabner and Amelung 2014), mainly by ligand exchange and formation of polyvalent cation bridges (von Lützow et al. 2006).
However, our knowledge on the complex SOM stabilization processes of the soil minerals is incomplete (Barré et al. 2014; Wiesmeier et al. 2019) and it is often hard to differentiate the overlapping effects of the different type of minerals (Gartzia-Bengoetxea et al. 2020), oxides, hydroxides, oxy-hydroxides and poorly crystalline phases of Al and Fe are of particular interest because in some cases, the SOM stabilizing effect of these materials was found to be more important than that of other soil minerals (Jones and Edwards 1998; Kaiser et al. 2002; Wiseman and Puttmann 2005; Ringer et al. 2021). Fang et al. (2019) also showed that Al and Fe (hydr) oxides had a greater effect on soil organic carbon stocks than climatic and edaphic parameters.
The 2:1 type clay minerals (e.g. smectites, vermiculites, chlorites and illite) are another type of soil minerals with significant role in SOM stabilization. It is generally believed that the capacity of soil dominated by 2:1 clay minerals to bond SOM is higher than that of soils dominated by 1:1 clay minerals (e.g. kaolinite) (Schulten and Leinweber 2000; Six et al. 2002; Feng et al. 2013). In line with this, Saggar et al. (1996) and Wattel-Koekkoek et al. (2003) found that the mean residence time of soil organic carbon (SOC) was higher in smectitic than in kaolinitic soils. On the contrary, Bruun et al. (2010) reported higher stability of SOC in kaolinitic than in smecitic soil.
Therefore, there is a growing need for not only the determination of the clay content but also the study of the mineralogical characteristics of the soils and to examine the role of the different type of minerals in SOM turnover in order to better understand SOM stabilization processes. In addition, it is especially important to study the stabilization of organic matter in temperate soils, since these soils often contain a mixture of the above-mentioned minerals. Thus, the different SOM stabilization capacities of the various minerals can thus be compared in situ regardless of the different environmental conditions (climate, pH and redox conditions) in temperate soils. Therefore, the aim of this study was to determine the type and quantity of clay-sized minerals in 13 temperate forest soils with different mineral composition to study their role in SOM turnover. Specifically, the study aimed to (i) address the role of soil texture in affecting the decomposition of SOM and to (ii) identify the mineral groups with the greatest effect on SOM turnover in the short and long term in the acid forest soils studied. In addition, the study also aimed to (iii) analyse how the addition of plant material to the soil affected the decomposition rates of SOM.
2 Materials and methods
2.1 Soil samples
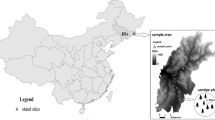
Thirteen topsoils (0‒20 cm with one exception: sample code BAT, 0‒3 cm, where the bedrock was near the surface) were collected as point samples from seven regions in Hungary (Fig. 1). Samples were collected from non-arable forest sites from different regions of the country in order to represent soils with various parent materials (Table 1) and textural and mineralogical characteristics (Table 2). Samples collected from the same region also represent different basic soil properties (Table 3) and mineralogical parameters (Table 2).
Undisturbed soil samples were taken in order to determine the water-holding capacity (WHC). The soils were air-dried, homogenized, passed through a 2-mm sieve and stored at room temperature in the dark for 9 months before the incubation experiment.
2.2 Laboratory analysis of soil properties and soil minerals
The soil texture, the total and dissolved organic carbon and nitrogen contents, the iron and aluminium contents and the cation exchange capacity (CEC) were measured before the 163-day incubation experiment. The soil texture was determined by the pipette method (Gee and Bauder 1986). The total organic carbon (TOC) content was analysed using an NDIR-chemiluminescent analyser (Apollo 9000, Tekmar Dohrmann). The total N content was determined by the Kjeldahl method (Conklin 2014). The CEC was determined according to the method of Gillman (1979). A detailed description of the procedure was reported in a previous study (Zacháry et al. 2018).
The iron and aluminium concentrations were determined in acid ammonium oxalate (Schwertmann 1973) and dithionite–citrate–bicarbonate extracts (Holmgren 1967), and measured using a microwave plasma-atomic emission spectrometer (4200, Agilent Technologies). Ammonium oxalate extraction was used to assess the contents of poorly crystalline Fe- and Al-oxides, whereas the dithionite–citrate–bicarbonate extraction was applied to determine the total “free” Fe- and Al-oxide concentrations. Thus, the difference of FeDCB and FeOX (FeDCB‒FeOX) represents crystalline Fe oxides (Mikutta et al. 2005).
The clay fraction (< 2 µm) of the soil samples was separated by sedimentation. X-ray powder diffraction (XRD) measurements were carried out using a Philips PW 1710 diffractometer with CuKα radiation at 45 kV and 35 mA. Clay minerals were identified by XRD patterns obtained from parallel-oriented specimens sedimented on glass slide. The following diagnostic treatments were carried out for all the samples: ethylene glycol solvation at 60 °C overnight, Mg saturation followed by glycerol solvation at 95 °C overnight, K saturation, and heating at 350 and 550 °C for 2 h.
2.3 Incubation experiment
A detailed description of the incubation experiment was reported in a previous study (Zacháry et al. 2018). Briefly, 200 g of sieved, air-dried soil was weighed into 1 l Duran® glass bottles and pre-incubated at 50% WHC at 20 °C for 2 weeks, because soil disturbances such as rewetting and sieving may cause a flush of C mineralization (Franzluebbers 1999). The pre-incubated soils were thoroughly mixed with 1 g air-dried, shredded and sieved (< 2 mm) maize residues and incubated for 163 days. Maize residues were added to the forest soils in order to study the effect of new organic matter amendment and to get a δ13C isotopic difference between the maize residues and the forest soils. Three replicates of amended soil and one control with no residue addition were used for each soil type. One blank sample without soil or residue was used for the whole incubation experiment. The samples were kept in an incubator (KBW400 E5.1, Binder) at 20 °C for 163 days at 70% WHC. Soil respiration was trapped in plastic tubes containing 15 ml of 2 M NaOH, placed in the air-tight incubation bottles. The NaOH traps were replaced on days 3, 8, 15, 30, 51, 79, 107, 135 and 163. The amount of CO2 evolved was measured by titrating the remaining NaOH with 1 M HCl after adding BaCl2 (Anderson 1982). Soil moisture losses were measured by weighing the mass of the samples on day 0 and the days of the NaOH replacement. The mass differences were calculated and the losses were compensated on the NaOH replacement days by adding distilled water to the samples.
2.4 Kinetic model fitting and calculations
Carbon mineralization kinetics (decomposition rate constants) was modelled by fitting the cumulative CO2 efflux measured during the 163-day incubation period to a first-order two pools model (Molina et al. 1980):
where C is the cumulative carbon mineralized over t time, C1 is the size of the easily mineralizable carbon pool, C2 is the size of the slowly mineralizable carbon pool, k1 is the decomposition rate constant of the easily mineralizable carbon pool, k2 is the decomposition rate constant of the slowly mineralizable carbon pool and t is the time from the start of incubation.
The quantity of organic matter (soil plus added residue in case of the amended samples) that is decomposed under the full length of incubation (TMC, total mineralized carbon) was calculated as the percentage of the quantity of CO2 respired during the incubation relative to the initial TOC content of the samples:
Statistical analysis was conducted using IBM SPSS Statistics 22.0 (Armonk, NY, USA). One-way analysis of variance (ANOVA) with post hoc Tukey’s HSD test was used to evaluate differences between the soils. Linear regression was applied to determine how the mineral properties were related to the quantity of TMC and to the decomposition rate constants of the easily (k1) and slowly (k2) mineralizable carbon pools. Statistical outliers, where standardized values (with zero mean and one standard deviation) were less than − 2 or greater than 2, were excluded from the analysis.
3 Results
3.1 Mineralogical characterization of soils
The three soils developed on red clay sediment in the north-eastern part of Hungary (JOS2, JOS1, JOS3) and one sample from Western Hungary (SOP3) had the highest dithionite-citrate-bicarbonate-extractable Fe concentrations and FeDCB‒FeOX contents (Table 2). The dithionite-citrate-bicarbonate-extractable Al concentrations were also high in these samples. The three coarsest samples (CEG, NYIR1 and NYIR2) had the lowest FeDCB and AlDCB concentrations and FeDCB‒FeOX contents.
Primary minerals (quartz and feldspars) were found in the clay fraction (< 2 µm) of all the samples (Table 2). The three coarsest samples (CEG, NYIR1 and NYIR2) contained only illite and chlorite phases with the dominance of illite. Illite was also the dominant phase in the finer grain-sized samples with one exception (KAR). The three samples from the north-eastern part of Hungary (JOS2, JOS1, JOS3), which is covered with red clay sediments, had significant quantities (approx. 38% of all clay minerals) of kaolinite. Two samples, KIS and KAR, had the highest amount of swelling clay minerals (63% of all clay minerals) as mixed layer species in the KAR sample and as mixed layer phases plus smectite only in the KIS sample. Swelling clay minerals (illite/vermiculite and/or illite/smectite mixed layer species + chlorite/vermiculite and/or chlorite/smectite mixed layer species) were detected in the smallest amount (below 20% of all clay minerals) in samples SOP2, JOS3 and JOS2 and were not detected at all in samples CEG, NYIR1, NYIR2 and JOS1.
3.2 Parameters affecting the quantity of total mineralized carbon (TMC) in soils
In the present study, 1‒6% and 2‒18% of the initial SOC content were mineralized during the 6-month incubation in the control and amended samples, respectively (Fig. 2). In general, the C mineralization of the amended samples was double that of their control pairs. The CEG (fourfold), NYIR1 (threefold), NYIR2 (2.5-fold) and SOP1 (2.5-fold) samples had the highest difference between the C mineralization of the amended and control samples. Residue addition had the least effect on the mineralization of the BAT, JOS2, JOS1 and SOP2 samples, with 1.1, 1.2, 1.4 and 1.5-fold differences between the amended and control samples, respectively.
Soil parameters related to the mineral phases showed significant relationships with the values of TMC of the amended samples (Fig. 3). Neither the FeOX, swelling clay minerals (smectite + illite/vermiculite and/or illite/smectite mixed layer species + chlorite/vermiculite and/or chlorite/smectite mixed layer species), chlorite and kaolinite contents nor CEC exhibited a significant linear relationship with the quantity of TMC, while the sand, clay, non-swelling clay mineral (illite + kaolinite + chlorite), illite, AlDCB, AlOX and FeDCB contents were found to be significant parameters in this respect (Fig. 3). Among these mineral parameters, only the sand content had a positive relationship with the quantity of TMC (Fig. 3a), whereas an inverse relationship was detected for the other parameters (Fig. 3b–g). The illite (Fig. 3c), non-swelling (Fig. 3d), AlDCB (Fig. 3e) and AlOX (Fig. 3f) contents had the strongest negative relationship with the quantity of TMC of the soils.
Significant (p < 0.05) linear relationships between the sand (a), clay (b), illite (c), non-swelling clay mineral (illite + kaolinite + chlorite) (d), AlDCB (e), AlOX (f) and FeDCB (g) contents and the quantity of total mineralized carbon (TMC) in amended samples. Samples designated as ⊗ represent statistical outliers, which were excluded from the analysis
3.3 Parameters affecting the decomposition rates (k1 and k2) of SOM
The addition of maize considerably accelerated the rate of SOM decomposition, which was 3.6 times faster on average than in the control samples for the easily mineralizable carbon pool and 1.9 times faster on average for the slowly mineralizable carbon pool. The addition of maize accelerated the decomposition of SOM to the greatest extent in the amended samples CEG, NYIR2, SOP1 and KIS, where the k1 values were 8.2-fold, 5.5-fold, 5.5-fold and 4.5-fold higher, respectively, than in the control samples (Table 4). The SOM decomposition of the BAT, JOS2, KAR and SOP2 samples gave the poorest response to the addition of new organic matter, the difference between the k1 values of amended and control samples being 1.4-fold, 1.9-fold, 2.1-fold and 2.1-fold, respectively.
Only the NYIR1 (5.2-fold), CEG (2.3-fold), NYIR2 (2.2-fold) and KAR (2.1-fold) samples showed medium to large difference between the k2 values of the amended and control samples, indicating that the SOM decomposition of the slowly mineralizable carbon pool was less affected by the addition of maize residues than that of the easily mineralizable carbon pool.
Among the mineral parameters studied, the FeOX, FeDCB, swelling clay mineral (smectite + illite/vermiculite and/or illite/smectite mixed layer species + chlorite/vermiculite and/or chlorite/smectite mixed layer species), chlorite and kaolinite contents and CEC showed no significant linear relationship with the decomposition rate constant of the amended samples in either carbon pool, whereas the sand, clay, non-swelling clay mineral (illite + kaolinite + chlorite), illite, AlDCB and AlOX contents had a significant influence on the SOM decomposition rate of the easily mineralizable carbon pool (Fig. 4). Of these parameters, only the sand content exhibited a positive relationship with the decomposition rate constant of the easily mineralizable carbon pool (Fig. 4a), whereas an inverse relationship was detected for the other parameters (Fig. 4b–f). The AlDCB (Fig. 4c), AlOX (Fig. 4d) and illite (Fig. 4c) contents had the strongest negative relationship with the decomposition rate constant of the easily mineralizable carbon pool.
Significant (p < 0.05) linear relationships between the sand (a), clay (b), AlDCB (c), AlOX (d), illite (e) and non-swelling clay mineral (illite + kaolinite + chlorite) (f) contents and the decomposition rate constant of the easily mineralizable carbon pool (k1) of amended samples. Samples designated as ⊗ represent statistical outliers, which were excluded from the analysis
The SOM decomposition rate of the slowly mineralizable carbon pool in the samples was only affected by the non-swelling clay mineral (Fig. 5a) and illite (Fig. 5b) contents of the samples in the present study. The results showed that the decomposition rate constant of the slowly mineralizable carbon pool decreased as the non-swelling clay mineral and illite content increased.
Significant (p < 0.05) linear relationships between the non-swelling clay mineral (illite + kaolinite + chlorite) (a) and illite (b) contents and the decomposition rate constant of the slowly mineralizable carbon pool (k2) of amended samples. Samples designated as ⊗ represent statistical outliers, which were excluded from the analysis
4 Discussion
4.1 Organic matter addition caused enhanced SOM mineralization
Maize addition was found to increase the rate of SOM mineralization, resulting in a higher quantity of TMC (Fig. 2) and higher decomposition rate constants (Table 4) for amended soil samples than for their control pairs. This is in agreement with other studies that demonstrated accelerated SOM mineralization due to fresh plant residue addition (Helfrich et al. 2008; Stewart et al. 2009; Shahbaz et al. 2017). This could be attributed to the fact that the addition of plant residues and fertilizers stimulates the microbiological activity of the soil (Wutzler and Reichstein 2008; Blagodatsky et al. 2010), resulting in increased CO2 emissions (Thiessen et al. 2013; Kotroczó et al. 2020). It was also found that the turnover rate of the easily mineralizable carbon pool accelerated to a greater extent after plant residue addition than that of the slowly mineralizable carbon pool, suggesting that the carbon in the slowly mineralizable carbon pool is more resistant to decomposition. Although, as expected, different soils reacted differently to the addition of maize residues, the carbon decomposition of the soil samples with the coarsest particle size (NYIR1, NYIR2 and CEG) exhibited the best response to maize addition, resulting in the greatest differences between the mineralization of the control and amended samples and the highest SOM mineralization and k1 values in the amended samples.
4.2 Mineralogical parameters that control SOM turnover
Although texture was found to be an important controlling factor of carbon decomposition, more accurate and robust predictions can be achieved taking account the mineral composition of the soils, as indicated by the strength of the regression relationships (Figs. 3, 4 and 5). Vogel et al. (2014) showed that less than 19% of the clay-sized mineral surfaces had organic matter coverage which could explain why it may be misleading to consider only the clay or sand content when investigating the stabilization of organic matter. Confirming this, linear regression indicated that SOM stabilization was more closely related to the quality of the mineral phases than to the content of fine grain-sized particles, as the non-swelling clay mineral, illite and Al contents proved to be the most important mineral-related parameters inhibiting the decomposition of SOM in the present study.
4.2.1 Al content found better indicator for SOM stabilization than Fe
It was reported by Wiseman and Puttmann (2005) that no significant correlation was detected between soil clay mineral composition (smectite versus non-smectite domination) and organic matter content, whereas the FeOX and AlOX concentrations tended to affect the amount of organic matter in the soils investigated. Many studies (Eusterhues et al. 2005; Kleber et al. 2015) concluded that metallic oxides play a major role in the binding of organic matter in acidic soils due to their large specific area and large number of reactive surface sites. According to Gu et al. (1994), the maximum sorption capacity is observed between pH 4.3 and 4.7, where ligand exchange between the OH functional groups of Fe and Al oxides and the carboxyl and phenolic OH functional groups of the organic matter is the main process responsible for organic matter adsorption. Therefore, this binding mechanism is a typical organo-mineral interaction in soils rich in acidic protonated hydroxyl groups (Shen 1999; Kögel-Knabner and Amelung 2014). Furthermore, in addition to ligand exchange, Fe and Al ions are able to form cation bridges with organic ligands (mainly carboxyl groups) in acidic soils, thereby building organo-mineral complexes (Oades 1988; von Lützow et al. 2006). As the 13 soils investigated in the present study were slightly (pH 6.2) to extremely acidic (pH 3.7), it is likely that the Al content of the soils was one of the main parameters controlling the decomposition of organic matter, because Al-containing minerals adsorb organic matter via ligand exchange and the formation of cation bridges. These mechanisms could be particularly important in highly weathered soil containing high amounts of crystalline Al and Fe oxides. From this point of view, samples JOS1, JOS2 and JOS3, derived from the north-eastern part of Hungary, where the soil is covered with red clay sediments (Kiss 2012), and therefore having the highest Al and Fe oxide content (Table 2) were of special interest in the present study. In these samples, the degree of mineral weathering was high, as indicated by the significant amount of kaolinite (approx. 38% of all clay minerals) accompanied by largest quantities of crystalline Fe oxide content (FeDCB‒FeOX) (Table 2). In contrast, the coarsest textured soils (CEG, NYIR1 and NYIR2) had the smallest FeDCB‒FeOX concentrations and the simplest bimodal (illite plus chlorite) mineral composition as a consequence of the small degree of weathering in these samples.
Although the Fe content can be considered a strong controlling factor in SOM turnover (Kaiser et al. 2002; Kiem and Kögel-Knabner 2002; Mikutta et al. 2006; Bruun et al. 2010), in the present study, it was found that the Al content of the soils is better proxy for the SOM stabilization than the Fe content: the FeDCB content only affected the quantity of the total mineralized carbon (TMC), whereas the TMC values of soils and the decomposition rate of SOM in the easily mineralized carbon pool decreased with increasing Al content (with both the AlDCB and AlOX content). Lawrence et al. (2015) and Fang et al. (2019) also found more significant relationships between the AlDCB content and the SOC variables, whereas they reported no or less significant relationship between the FeDCB content and the SOC variables of the samples studied. This is in agreement with the findings of Kaiser and Zech (1998), who reported the highest adsorption of dissolved organic matter on amorphous Al(OH)3 rather than on ferrihydrite or goethite.
4.2.2 Non-swelling clay minerals can affect the decomposition rate of SOM of forest soils
While the content of clay-sized particles showed only a weak negative linear relationship with the quantity of TMC of soils (Fig. 3b) and with k1 values (Fig. 4b), the quantity of illite and non-swelling clay minerals had a strong negative relationship with these parameters (Figs. 3 and 4). Moreover, the illite and non-swelling clay mineral contents were the two parameters that affected the turnover and the stabilization of SOM in the slowly mineralized carbon pool (Fig. 5). Although the specific surface area of illite is generally smaller than that of smectites and its negative charge excess is mainly compensated by K+, the sorption capacity of illite may also be significant due to the formation of amphoteric surfaces (silanol and aluminol) at the edges of minerals and to ion exchange on the basal flat surfaces of siloxane and on “frayed edges” (Kulik et al. 2000; Sinitsyn et al. 2000). In addition, Kubicki et al. (1999) found that illite is more likely to form strong surface complexes with organic acids (oxalic acid, benzoic acid, salicylic acid and phthalic acid) than kaolinite and montmorillonite, which they explained by the fact that illite may have more reactive areas due to the presence of Fe hydroxides. Kögel-Knabner and Amelung (2014) also mentioned the amphoteric AlOH groups on the edges of illite particles as potential organic matter sorbents. In addition, Kögel-Knabner and Amelung (2021) reported that silicate minerals (montmorrilonite > vermiculite > illite > kaolinite) are much more important SOM stabilizers than Fe oxides in the coarse clay fraction of temperate soils.
The present findings highlight the importance of the non-swelling clay mineral and illite contents of soils in controlling the stabilization of organic matter in the longer term. This is particularly important, as illite is a common mineral in almost all soil types.
5 Conclusions
The results confirmed the significance of detailed mineralogical analysis in the studies of soil organic matter turnover. Present study supported the crucial role of the crystalline and poorly crystalline mineral phases of Al and Fe in the binding of SOM in acid forest environments, and highlighted the effect on SOM stabilization of mineral groups that usually receive comparatively less attention. Accordingly, illite minerals are able to provide efficient binding surfaces for organic matter even over a longer timescale, since the decomposition rate constant of the slowly mineralizable carbon pool was only negatively related to the content of non-swelling clay minerals and illite, which was the dominant clay mineral in almost all the 13 forest soil samples investigated.
It was concluded that detailed knowledge on the role of individual mineral phases on the binding of SOM is necessary for a more exact determination of the dynamics of SOM decomposition and sequestration.
References
Anderson JPE (1982) Soil respiration. In: Page AL (ed) Methods of soil analysis. Part 2. Chemical and microbiological properties, Page, A. L. American Society of Agronomy, Madison, WI, pp 831–871
Angst G, Mueller KE, Kögel-Knabner I et al (2017) Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter. Biogeochemistry 132:307–324. https://doi.org/10.1007/s10533-017-0304-2
Angst G, Mueller KE, Nierop KGJ, Simpson MJ (2021) Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol Biochem 156:108189
Barré P, Fernandez-Ugalde O, Virto I et al (2014) Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: incomplete knowledge and exciting prospects. Geoderma 235–236:382–395. https://doi.org/10.1016/j.geoderma.2014.07.029
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. https://doi.org/10.1016/j.soilbio.2010.04.005
Bruun TB, Elberling B, Christensen BT (2010) Lability of soil organic carbon in tropical soils with different clay minerals. Soil Biol Biochem 42:888–895. https://doi.org/10.1016/j.soilbio.2010.01.009
Conklin ARJ (2014) Introduction to soil chemistry: analysis and instrumentation, 2nd editio. John Wiley & Sons, Hoboken, New Jersey
Eusterhues K, Rumpel C, Kögel-Knabner I (2005) Organo-mineral associations in sandy acid forest soils: importance of specific surface area, iron oxides and micropores. Eur J Soil Sci 56:753–763. https://doi.org/10.1111/j.1365-2389.2005.00710.x
Fang K, Qin S, Chen L et al (2019) Al/Fe mineral controls on soil organic carbon stock across tibetan alpine grasslands. J Geophys Res Biogeosciences 124:247–259. https://doi.org/10.1029/2018JG004782
Fekete I, Berki I, Lajtha K et al (2021) How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe? Biogeochemistry 152:13–32. https://doi.org/10.1007/s10533-020-00728-w
Feng W, Plante AF, Six J (2013) Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 112:81–93. https://doi.org/10.1007/s10533-011-9679-7
Franzluebbers AJ (1999) Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biol Biochem 31:1083–1090. https://doi.org/10.1016/S0038-0717(99)00022-X
Gartzia-Bengoetxea N, Virto I, Arias-González A et al (2020) Mineral control of organic carbon storage in acid temperate forest soils in the Basque Country. Geoderma 358:113998. https://doi.org/10.1016/j.geoderma.2019.113998
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis: part 1—physical and mineralogical methods, 2nd edn. Soil Science Society of America, Madison, WI, pp 383–411
Gillman GP (1979) A proposed method for the measurement of exchange properties of highly weathered soils. Aust J Soil Res 17:129–139
Gu B, Schmitt J, Chen Z et al (1994) Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ Sci Technol 28:38–46. https://doi.org/10.1021/es00050a007
Guidi P, Falsone G, Wilson C et al (2021) New insights into organic carbon stabilization in soil macroaggregates: an in situ study by optical microscopy and SEM-EDS technique. Geoderma 397:115101. https://doi.org/10.1016/j.geoderma.2021.115101
Hassink J (1997) The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 191:77–87. https://doi.org/10.1023/A:1004213929699
Helfrich M, Ludwig B, Potthoff M, Flessa H (2008) Effect of litter quality and soil fungi on macroaggregate dynamics and associated partitioning of litter carbon and nitrogen. Soil Biol Biochem 40:1823–1835. https://doi.org/10.1016/J.SOILBIO.2008.03.006
Holmgren GGS (1967) A rapid citrate-dithionite extractable iron procedure. Soil Sci Soc Am J 31:210–211. https://doi.org/10.2136/sssaj1967.03615995003100020020x
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Rome
Jones DL, Edwards AC (1998) Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biol Biochem 30:1895–1902. https://doi.org/10.1016/S0038-0717(98)00060-1
Kaiser K, Eusterhues K, Rumpel C et al (2002) Stabilization of organic matter by soil minerals — investigations of density and particle-size fractions from two acid forest soils. J Plant Nutr Soil Sci 165:451–459. https://doi.org/10.1002/1522-2624(200208)165:4%3c451::AID-JPLN451%3e3.0.CO;2-B
Kaiser K, Zech W (1998) Soil dissolved organic matter sorption as influenced by organic and sesquioxide coatings and sorbed sulfate. Soil Sci Soc Am J 62:129–136. https://doi.org/10.2136/sssaj1998.03615995006200010017x
Kiem R, Kögel-Knabner I (2002) Refractory organic carbon in particle-size fractions of arable soils II: organic carbon in relation to mineral surface area and iron oxides in fractions < 6 µm. Org Geochem 33:1699–1713. https://doi.org/10.1016/S0146-6380(02)00112-2
Kiss K (2012) Vörösagyag-talajok vizsgálata az Aggteleki-karszton (a Béke-barlang vízgyűjtőjén). Karsztfejlődés XVII:89–103
Kleber M, Bourg IC, Coward EK et al (2021) Dynamic interactions at the mineral–organic matter interface. Nat Rev Earth Environ 2:402–421
Kleber M, Eusterhues K, Keiluweit M et al (2015) Mineral-organic associations: formation, properties, and relevance in soil environments. Adv Agron 130:1–140. https://doi.org/10.1016/bs.agron.2014.10.005
Kögel-Knabner I, Amelung W (2014) Dynamics, chemistry, and preservation of organic matter in soils. In: Holland H, Turekian K (eds) Treatise on Geochemistry. Elsevier, Amsterdam, pp 157–215
Kögel-Knabner I, Amelung W (2021) Soil organic matter in major pedogenic soil groups. Geoderma. https://doi.org/10.1016/j.geoderma.2020.114785
Kotroczó Z, Juhos K, Biró B et al (2020) Effect of detritus manipulation on different organic matter decompositions in temperate deciduous forest soils. Forests 11:675. https://doi.org/10.3390/f11060675
Kubicki JD, Schroeter LM, Itoh MJ et al (1999) Attenuated total reflectance Fourier-transform infrared spectroscopy of carboxylic acids adsorbed onto mineral surfaces. Geochim Cosmochim Acta 63:2709–2725. https://doi.org/10.1016/S0016-7037(99)00194-5
Kulik DA, Aja SU, Sinitsyn VA, Wood SA (2000) Acid-base surface chemistry and sorption of some lanthanides on K+-saturated, Marblehead illite: II. A multisite-surface complexation modeling. Geochim Cosmochim Acta 64:195–213. https://doi.org/10.1016/S0016-7037(99)00174-X
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38:425–448. https://doi.org/10.1016/j.soilbio.2005.08.020
Lawrence CR, Harden JW, Xu X et al (2015) Long-term controls on soil organic carbon with depth and time: a case study from the Cowlitz River Chronosequence, WA USA. Geoderma 247–248:73–87. https://doi.org/10.1016/j.geoderma.2015.02.005
Mikutta R, Kleber M, Jahn R (2005) Poorly crystalline minerals protect organic carbon in clay subfractions from acid subsoil horizons. Geoderma 128:106–115. https://doi.org/10.1016/j.geoderma.2004.12.018
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56. https://doi.org/10.1007/s10533-005-0712-6
Molina JAE, Clapp CE, Larson WE (1980) Potentially mineralizable nitrogen in soil: the simple exponential model does not apply for the first 12 weeks of incubation. Soil Sci Soc Am J 44:442–443. https://doi.org/10.2136/sssaj1980.03615995004400020054x
Németh T, Sipos P (2006) Characterization of clay minerals in brown forest soil profiles (Luvisols) of the Cserhát Mountains (North Hungary). Agrokémia És Talajt 55:39–48. https://doi.org/10.1556/agrokem.55.2006.1.5
Németh T, Sipos P, Balázs R et al (2010) Adsorption of copper on the illuviation and accumulation horizons of a Luvisol. Carpathian J Earth Environ Sci 5:19–24
Oades JM (1988) The retention of organic matter in soils. Biogeochemistry 5:35–70. https://doi.org/10.1007/BF02180317
Rasmussen C, Heckman K, Wieder WR et al (2018) Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137:297–306. https://doi.org/10.1007/s10533-018-0424-3
Ringer M, Jakab G, Sipos P et al (2021) Vertical differentiation of pedogenic iron forms – a key of hydromorphic soil profile development. Hungarian Geogr Bull 70:369–380. https://doi.org/10.15201/hungeobull.70.4.6
Saggar S, Parshotam A, Sparling GP et al (1996) 14C-labelled ryegrass turnover and residence times in soils varying in clay content and mineralogy. Soil Biol Biochem 28:1677–1686. https://doi.org/10.1016/S0038-0717(96)00250-7
Saviozzi A, Vanni G, Cardelli R (2014) Carbon mineralization kinetics in soils under urban environment. Appl Soil Ecol 73:64–69. https://doi.org/10.1016/j.apsoil.2013.08.007
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Schulten HR, Leinweber P (2000) New insights into organic-mineral particles: composition, properties and models of molecular structure. Biol Fertil Soils 30:399–432
Schwertmann U (1973) Use of oxalate for Fe extraction from soils. Can J Soil Sci 53:244–246. https://doi.org/10.4141/cjss73-037
Shahbaz M, Kuzyakov Y, Heitkamp F (2017) Decrease of soil organic matter stabilization with increasing inputs: mechanisms and controls. Geoderma 304:76–82. https://doi.org/10.1016/j.geoderma.2016.05.019
Shen Y-H (1999) Sorption of natural dissolved organic matter on soil. Chemosphere 38:1505–1515. https://doi.org/10.1016/S0045-6535(98)00371-3
Sinitsyn VA, Aja SU, Kulik DA, Wood SA (2000) Acid-base surface chemistry and sorption of some lanthanides on K+-saturated, Marblehead illite: I. Results of an experimental investigation. Geochim Cosmochim Acta 64:185–194. https://doi.org/10.1016/S0016-7037(99)00175-1
Sipos P (2004) Geologic and pedogenic effects on heavy metal distributions in forest soils from the Cserhát Mts and the Karancs area, NE Hungary. Acta Geol Hungarica 47:411–429. https://doi.org/10.1556/AGeol.47.2004.4.5
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176. https://doi.org/10.1023/A:1016125726789
Stewart CE, Paustian K, Conant RT et al (2009) Soil carbon saturation: implications for measurable carbon pool dynamics in long-term incubations. Soil Biol Biochem 41:357–366. https://doi.org/10.1016/j.soilbio.2008.11.011
Thiessen S, Gleixner G, Wutzler T, Reichstein M (2013) Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass - an incubation study. Soil Biol Biochem 57:739–748. https://doi.org/10.1016/j.soilbio.2012.10.029
Trumbore SE (2000) Age of soil organic matter and soil respiration. Ecol Appl 10:399–411
Trumbore SE, Chadwick OA, Amundson R (1996) Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science (80- ) 272:393–396. https://doi.org/10.1126/science.272.5260.393
Vogel C, Mueller CW, Höschen C et al (2014) Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat Commun 5:1–7. https://doi.org/10.1038/ncomms3947
von Lützow M, Kögel-Knabner I, Ekschmitt K et al (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review. Eur J Soil Sci 57:426–445. https://doi.org/10.1111/j.1365-2389.2006.00809.x
von Lützow M, Kögel-Knabner I, Ludwig B et al (2008) Stabilization mechanisms of organic matter in four temperate soils: development and application of a conceptual model. J Plant Nutr Soil Sci 171:111–124. https://doi.org/10.1002/jpln.200700047
Wattel-Koekkoek EJW, Buurman P, Van Der Plicht J et al (2003) Mean residence time of soil organic matter associated with kaolinite and smectite. Eur J Soil Sci 54:269–278. https://doi.org/10.1046/j.1365-2389.2003.00512.x
Wiesmeier M, Urbanski L, Hobley E et al (2019) Soil organic carbon storage as a key function of soils - a review of drivers and indicators at various scales. Geoderma 333:149–162. https://doi.org/10.1016/J.GEODERMA.2018.07.026
Wiseman CLS, Puttmann W (2005) Soil organic carbon and its sorptive preservation in central Germany. Eur J Soil Sci 56:65–76. https://doi.org/10.1111/j.1351-0754.2004.00655.x
Wutzler T, Reichstein M (2008) Colimitation of decomposition by substrate and decomposers – a comparison of model formulations. Biogeosciences Discuss 5:163–190. https://doi.org/10.5194/bgd-5-163-2008
Zacháry D, Filep T, Jakab G et al (2018) Kinetic parameters of soil organic matter decomposition in soils under forest in Hungary. Geoderma Reg 14:e00187. https://doi.org/10.1016/J.GEODRS.2018.E00187
Acknowledgements
The authors are grateful to Klaudia Kiss and Péter Sipos for their help in the field. Thanks are also due to Annamária Nagy for her help in the laboratory work.
Funding
Open access funding provided by ELKH Research Centre for Astronomy and Earth Sciences. This work was supported by the Széchenyi 2020 programme, the European Regional Development Fund and the Hungarian Government [grant No. GINOP-2.3.2–15-2016–00028]; and by the Development and Innovation Fund of Hungary [grant No. NKFIH 123953].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Jianming Xue
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zacháry, D., Filep, T., Jakab, G. et al. The effect of mineral composition on soil organic matter turnover in temperate forest soils. J Soils Sediments 23, 1389–1402 (2023). https://doi.org/10.1007/s11368-022-03393-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03393-8