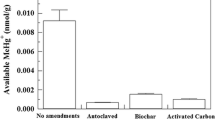
Abstract
Purpose
As the formation of toxic and bioaccumulative methylmercury (MeHg) in Hg-contaminated sediments is of great concern worldwide, suitable remediation options are needed. Activated carbon (AC) amendment is a contested alternative due to uncertainties surrounding sorption efficiency and its potential role in aiding MeHg formation. The purpose of this study was therefore to demonstrate AC performance under favourable conditions for Hg-methylation and to further understand the role AC plays in the methylation process.
Materials and methods
Mercury-contaminated sediment (57.1 mg kg−1) was sampled from the Gunneklev fjord, a site known as the most heavily contaminated fjord in Norway. In a laboratory experiment, lignite AC (A-AC, 5%) or activated biochar (A-BC, 5%) along with dried algae biomass, serving as an excess source of easily degradable organic matter (OM) and sulphate, were added to sediment samples that were kept anoxic and dark over a period of 12 months.
Results and discussion
The amount of MeHg in sediment and porewater of the amended samples were measured at 0, 1, 3, 6, and 12 months and compared to an unamended control. A net increase of MeHg in the sediment was observed in both control and amended samples, but contrary to expectations, sediment MeHg was 5 and 3 times higher in the A-AC and A-BC treatments, respectively, relative to the control after 12 months. As the stimulation of Hg-methylation could not be attributed to the sorbents supplying more available OM or sulphate for dissimilatory sulphate reduction, it is speculated that the sorbents rather aid this process through shuttling of electrons between the substrates involved. Meanwhile, the A-AC and A-BC amendments strongly reduced the available MeHg-concentration in porewater (by 87% for A-AC and by 93% for A-BC after 12 months), confirming that AC sorbents can be used to effectively limit the transport of MeHg from sediments.
Conclusion
When considering remediation of OM-rich Hg-contaminated sediments with AC, caution is thus warranted, as the overall effect of reducing MeHg-transport out of the sediment could partly be offset by an increased fraction of MeHg in the sediment. Thin-layer capping with AC might therefore be preferable to complete mixing of AC and sediment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg) pollution is a widespread contamination issue throughout the world, in industrial as well as remote areas (Fernandez-Luqueno et al. 2013). Methylation of inorganic Hg to methylmercury (MeHg) takes place under anoxic conditions and increases the overall risk of Hg due to the higher toxicity and bioaccumulation potential of MeHg as compared to inorganic Hg (Magos et al. 1985; Ullrich et al. 2001; Clarkson et al. 2003). Biotic methylation processes dominate overall MeHg production in most systems (Ullrich et al. 2001), and sulphur reducing bacteria (SRB) have been identified as the main methylators in marine sediments (Regnell and Watras 2019).
Below the top millimetres to centimetres, contaminated coastal marine sediments are often anoxic (Dell’Anno et al. 2009), and conducive to the formation of MeHg, especially in the presence of fresh, easily degraded organic matter (OM) that can serve as electron donor (Ndungu et al. 2016). Hg contamination is often so widespread that traditional dredging and containment technologies are not feasible. However, the placement of sand or clay caps as remediation measures can lead to an upward extension of anoxic conditions in initially oxic sediments and subsequently result in increased MeHg formation (Johnson et al. 2010). On the other hand, Ndungu et al. (2016) demonstrated that although a thin clay capping layer increased MeHg formation, OM added as algae was the limiting factor in this process and the cap succeeded in reducing the release of said MeHg. Due to the contentious effect of other remediation approaches, addition of actively sorbing materials such as biochar or activated carbon (AC) has been suggested as a methodology to reduce total Hg (THg) and MeHg porewater concentrations, and thus risk (Gomez-Eyles et al. 2013).
Biochar is a highly carbonaceous material made through the heating of biomass in the absence of oxygen (pyrolysis), while AC is a term used to describe carbonaceous materials (often hard coals such as lignite or anthracite) that have been subjected to an activation process to increase porosity and optimize surface chemistry (Hagemann et al. 2018). Traditional AC manufactured from fossil hard coal has been shown to exhibit effective sorbent properties (Cornelissen et al. 2012; Patmont et al. 2015), but biochar has the advantage of being a more sustainable option for sediment remediation in a life cycle perspective (Sparrevik et al. 2011; Alhashimi and Aktas 2017). It should be noted however that the sorbent properties of biochars vary greatly with feedstock, pyrolysis temperature and retention time (Lehmann and Joseph 2015). AC has been proven to function well for hydrophobic organic chemicals (Ghosh et al. 2011; Patmont et al. 2015). A few studies have shown that AC, and to a slightly lesser extent biochar, also bind Hg, and especially MeHg, very strongly (Kong et al. 2011; Gomez-Eyles et al. 2013). This is especially true for sediments that show intrinsically weak sorption of Hg and MeHg (Gilmour et al. 2013).
There is no scientific consensus on the effect of biochar and AC on total MeHg in sediments. For example, Bussan et al. (2016) measured reduced Hg-methylation rates in environmentally contaminated sediments with both AC and biochar. However, no measurements of porewater or bioavailability were done. One study found total sediment concentration of MeHg to stay constant upon AC amendment (Gilmour et al. 2013), whereas another field study on AC and biochar amendment to sediment by the same group observed enhanced MeHg formation, especially in the presence of biochar that added more degradable OM than AC (Gilmour et al. 2018). Around 20% of biochar carbon is not stable over prolonged time periods of more than a decade (Lehmann et al. 2009), and the labile carbon released may serve as an electron donor for bacteria in the Hg methylation process Also Liu et al. (2018), in a 500-day microcosm study where various biochars were added to Hg-contaminated sediment, observed 3- to tenfold increase in total MeHg contents in the sediment, especially for high-temperature chars (600–700 °C). Two studies on inundated rice paddies found increases in overall MeHg concentrations in the paddy soils upon the addition of biochars, by 20–80% (Shu et al. 2016) and 5–75% (Zhang et al. 2018). It is unknown why AC and biochar have been observed to stimulate MeHg production, but the main hypothesis seems to be that the addition of AC or biochar might provide additional OM or sulphate that in many systems are limiting factors for Hg-methylation.
The net MeHg porewater concentration following amendment is expected to be the result of the extent of MeHg produced through methylation and the amount of MeHg removed through the partitioning to the sorbents. Previously, the extensive sorption effect was observed to be stronger than the MeHg formation effect, leading to overall lower MeHg bioavailability despite the formation of MeHg (Shu et al. 2016; Gilmour et al. 2018; Liu et al. 2018; Zhang et al. 2018). As mentioned above, however, methylation was likely limited in these studies by the access of methylating bacteria to suitable electron donors and acceptors.
More work is thus needed to better understand the possible limitations of AC or biochar amendment of Hg-contaminated sediment. To this end, a highly Hg-contaminated marine sediment was amended with AC made from fossil hard coal (A-AC), as well as an activated biochar made from coconut shell (A-BC), and analysed for MeHg concentrations in both the sediment and the porewater at various time intervals.
In contrast to previous studies on sorbent amendments of Hg contaminated sediments where MeHg production was limited by the presence of OM or sulphate, sorbent effectiveness is here tested in a scenario where there are no limiting factors for methylation. Such a scenario is considered relevant in a purely mechanistical perspective but also relevant as a scenario where an OM-rich sediment with favourable conditions for methylation is amended. Furthermore, this study offers a comparison between the efficiency of the more sustainable activated biochar sorbent compared to a traditional activated carbon made from fossil hard coal. Porewater concentrations were used as a measure of amendment efficiency as this fraction is both relevant for uptake in organisms (Amirbahman et al. 2013; Gilmour et al. 2013) and transport from the sediment to the water column (Ndungu et al. 2016).
The following hypotheses were tested: (H1) A-AC and A-BC sorbent amendment will not enhance MeHg production in a system where excess amounts of OM and sulphate are present and (H2) Both A-AC and A-BC amendments will prove effective for sediment Hg-remediation by strongly reducing porewater MeHg concentrations.
2 Materials and methods
2.1 Study site
The Gunneklev fjord is a 0.76-km2 fjord in the inner part of the larger Inner Grenlandsfjord (Frierfjord) in the province of Telemark, Norway (Fig. 1). The Gunneklev fjord is closed off between the Herøya peninsula and the mainland, with a small inlet at the outlet of the Skien River. There is also a small outlet in the other end of the fjord through a small channel, built as an exit from the Gunneklev fjord into the Inner Grenlandsfjord for small recreational boats from the marina situated in the inner part of the Gunneklev fjord. The Gunneklev fjord is a brackish fjord (salinity 5–20 PSU in the bottom water (Olsen et al. (2018) and references therein) with an average depth of 4.6 m and water volume of 3.5 × 106 m3. It is one of the most polluted fjord systems in Norway due to historic release of Hg-containing sludge from a chlor-alkali plant (Olsen et al. 2018). Previous studies have shown sediment concentration ranges of total mercury (THg) ranging from 2 to 5 mg kg−1 at the 0–5 cm surface layer to 500 mg kg−1 at 10–20 cm depth, and MeHg of 1–3 µg kg−1 at the surface to 300 µg kg−1 at 10–20 cm depth (Olsen et al. 2018). It has been estimated that the fjord contains about 20 to 30 tonnes of Hg, of a total of 80 tonnes released from Herøya Industrial Complex between 1947 and 1987 (Olsen et al. 2019). Despite the high sediment concentrations, Hg concentrations in the water column are not significantly higher than in nearby freshwater lakes fed by atmospheric Hg-transport, but the Hg concentrations in fish are elevated, indicating that the legacy pollution is still accumulating in the food webs (Braaten et al. 2019).
2.2 Sediment sampling
Surface sediment was sampled using a Van Veen grab sampler at approximately 0–10 cm depth. The sample was collected at latitude 59.12528859 and longitude 9.636577517 (WGS 84, UTM32), in a PE bucket (20 L) with a sealed lid, which was stored cold (4 °C) and dark until use.
2.3 Experimental setup
The bulk sediment sample was homogenized through slow stirring (≈10 min) and split into three subsamples (≈5 L each): (1) Control, (2) A-AC amendment, and (3) A-BC amendment. Each of the three subsamples was added chlorella algae (2% per d.w. sediment). Similar to the study by Ndungu et al. (2016), Chlorella algae was obtained from a Naturalis AS, Norway — a pure (100% algae) and dried powder, sold as a dietary supplement.
The A-AC and A-BC were then added (5% of d.w.) to subsamples (2) and (3), respectively (see section below for more details about the sorbents). All three subsamples were then homogenized thoroughly by stirring (≈10 min) before they each were split into five identical, pre-cleaned glass jars (500 mL) that were to represent a time series of 0, 1, 3, 6, and 12 months. The glass jars were filled nearly to the rim and topped off with saltwater from 60 m depth collected at the Norwegian Institute for Water Research (NIVA) research station at Solbergstrand, Norway, before they were sealed with lids and stored dark at 20 °C until the completion of the respective time series. Gas produced in the sealed jars was released at regular intervals by carefully loosening the lid.
This experimental setup was chosen to mimic stratified marine waters with little or no exchange in the water column above the anoxic sediment surface. When contaminated with Hg, such locations are considered among potential hot spots for methylmercury production (Ullrich et al. 2001; Hammerschmidt and Fitzgerald 2004; Merritt and Amirbahman 2009; Dai et al. 2021). Not exchanging the water above the surface sediment might lead to a higher accumulation of MeHg in porewater and sediment, as according to Fick’s first law (Fick 1855), diffusive flux is controlled by the concentration gradient. This potential artefact is not considered an issue as it would further promote the high MeHg environment; the experimental setup was created to promote.
2.4 Sorbent materials
One fossil hard coal-based AC material fabricated from lignite (A-AC; BP2 fine powder) and one activated biomass-based material fabricated from coconut shells (A-BC; activated biochar; CP1 fine powder) were obtained from Jacobi Carbons, Kalmar, Sweden. Both AC qualities were of the same particle size range (average particle size 20 µm, 80% smaller than 45 µm) and were produced using high temperature steam activation. Steam activation generally requires temperatures > 850 °C (Marsh and Reinoso 2006). The characteristics of these materials have extensively been described in Amstaetter et al. (2012). A-AC exhibited a large portion of pores > 15 Å (541 m2 g−1). In contrast, a clear dominance of narrow pores in the size range of 3.5–15 Å was observed for the A-BC, and almost 10 times lower pore surface area > 15 Å (61 m2 g−1) than A-AC. The volume of larger pores > 15 Å in A-BC (0.053 cm3 g−1) was much lower than that in A-AC (0.602 cm3 g−1). In contrast, both the 3.5–15 Å pore surface area and volume were larger for the biomass-based A-BC (977 m2 g−1 and 0.322 cm3 g−1, respectively) than for the coal-based A-AC (726 m2 g−1 and 0.268 cm3 g−1, respectively) (Amstaetter et al. 2012).
2.5 Sediment and porewater characterization
Porewater was extracted from sediment samples through centrifugation (5000 rpm) of approximately 400 g sediment in pre-cleaned Teflon vials. The separated pore water phase was pipetted off into pre-cleaned glass vials (50 mL). The samples could not be analysed immediately and were therefore frozen after centrifugation to inhibit further methylation/demethylation of Hg and stored dark until analysed. Freezing samples is an accepted way of inhibiting changes in speciation post sampling induced by bacterial activity (Carr et al. 2001), and is commonly used for sediment samples for Hg analysis (Lutz et al. 2008). Freezing unfiltered porewater could alter distribution between dissolved and particle bound species, but post centrifugation particle content will be low and negligible effects are assumed (Carr et al. 2001). Freezing centrifuged porewater samples could potentially alter the DOC content however (Peacock et al. 2015), but as DOC was expected to be present in excess, this potential artefact was considered an acceptable trade off.
Aqueous (porewater) total Hg (THg) and MeHg was analysed following USEPA method 1631 and method 1630 as described in Braaten et al. (2014), including filtration at 0.45 µm, oxidation (for THg) and distillation and ethylation (for MeHg) followed by purge/trap and cold vapour atomic fluorescence (CVAFS) detection. Method detection levels (MDLs) were 0.02 ng L−1 and 0.1 ng L−1 for MeHg and THg, respectively (3 standard deviations of method blanks). For both aqueous Hg species, automated systems were used for analysis (Brooks Rand Instruments MERX). Relative standard deviation (RSD) of sample duplicates was < 10% and < 20% for THg and MeHg, respectively. Recoveries of matrix spikes were 80–120% for MeHg and 90–110% for THg.
Sediment THg determination was done according to USEPA method 7473 using thermal decomposition and direct atomic absorption spectrophotometry (AAS) on a DMA-80 instrument from Milestone. Analysis of a THg certified reference material (CRM, Mess-4, marine sediment) was within the reported range (0.09 ± 0.04 mg kg−1). MDL for THg was 0.7 µg kg−1 (3 standard deviations of method blanks) and RSD of sample duplicates was < 20%.
The MeHg in sediments was extracted by methods described in detail by Bloom et al. (1997). The method includes leaching of MeHg from the sediment with potassium bromide (KBr, 18%), sulphuric acid (H2SO4, 5%), and copper sulphate (CuSO4, 1 M) before extraction of the MeHg in the leachates into dichloromethane (DCM). The Hg was then back-extracted into DI water (by use of Whatman 1 PS silicone treated filter paper) before heating (≈70 °C for 5 h). Determination followed USEPA Method 1630 as described above. Analysis of a MeHg CRM (ERM-CC580; estuarine sediment) was within the reported range (75 ± 4 ng g−1). MDL was 20 pg g−1 (3 std.dev. of blank extractions) based on a 0.05-g sample weight.
For dissolved organic carbon (DOC) determination, porewater samples were filtered through 45 µm syringe filters, before being analysed using catalytic combustion and infrared detection on an Apollo 9000 from Teledyne Tekmar as described in NS-EN 1484. Limit of quantification (LOQ) was 0.5 mg L−1 and method uncertainty < 20%.
The SO42− in porewater was determined by photometry at 420 nm after precipitation of BaSO4 according to ISO15923 by the accredited laboratory ALS Laboratory Group Norge AS (ALS). LOD was 0.5 mg L−1 and method uncertainty < 10%. pH of porewater was determined by potentiometry according to NS-EN-ISO10523 by ALS.
Sediment total water content was done by titration with Karl Fischer reagent (ISO760) with LOQ of 0.01% and method uncertainty of ± 9% by ALS. Sediment particle size distribution (> 63 µm and < 2 µm) according to internal standard CZ_SOP_D06_07_N11 with LOQ of 0.1% and measurement uncertainty of ± 0.3% µm for particles > 63 m and ± 0.5% for particles < 2 µm by ALS. Total organic carbon (TOC) was determined coulometrically according to standard DIN-ISO10694 with LOQ of 0.01% by ALS.
2.6 Data analysis
In this experiment, remediation efficiency (MeHgreduction, THgreduction, %) was defined as the ability of the sorbent to reduce the MeHg and THg porewater concentration (Cpw, amended,) relative to the control (Cpw,control):
As another measure of degree of sorption of MeHg to the sorbents, partitioning coefficients between water and sediment (KD) were calculated for the control and the sediment and AC sorbent mixtures using the following equation:
where Csediment is the concentration in the sediment or sediment + sorbent mixtures and Cpw is the concentration in extracted pore water. Under the experimental conditions, an anoxic sediment with available substrates for methylating bacteria, it should however be expected that MeHg does not reach a full equilibrium partitioning between sediment and water at any point due to continuous methylation and demethylation processes (Ullrich et al. 2001). In comparison to the fast adsorption processes of Hg species to AC, where > 80% of the adsorption happens within 3–6 h according to two studies by Ting et al. (2018) and Li et al. (2013), methylation rates are relatively slow — 0.01 day−1 (Hintelmann et al. 2000). It is therefore realistic to expect that the systems will be close to equilibrium. The partitioning coefficients, that assume equilibrium, will therefore be pseudo-KD that can be used as a conservative and comparative estimate of the sorption strength of the different sorbent amendments.
Single linear regression analyses and t-tests were done using the software R (v.3.4.3), and results were considered significant for p < 0.05.
2.7 Quality control and assurance
Due to economic constraints, sample time points (sediment jars) were not replicated. The method uncertainty, which is related to the degree of homogenization of bulk samples before splitting and whether splitting and subsequent treatment of individual sample jars led to variation in contents or conditions, was tested by analysing sediment THg in all sample jars. THg in the sediment is not expected to change. As Fig. 2H shows, there were no apparent trends in sediment THg and no statistically significant differences between the control and the two amendments (t-test, p < 0.05). The average sediment THg concentration across all treatments and time points (n = 15) was 52.5 ± 5.3 mg kg−1, amounting to a 10.1% relative standard deviation of the method. The analytical uncertainty however (Sect. 2.4) was equal to or higher than 10% for all parameters in the time series studied (THg — 10%, MeHg — 20%, DOC — 20%, and SO42− — 10%). In the absence of replications and since the analytical error was higher than the method error estimation, the analytical error was used as a measure of uncertainty for the individual sampling points.
A MeHg (ng L−1), B THg (μg L−1), C reduction of MeHg and THg (%) — as per Eq. 1, D pH, E DOC (mg L−1) and F SO4 2- (mg L−1) in porewater, and G MeHg (μg kg−1) and H) THg (mg kg−1) in sediment. Error bars show the analytical uncertainty
3 Results
3.1 Sediment characterization
The sediment from the Gunneklev fjord had a high water content (84 ± 9%) and a large amount of fine particles, with 90.6% of the particle diameter distribution being between 63 and 2 µm, and 3.0% below 2 µm. Furthermore, the sediment had an original TOC content of 5.56% d.w. and a porewater pH of 8.1, with SO42− and S2− concentrations of 900 mg L−1 and 0.12 mg L−1, respectively. Adding algae biomass (2% d.w.) to the samples supplied the sediment with about 1% of d.w. organic carbon, resulting in an immediate porewater DOC concentration of 90 mg L−1, while increasing the porewater SO42− concentration to 1600 mg L−1 and lowering the pH to 7.6. At the onset of the experiment time series (t0, 0 months), THg sediment content was 57.1 mg kg−1, of which 0.9 µg kg−1 (0.0015%) was MeHg. The MeHg concentration is in the lower range of what has previously been reported for the Gunneklev fjord (Olsen et al. 2018), and could have been affected by of aerobic demethylation during sample preparation (Ullrich et al. 2001).
3.2 Methylmercury production in porewater and sediment
Figure 2 shows development of porewater MeHg, THg, DOC and SO42−, and sediment MeHg and THg over the duration of the experiment (0–12 months), while a full list of concentrations is presented in the supporting information (SI, Table S1).
The initial concentrations of MeHg in pore water and sediment were 9 ± 2 ng L−1 and 0.9 ± 0.2 µg kg−1, respectively as measured in the unamended control sample (i.e. in the presence of algae but without sorbent) at time zero (Fig. 2A). Conditions proved favourable for Hg-methylation under the regime of the experimental setup, as a sharp increase in MeHg porewater concentration was observed in the control during the first month of the trial, increasing from the initial 9 ± 2 to 393 ± 79 ng L−1. In the following months, the porewater concentration varied, by first dropping to 147 ± 29 and 18 ± 4 ng L−1 after 3 and 6 months, respectively, before increasing to 63 ± 13 ng L−1 after 12 months (Fig. 2). The MeHg sediment concentration showed a similar trend: first a sharp increase, from 0.9 ± 0.2 to 18 ± 4 µg kg−1, after 1 month, then a drop to 8 ± 2 and 6 ± 1 µg kg−1 after 3 and 6 months, respectively, before a final increase to 38 ± 8 µg kg−1 after 12 months (Fig. 2G).
The porewater concentrations of MeHg in the A-AC treated sediment were 8–100 times lower than those of the control but showed a similar trend over time: 1.2 ± 0.2, 3.7 ± 0.7, 7 ± 1, 0.8 ± 0.2, and 8 ± 2 ng L−1 after 0, 1, 3, 6, and 12 months, respectively (Fig. 2A). In the A-AC treatment however, MeHg concentrations in the sediment increased exponentially over the time of the experiment (R2 = 0.8299, p = 0.031), ending up with a concentration (199 ± 40 µg kg−1) which was 5 times higher than in the unamended control (38 ± 8 µg kg−1) after 12 months. The difference between control and A-AC treatment was apparent after 3 months, at which sediment MeHg concentration was twice that of the control.
The A-BC treatment showed a slightly different trend than the A-AC treatment (Fig. 2A), as MeHg porewater concentrations decreased from the onset (3.8 ± 0.8 ng L−1) all the way to the 6-month mark where the concentration was 8 times lower than in the A-AC treatment (A-AC 0.8 ± 0.1 ng L−1 vs. A-BC 0.06 ± 0.01 ng L−1). Similar to the A-AC treatment, an increase in porewater concentration was seen in the A-BC treatment between 6 and 12 months, from 0.06 ± 0.01 to 4.5 ± 0.9 ng L−1. Overall, sediment MeHg concentrations were lower in the A-BC treatment compared to the A-AC treatment, but as for the A-AC treatment, MeHg sediment contents in the A-BC treatment increased exponentially over time (R2 = 0.8122, p = 0.037) and ended with a MeHg concentration 3 times higher than the control after 12 months (A-BC 116 ± 23 µg kg−1 vs. control 38 ± 8 µg kg−1). Unlike the A-AC treatment however, there was no apparent difference between MeHg sediment concentrations until after 12 months.
The trends of THg concentration in porewater were similar to those of MeHg in porewater for both the control and the two treatments over the 12-month span (Fig. 2B). In the control sample, there was a strong positive linear correlation between THg and MeHg in porewater (R2 = 0.9828, p < 0.001). This correlation was less strong in the amended samples (R2 = 0.6728, p = 0.033).
The pH did not change notably over the course of the experiment however (Fig. 2D) — an initial increase was seen between the onset and 1 month, but thereafter pH stayed at approximately 9.5 in the control and both treatments. Alkaline ash components usually found in carbonaceous materials (Lehmann and Joseph 2015) are likely responsible for the difference in pH between control and treatments at t0, but the increase to pH ≈ 9.5 is probably the cause of consumption of H+ during the anaerobic reduction of labile OM (Canfield et al. 1993).
Over the course of the first month, the DOC-concentration in porewater increased 10–100 times in both the control sediment and the two sorbent-amended ones (Fig. 2E), most likely due to the labile fraction of OM in the added algae dissolving as has previously been observed by Ndungu et al. (2016). In the control sample, the 1-month spike in DOC-concentration was followed by an increase in both THg and MeHg of 15 and 44 times the concentration at t0. Ndungu et al. (2016) recorded a similar effect in a microcosm study of an isolation capping where excess OM was added, and attributed it to ionic Hg partitioning from the sediment into the porewater DOC. Fluctuations in DOC-concentrations of about 10–20% were observed between 1 and 12 months: 1200 ± 270 mg L−1 in the control, 273 ± 53 mg L−1 in the A-AC treatment, and 663 ± 59 mg L−1 in the A-BC treatment. In the control, these fluctuations are assumed to be mainly related to anaerobic degradation of OM (Canfield et al. 1993). The approximately four- and two-times lower DOC concentrations in the A-AC and A-BC treatments, respectively, compared to the control, are attributed to sorption of OM to the ACs (Bjelopavlic et al. 1999; Schreiber et al. 2005; Schwartz et al. 2019). The higher sorption efficiency of A-AC compared to A-BC could be due to the larger share of pores > 15 Å (upper micropore range and higher) in the A-AC that allows for better accommodation of large organic macromolecules (Bjelopavlic et al. 1999).
The porewater concentration of SO42− however was gradually reduced over the course of the whole experiment in both control and treatments — the concentrations were about 10 times lower after 12 months than at the start (t0) (Fig. 2F). This demonstrates the presence and activity of SRB in the sediment.
Overall these data show that the addition of fresh organic matter in the form of algae strongly stimulated MeHg formation — after 12 months, the MeHg sediment concentration in the control had increased to 45 times the initial concentration. It appears that the addition of A-AC and A-BC in combination with the algae further stimulated the formation of MeHg, as sediment MeHg concentrations were 650 and 300 times higher in the A-AC and A-BC treatments, respectively, compared to the control at time zero. The apparent effect was furthermore strongest in the A-AC treatment, where the difference between control and treatment was significant after 3 months.
3.3 Remediation efficiency
The A-AC treatment had a strong, immediate remediation effect as 86% of MeHg was removed from the pore water at t0 (Fig. 2C). The effect was at maximum after 1 month (99%) and then declined towards 87% after 12 months. The effect of the A-BC treatment had a slower onset as only 56% was immediately sorbed at t0. From 1 month onwards to the 6-month mark, the effect was strong (> 99%), before a drop to 93% at 12 months.
The remediation effect for THg was highly variable (Fig. 2C). The pool of inorganic Hg in the sediment was high compared to MeHg (0.012% MeHg at t0) and porewater THg in the control fluctuated over the 12 months of the experiment. It is likely that the ACs did not have the capacity to continuously sorb all the inorganic Hg released from the sediment through desorption processes. Furthermore, the apparent high THg-remediation effect seen in the first half of the experiment, i.e. 91% and 84% after 1 month and 93% and 88% after 6 months for A-AC and A-BC treatments, respectively (Table S1), might be partly due to biosorption of inorganic Hg by the Chlorella algae biomass (Kumar et al. 2020; Spain et al. 2021). However, as the algae biomass decomposes over time, biosorbed Hg could be released back to solution, contributing to the low remediation effect recorded at the end of the experiment (A-AC 53% and A-BC 0%; Table S1).
At t0, the partitioning between porewater, sediment, and sorbent had not reached pseudo-equilibrium as there were no significant differences in pseudo-log KD between the control, A-AC, and A-BC treatments (2.0, 2.4, and 2.0 log L kg−1, respectively (p < 0.05)). The effect of the amendments is illustrated by an increase in KD over time as MeHg is produced — between 3 and 12 months, the KD for the amended systems were 1.5–2 log units higher than the control. At 12 months, both amendments resulted in the same log KD (4.4 L kg−1).
4 Discussion
4.1 Methylmercury formation
The experimental conditions, where OM and sulphate were in excess, strongly stimulated Hg methylation, as the MeHg concentrations of the control sample was 45 times higher after 12 months compared to t0. The sorbent amendments seemed to stimulate methylation even further; the difference between MeHg sediment concentration at t0 and 12 months were almost an order of magnitude higher in the amended samples compared to the control.
The observation that the DOC concentrations were not depleted over the course of the experiment (Fig. 2) indicates that Hg methylation was not limited by access to organic carbon as an electron donor in this system. Access to sulphate as an electron acceptor for SRB also did not limit MeHg formation in this experiment, as sulphate was not depleted after 12 months (Fig. 2). The observed gradual reduction of SO42− (Fig. 2) however shows that SO42− likely would limit methylation in this system in the long term.
The enhanced methylation seen in the amended samples is the opposite of what was hypothesized (H1). Based on observations of enhanced Hg-methylation following biochar amendment to a salt marsh (Gilmour et al. 2018) and inundated rice paddy soils (Shu et al. 2016; Zhang et al. 2018) explained by biochar containing additional labile OM and/or sulphate that aided methylation, it was believed that A-AC and A-BC would not enhance methylation in our system where these parameters were in excess.
The exact mechanisms of Hg-methylation by the numerous strains of SRB that exist are still not well known, but it is generally understood that MeHg is a dissimilatory product of the electrochemical process of anaerobic sulphate reduction where OM typically acts as an electron donor (King et al. 1999; Merritt and Amirbahman 2009; Gilmour et al. 2011). A possible explanation of the increased MeHg formation in the present study is that the carbonaceous sorbents aid dissimilatory Hg methylation by SRB by contributing to electron shuttling between the electron donors and acceptors via their extensive aromatic free pi-electron systems (Chen et al. 2014; Yuan et al. 2017). This speculation is supported by the fact that both OM and Hg was found to sorb to the AC-sorbents used in this study, meaning that electron transfer through the AC-material between OM and Hg is a viable alternative mechanism to electron transfer during direct contact between dissolved OM and Hg. Biochar has been shown to greatly affect soil redox conditions and can act as both an electron acceptor and donor due to a diverse surface functional group chemistry (Joseph et al. 2015).
Both biochar (Chen et al. 2014; Yuan et al. 2018) and AC (Liu et al. 2012) have been found to enhance methanogenesis through an electron shuttling mechanism. In biochar applied to soil, the shuttling effect was found to be augmented by quinone groups on the biochar surface that both accept and donate electrons (Yuan et al. 2018). The same mechanism has been used to explain biochar’s role in contributing to increased reduction of Fe(III) minerals (Kappler et al. 2014) and Cr(VI) in soils (Xu et al. 2019). Furthermore, electron shuttling has been linked to biochar-assisted reductive debromination in sediments (Chen et al. 2018). The fact that A-AC seemed to stimulate MeHg production to a larger degree than A-BC could be explained by A-AC having a higher potential for direct interspecies electron transfer as Chen et al. (2014) found when comparing biochars to previously tested ACs. To the authors’ knowledge, the connection between electron shuttling and AC induced Hg methylation in sediments has not yet been demonstrated. The results from the present study are only indicative of such a mechanism, so future effort should be put into exploring this aspect.
4.2 Sorbent amendment efficiency
The sorption effect of both AC types for the MeHg was so strong that porewater concentrations were 1 to 2 orders of magnitude lower than those in the control, and even lower than those in the control at time zero before the labile OM started stimulating MeHg formation. It should be noted that the strong sorbent effect is observed despite the presence of high porewater DOC concentrations (> 200 mg L−1; Table S1). Organic matter is known to clog pores and block potential binding sites on the sorbent surfaces (Pignatello and Xing 1996; Cornelissen et al. 2005). However, it is expected that both Hg and MeHg will be complexed with OM considering the high DOC porewater concentrations (> 200 mg L−1; Fig. 2E) and the low sulphide concentration (0.12 mg L−1; Table 1) (Ravichandran 2004), resulting in co-sorption of Hg and OM. It has previously been shown that Hg-OM and MeHg-OM complexes have lower partitioning coefficients to AC than free Hg(II) and MeHg as complexation limits HgS precipitation on AC surfaces (Schwartz et al. 2019). Size exclusion of large Hg-OM and MeHg-DOC complexes from small pores should also be expected (Bjelopavlic et al. 1999). The activated biochar has a higher volume of extremely small nano/micropores (3.5–15 Å) compared to the A-AC, that has the majority of the pore volume in the higher micropore and mesopore range (> 15 Å). Considering that both sorbents performed equally well in removing porewater MeHg, size exclusion of MeHg-OM complexes from narrow pores does not appear to be a limiting factor for sorbent efficiency in this case.
Strong binding of MeHg to AC and biochar has previously been reported. Gomez-Eyles et al. (2013) observed strong binding of MeHg to both AC and biochar, with an up to 92% predicted reduction in porewater concentrations upon biochar amendment. Biochar was only effective for MeHg, not for THg. Gilmour et al. (2013) measured MeHg and Hg sorption and bioaccumulation with AC, and found that AC bound MeHg strongly, especially for sediments with intrinsically weak sorption. KD of MeHg for the whole system increased at least 1 order of magnitude at AC dosages of up to 8%. In contrast to the present study, formation of MeHg was not stimulated by the sorbent amendments — total MeHg sediment concentration remained constant. In another study by the same team (Gilmour et al. 2018), one of the first thin-layer capping field tests for Hg and MeHg, a strong effect of AC was observed after 1 month (90% reduction in porewater concentration), but this effect was not sustained for 2 years (reduced to 40% effect). Biochar, in contrast to AC, sustained a moderate effect of 40–60% reduction in porewater concentrations (Table 2).
Liu et al. (2018) carried out a 500-day microcosm experiment including pyrosequencing to study the microorganisms present. They observed clearly lower MeHg in porewater in the presence of AC and various biochar amendments. Overall KD of the amended systems was strongly variable (2.3 to 4.3 log L kg−1), similar to what was observed in the present study (2.9–5.0 log L kg−1). There was no clear pattern of stimulation of MeHg formation by the amendments — MeHg sediment concentrations were not significantly different across controls and amendments and ranged from 8 to 35 µg kg−1 and are rather similar to values observed in the control of the present study (0.9 ± 0.2—38 ± 8 µg kg−1; Table S1). However, some spikes were observed, especially for the high-temperature biochars, up to 260 µg kg−1. Such spikes are in line with observations in the amended samples in the present study.
Observations of stimulation of MeHg formation in the sediment (Sect. 4.1) at the same time as removal of the majority of MeHg from the porewater by the amendments are mirrored by the trends seen in two studies on inundated anaerobic rice paddy soils. Shu et al. (2016) observed that non-activated straw biochar reduced MeHg uptake by rice grains (49–92%), even though more MeHg was formed (20–80% more). Thus, MeHg was strongly bound to biochar (MeHg in overlying water was 95% lower) and diluted in the plants because of stimulation of crop production by 35–79%. Overall, KD between solid and overlying water was a factor 10 higher with biochar, offsetting the 20–80% stimulation of MeHg formation. Similar to this study, Zhang et al. (2018) observed that biochar reduced MeHg levels in rice plants by 60% due to 64–99% lower bioavailability (as measured by thiosulfate extraction), even though soil MeHg levels were increased by 5–75%.
All in all, the latter two rice paddy studies observed a stimulation of MeHg formation by sorbent amendment in the same order of magnitude as the present study. However, the overall remediation effect was less strong for MeHg in these studies (50–90%) than in the present study (> 95%), probably because non-activated biochars were used, in contrast to the activated materials deployed in the present study. Activation usually leads to an increase in sorption strength of around 1 order of magnitude for hydrophobic organic compounds (Kupryianchyk et al. 2016).
Even though this study and others have shown that AC amendment can significantly reduce MeHg porewater concentrations, the accompanying increase of total MeHg contents in the sediment could be an issue of concern. Whether an AC amendment will be able to sufficiently counter such an increase over time by sorbing new MeHg desorbing from the sediment will depend on multiple factors: the total sorption capacity of the AC, the availability of substrates that drive methylation in the sediment, and the extent of degradation of OM and subsequent release of MeHg associated with it. Long-term studies (> 12 months) or modelling such scenarios might provide the insight needed to properly understand the limitations of AC amendment of Hg-contaminated sediment.
Given these considerations, the second hypothesis (H2) was confirmed: the AC sorbents efficiently reduced porewater MeHg concentrations, but further studies are needed to increase the understanding of to what extent AC increase Hg-methylation in sediments, and how this will affect sorbent performance in the long term.
5 Conclusion
Under conditions with high DOC-concentrations, that lead to complexation with MeHg and probably reduce the sorption of MeHg due to size exclusion from micropores in the sorbents, the A-AC and A-BC amendments were still able to strongly reduce the porewater concentration of MeHg. However, these carbonaceous sorbents also seemed to contribute to an increase in the total MeHg-contents in the sediment over time. It is speculated that the effect is mainly due to the AC amendments assisting the methylation processes through electron shuttling effects, rather than the direct addition of labile OM. Future research efforts should be directed towards exploring the potential mechanisms and conditions that might allow carbonaceous sorbents to assist Hg-methylating bacteria.
It should be noted that in addition to the mechanistic insight this study provides, it demonstrates what could happen in a system where OM is in excess, providing good conditions for bacterial methylation of Hg, that should be considered representative either for an organic and sulphate rich sediment, or for a scenario where large algal blooms provide such a carbon and nutrient source to a more OM poor sediment. During an event like this, there is also a risk of excess OM in the porewater attenuating the effect of the sorbent.
These findings demonstrate that caution is warranted when considering the use of carbonaceous sorbents for Hg-contaminated sediment remediation. The optimal amendment scenario to avoid the formation of MeHg would therefore be a solution where the sorbents are not in direct contact with the Hg-contamination in the sediment, thus limiting transport out of the sediment, but not contributing to increased MeHg formation in the contaminated sediment. Thin capping with a layer of active sorbents (Cornelissen et al. 2012; Patmont et al. 2015) may in the case of Hg contamination be a better solution than complete mixing of sorbent and sediment (Ghosh et al. 2011).
Finally, this study has shown that biomass-based A-BC outperformed traditional fossil-based A-AC, as A-BC was equally effective as A-AC in MeHg removal from porewater, while leading to a smaller increase in sediment MeHg. Considering in addition that biochars offer a more sustainable sorbent remediation alternative in a life cycle perspective (Sparrevik et al. 2011; Alhashimi and Aktas 2017), activated biochars might be the best alternatives for sorbent-assisted remediation of Hg-contaminated sediments.
Data availability
All raw data produced for this study is included in the supplementary material.
References
Alhashimi HA, Aktas CB (2017) Life cycle environmental and economic performance of biochar compared with activated carbon: a meta-analysis. Resour Conserv Recycl 118:13–26. https://doi.org/10.1016/j.resconrec.2016.11.016
Amirbahman A, Massey DI, Lotufo G, Steenhaut N, Brown LE, Biedenbach JM et al (2013) Assessment of mercury bioavailability to benthic macroinvertebrates using diffusive gradients in thin films (DGT). Environ Sci Process Impacts 15:2104–2114. https://doi.org/10.1039/C3EM00355H
Amstaetter K, Eek E, Cornelissen G (2012) Sorption of PAHs and PCBs to activated carbon: coal versus biomass-based quality. Chemosphere 87:573–578. https://doi.org/10.1016/j.chemosphere.2012.01.007
Bjelopavlic M, Newcombe G, Hayes R (1999) Adsorption of NOM onto activated carbon: effect of surface charge, ionic strength, and pore volume distribution. J Colloid Interface Sci 210:271–280. https://doi.org/10.1006/jcis.1998.5975
Bloom N, Colman J, Barber L (1997) Artifact formation of methyl mercury during aqueous distillation and alternative techniques for the extraction of methyl mercury from environmental samples. Fresenius J Anal Chem 358:371–377. https://doi.org/10.1007/s002160050432
Braaten HFV, de Wit HA, Fjeld E, Rognerud S, Lydersen E, Larssen T (2014) Environmental factors influencing mercury speciation in subarctic and boreal lakes. Sci Total Environ 476–477:336–345. https://doi.org/10.1016/j.scitotenv.2014.01.030
Braaten HFV, Gundersen CB, Beylich B, Håvardstun J, Carlsson P, Bryntesen T et al (2019) Oppfølgingsundersøkelse av kvikksølv i fisk fra Gunneklevfjorden og nærliggende referanseinnsjøer. NIVA-rapport; 7371. Norwegian Institute for Water Research (NIVA)
Bussan DD, Sessums RF, Cizdziel JV (2016) Activated carbon and biochar reduce mercury methylation potentials in aquatic sediments. Bull Environ Contam Toxicol 96:536–539
Canfield DE, Thamdrup B, Hansen JW (1993) The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim Cosmochim Acta 57:3867–3883. https://doi.org/10.1016/0016-7037(93)90340-3
Carr RS, Nipper M, Adams WJ, Berry WJ, Burton Jr GA, Ho K et al (2001) Summary of a SETAC technical workshop. Porewater toxicity testing: biological, chemical, and ecological considerations with a review of methods and applications, and recommendations for future areas of research. In: Carr RS, Nipper M, Pensacola FL (eds), pp 38
Chen J, Wang C, Pan Y, Farzana SS, Tam NF-Y (2018) Biochar accelerates microbial reductive debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in anaerobic mangrove sediments. J Hazard Mater 341:177–186. https://doi.org/10.1016/j.jhazmat.2017.07.063
Chen S, Rotaru A-E, Shrestha PM, Malvankar NS, Liu F, Fan W et al (2014) Promoting interspecies electron transfer with biochar. Sci Rep 4:5019. https://doi.org/10.1038/srep05019
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Cornelissen G, Amstaetter K, Hauge A, Schaanning M, Beylich B, Gunnarsson JS et al (2012) Large-scale field study on thin-layer capping of marine PCDD/F-contaminated sediments in Grenlandfjords, Norway: physicochemical effects. Environ Sci Technol 46:12030–12037. https://doi.org/10.1021/es302431u
Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895. https://doi.org/10.1021/es050191b
Dai S-S, Yang Z, Tong Y, Chen L, Liu S-Y, Pan R et al (2021) Global distribution and environmental drivers of methylmercury production in sediments. J Hazard Mater 407:124700. https://doi.org/10.1016/j.jhazmat.2020.124700
Dell’Anno A, Beolchini F, Gabellini M, Rocchetti L, Pusceddu A, Danovaro R (2009) Bioremediation of petroleum hydrocarbons in anoxic marine sediments: consequences on the speciation of heavy metals. Mar Pollut Bull 58:1808–1814
Fernandez-Luqueno F, López-Valdez F, Gamero-Melo P, Luna-Suárez S, Aguilera-González EN, Martínez AI et al (2013) Heavy metal pollution in drinking water-a global risk for human health: A review. Afr J Environ Sci Technol 7:567–584
Fick A (1855) V. On liquid diffusion. London Edinburgh Dublin Phil Mag J Sci 10:30–39. https://doi.org/10.1080/14786445508641925
Ghosh U, Luthy RG, Cornelissen G, Werner D, Menzie CA (2011) In-situ sorbent amendments: a new direction in contaminated sediment management. Environ Sci Technol 45:1163–1168. https://doi.org/10.1021/es102694h
Gilmour C, Bell T, Soren A, Riedel G, Riedel G, Kopec D et al (2018) Activated carbon thin-layer placement as an in situ mercury remediation tool in a Penobscot River salt marsh. Sci Total Environ 621:839–848. https://doi.org/10.1016/j.scitotenv.2017.11.050
Gilmour CC, Elias DA, Kucken AM, Brown SD, Palumbo AV, Schadt CW et al (2011) Sulfate-reducing bacterium desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Appl Environ Microbiol 77:3938–3951. https://doi.org/10.1128/aem.02993-10
Gilmour CC, Riedel GS, Riedel G, Kwon S, Landis R, Brown SS et al (2013) Activated carbon mitigates mercury and methylmercury bioavailability in contaminated sediments. Environ Sci Technol 47:13001–13010
Gomez-Eyles JL, Yupanqui C, Beckingham B, Riedel G, Gilmour C, Ghosh U (2013) Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environ Sci Technol 47:13721–13729
Hagemann N, Spokas K, Schmidt H-P, Kägi R, Böhler AM, Bucheli DT (2018) Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water 10. https://doi.org/10.3390/w10020182
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495. https://doi.org/10.1021/es034528q
Hintelmann H, Keppel-Jones K, Evans RD (2000) Constants of mercury methylation and demethylation rates in sediments and comparison of tracer and ambient mercury availability. Environ Toxicol Chem 19:2204–2211. https://doi.org/10.1002/etc.5620190909
Johnson NW, Reible DD, Katz LE (2010) Biogeochemical changes and mercury methylation beneath an in-situ sediment cap. Environ Sci Technol 44:7280–7286
Joseph S, Husson O, Graber ER, Van Zwieten L, Taherymoosavi S, Thomas T et al (2015) The electrochemical properties of biochars and how they affect soil redox properties and processes. Agronomy 5. https://doi.org/10.3390/agronomy5030322
Kappler A, Wuestner ML, Ruecker A, Harter J, Halama M, Behrens S (2014) Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ Sci Technol Lett 1:339–344. https://doi.org/10.1021/ez5002209
King JK, Saunders FM, Lee RF, Jahnke RA (1999) Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environ Toxicol Chem 18:1362–1369. https://doi.org/10.1002/etc.5620180704
Kong H, He J, Gao Y, Wu H, Zhu X (2011) Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. J Agric Food Chem 59:12116–12123
Kumar M, Singh AK, Sikandar M (2020) Biosorption of Hg (II) from aqueous solution using algal biomass: kinetics and isotherm studies. Heliyon 6:e03321. https://doi.org/10.1016/j.heliyon.2020.e03321
Kupryianchyk D, Hale S, Zimmerman AR, Harvey O, Rutherford D, Abiven S et al (2016) Sorption of hydrophobic organic compounds to a diverse suite of carbonaceous materials with emphasis on biochar. Chemosphere 144:879–887
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of biochar in soil. In: Lehman J, Joseph S (ed) Biochar for environmental management: science and technology, 2nd edn. Routledge, New York, pp 183–206
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation, 2nd edn, Routledge, New York
Li Z, Wu L, Liu H, Lan H, Qu J (2013) Improvement of aqueous mercury adsorption on activated coke by thiol-functionalization. Chem Eng Sci 228:925–934. https://doi.org/10.1016/j.cej.2013.05.063
Liu F, Rotaru A-E, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR (2012) Promoting direct interspecies electron transfer with activated carbon. Energy Environ Sci 5:8982–8989. https://doi.org/10.1039/C2EE22459C
Liu P, Ptacek CJ, Blowes DW, Gould WD (2018) Control of mercury and methylmercury in contaminated sediments using biochars: a long-term microcosm study. Appl Geochemistry 92:30–44
Lutz MA, Brigham ME, Marvin-DiPasquale MC (2008) Procedures for collecting and processing streambed sediment and porewater for analysis of mercury as part of the national water-quallity assessment program. USGS Open-File Report 2008-1279. https://pubs.usgs.gov/of/2008/1279/. Accessed 12 Jan 2021
Magos L, Brown A, Sparrow S, Bailey E, Snowden R, Skipp W (1985) The comparative toxicology of ethyl-and methylmercury. Arch Toxicol 57:260–267
Marsh H, Reinoso FR (2006) Chapter 5 activation processes (thermal or physical), in Marsh H, Reinoso FR (ed) Activated carbon, 1st edn. Elsevier Science Books, pp 243–321
Merritt KA, Amirbahman A (2009) Mercury methylation dynamics in estuarine and coastal marine environments — A critical review. Earth Sci Rev 96:54–66. https://doi.org/10.1016/j.earscirev.2009.06.002
Ndungu K, Schaanning M, Braaten HFV (2016) Effects of organic matter addition on methylmercury formation in capped and uncapped marine sediments. Water Res 103:401–407. https://doi.org/10.1016/j.watres.2016.07.055
Olsen M, Fjeld E, Lydersen E (2019) The influence of a submerged meadow on uptake and trophic transfer of legacy mercury from contaminated sediment in the food web in a brackish Norwegian fjord. Sci Total Environ 654:209–217
Olsen M, Schaanning MT, Braaten HFV, Eek E, Moy FE, Lydersen E (2018) The influence of permanently submerged macrophytes on sediment mercury distribution, mobility and methylation potential in a brackish Norwegian fjord. Sci Total Environ 610–611:1364–1374. https://doi.org/10.1016/j.scitotenv.2017.08.136
Patmont CR, Ghosh U, LaRosa P, Menzie CA, Luthy RG, Greenberg MS et al (2015) In situ sediment treatment using activated carbon: a demonstrated sediment cleanup technology. Integr Environ Assess Manag 11:195–207. https://doi.org/10.1002/ieam.1589
Peacock M, Freeman C, Gauci V, Lebron I, Evans CD (2015) Investigations of freezing and cold storage for the analysis of peatland dissolved organic carbon (DOC) and absorbance properties. Environ Sci Process Impacts 17:1290–1301. https://doi.org/10.1039/C5EM00126A
Pignatello JJ, Xing B (1996) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30:1–11. https://doi.org/10.1021/es940683g
Ravichandran M (2004) Interactions between mercury and dissolved organic matter––a review. Chemosphere 55:319–331. https://doi.org/10.1016/j.chemosphere.2003.11.011
Regnell O, Watras CJ (2019) Microbial mercury methylation in aquatic environments: a critical review of published field and laboratory studies. Environ Sci Technol 53:4–19. https://doi.org/10.1021/acs.est.8b02709
Schreiber B, Brinkmann T, Schmalz V, Worch E (2005) Adsorption of dissolved organic matter onto activated carbon—the influence of temperature, absorption wavelength, and molecular size. Water Res 39:3449–3456. https://doi.org/10.1016/j.watres.2005.05.050
Schwartz GE, Sanders JP, McBurney AM, Brown SS, Ghosh U, Gilmour CC (2019) Impact of dissolved organic matter on mercury and methylmercury sorption to activated carbon in soils: implications for remediation. Environ Sci Process Impacts 21:485–496. https://doi.org/10.1039/C8EM00469B
Shu R, Wang Y, Zhong H (2016) Biochar amendment reduced methylmercury accumulation in rice plants. J Hazard Mater 313:1–8. https://doi.org/10.1016/j.jhazmat.2016.03.080
Spain O, Plöhn M, Funk C (2021) The cell wall of green microalgae and its role in heavy metal removal. Physiol Plant 173:526–535. https://doi.org/10.1111/ppl.13405
Sparrevik M, Saloranta T, Cornelissen G, Eek E, Fet AM, Breedveld GD et al (2011) Use of life cycle assessments to evaluate the environmental footprint of contaminated sediment remediation. Environ Sci Technol 45:4235–4241. https://doi.org/10.1021/es103925u
Ting Y, Chen C, Ch’ng B-L, Wang Y-L, Hsi H-C (2018) Using raw and sulfur-impregnated activated carbon as active cap for leaching inhibition of mercury and methylmercury from contaminated sediment. J Hazard Mater 354:116–124. https://doi.org/10.1016/j.jhazmat.2018.04.074
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293. https://doi.org/10.1080/20016491089226
Xu Z, Xu X, Tao X, Yao C, Tsang DCW, Cao X (2019) Interaction with low molecular weight organic acids affects the electron shuttling of biochar for Cr(VI) reduction. J Hazard Mater 378:120705. https://doi.org/10.1016/j.jhazmat.2019.05.098
Yuan H-Y, Ding L-J, Zama EF, Liu P-P, Hozzein WN, Zhu Y-G (2018) Biochar modulates methanogenesis through electron syntrophy of microorganisms with ethanol as a substrate. Environ Sci Technol 52:12198–12207. https://doi.org/10.1021/acs.est.8b04121
Yuan Y, Bolan N, Prévoteau A, Vithanage M, Biswas JK, Ok YS et al (2017) Applications of biochar in redox-mediated reactions. Bioresour Technol 246:271–281. https://doi.org/10.1016/j.biortech.2017.06.154
Zhang Y, Liu Y-R, Lei P, Wang Y-J, Zhong H (2018) Biochar and nitrate reduce risk of methylmercury in soils under straw amendment. Sci Total Environ 619–620:384–390. https://doi.org/10.1016/j.scitotenv.2017.11.106
Acknowledgements
Dr. Sarah E. Hale, the project manager of NFR 243789, is acknowledged for allocating the time of her postdoctoral researcher, and Dr. Ludovica Silvani to contribute to this study.
Funding
Open Access funding provided by Norwegian Geotechnical Institute. The authors received funding from the Norwegian Research Council (NFR) through the Klimaforsk project “Biochar as an adaptation strategy for climate change” (NFR 243789); the joint-industry sustainability (BIA-X) project “Valorization of Organic Waste into Sustainable Products for Clean-up of Contaminated Water, Soil, and Air” (VOW) (NFR 299070); and the NGI base funding.
Author information
Authors and Affiliations
Contributions
Erlend Sørmo: Funding acquisition, conceptualization, methodology, investigation, formal analysis, writing — original draft, writing — review and editing. Ludovica Silvani: Investigation, writing — review and editing. Hans Fredrik Veiteberg Braaten: Methodology, writing — review and editing. Tina Bryntesen: Investigation. Espen Eek: Conceptualization, writing — review and editing. Gerard Cornelissen: Supervision, funding acquisition, conceptualization, methodology, writing — original draft, writing — review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Nives Ogrinc
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sørmo, E., Silvani, L., Braaten, H.V. et al. Formation and availability of methylmercury in mercury-contaminated sediment: effects of activated carbon and biochar amendments. J Soils Sediments 22, 1041–1053 (2022). https://doi.org/10.1007/s11368-021-03134-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03134-3