Abstract
Purpose
The method using persulfate (PS) activation is effective for the remediation of organic pollutants contaminated soil. This study aims to evaluate the possibility of the application of green synthesized nanoscale zero valent iron (nZVI) activating PS for 1,2-dichlorobenzene (1,2-DCB) degradation in soil, investigate the effect of environmental factors such as nZVI dosage, PS concentration, and initial pH on 1,2-DCB removal efficiency and interpret the activation mechanism of nZVI for PS and degradation pathways of 1,2-DCB in soil.
Materials and methods
The contaminated soil was added to a brown bottle of 250 mL and dispersed in deionized water with a volume ratio of 1:5. Specified concentration of PS and nZVI were added to the bottle to initiate the degradation reaction. The bottle was placed on a reciprocating shaker at room temperature of 25 ºC. Control experiments containing PS or nZVI were also performed under the same reaction conditions. At desired time intervals, the well-mixed suspension of 10 mL slurry was transferred into 50 mL glass vials and centrifuged. 20 mL organic solvent with a volumetric ratio of hexane to acetone of 1:1 was added to 50 mL glass vials after the supernatant was discarded. 1,2-DCB and its degradation intermediates were analyzed by Gas Chromatograph-Mass Spectrometer (GC-MS) through extract.
Results and discussion
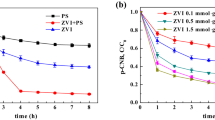
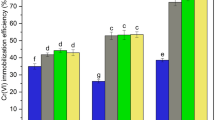
The maximum degradation efficiency of 97.3% with total organic carbon (TOC) removal of 61.3% for 1,2-DCB was achieved under the reaction conditions of initial 1,2-DCB concentration of 28.6 mg kg−1 in soil, 67.2 mg L−1 nZVI, 1.2 mmol L−1 PS and pH 7.5. Electron paramagnetic resonance (EPR) test indicated that SO4•− and •OH radicals were the dominant species being responsible for the degradation of 1,2-DCB. In addition, the GC-MS analysis showed that 3,4-dichlorophenol and o-chlorophenol were the primary intermediates, and the degradation pathways of 1,2-DCB were proposed subsequently.
Conclusions
nZVI was successfully synthesized through green processes based on extracted tea polyphenol, and utilized for the remediation of 1,2-DCB contaminated soil by activated PS. The generated free radicals were responsible for the efficient degradation of 1,2-DCB with high TOC removal. The findings might have significant implications for the remediation of 1,2-DCB contaminated soil utilizing the nZVI/PS system.
Graphical abstract







Similar content being viewed by others
References
Al-Shamsi MA, Thomson NR (2013) Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron. Ind Eng Chem Res 52:13564–13571
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Antizar-Ladislao B, Lopez-Real J, Beck AJ (2005) Laboratory studies of the remediation of polycyclic aromatic hydrocarbon contaminated soil by in-vessel composting. Waste Manage 25:281–289
Ayodhya D, Venkatesham M, Kumari AS, Reddy GB, Ramakrishna D, Veerabhadram G (2016) Photocatalytic degradation of dye pollutants under solar, visible and UV lights using green synthesised CuS nanoparticles. J Exp Nanosci 11:418–432
Burbano AA, Dionysiou DD, Suidan MT, Richardson TL (2005) Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res 39:107–118
Cano M, Guarín F, Aristizábal B, Villa AL, González LM (2017) Catalytic activity and stability of Pd/Co catalysts in simultaneous selective catalytic reduction of NOx with methane and oxidation of o-dichlorobenzene. Catal Today 296:105–117
Chen H, Liu S (2015) Cooperative adsorption based kinetics for dichlorobenzene dechlorination over Pd/Fe bimetal. Chem Eng Sci 138:510–515
Chen Z, Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2013) Multifunctional kaolinite supported nanoscale zero-valent iron used for the adsorption and degradation of crystal violet in aqueous solution. J Colloid Interface Sci 398:59–66
Conte P, Agretto A, Spaccini R, Piccolo A (2005) Soil remediation: humic acids as natural surfactants in the washings of highly contaminated soils. Environ Pollut 135:515–522
Conte P, Zena A, Pilidis G, Piccolo A (2001) Increased retention of polycyclic aromatic hydrocarbons in soils induced by soil treatment with humic substances. Environ Pollut 112:27–31
EPA U (1998) National pollutant discharge elimination system, Washington, DC
Fang GD, Dionysiou DD, Al-Abed SR, Zhou DM (2013) Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs. Appl Catal B: Environ 129:325–332
Furman OS, Teel AL, Watts RJ (2010) Mechanism of base activation of persulfate. Environ Sci Technol 44:6423–6428
Ghauch A, Ayoub G, Naim S (2013) Degradation of sulfamethoxazole by persulfate assisted micrometric Fe0 in aqueous solution. Chem Eng J 228:1168–1181
Hoag GE, Collins JB, Holcomb JL, Hoag JR, Nadagouda MN, Varma RS (2009) Degradation of bromothymol blue by “greener” nano-scale zero-valent iron synthesized using tea polyphenols. J Mater Chem 19:8671–8677
Hoigné J, Bader H (1976) The role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res 10:377–386
Jalil AA, Triwahyono S, Razali NAM, Hairom NHH, Idris A, Muhid MNM, Ismail A, Yahaya NAM, Ahmad NAL, Dzinun H (2010) Complete electrochemical dechlorination of chlorobenzenes in the presence of various arene mediators. J Hazard Mater 174:581–585
Li Q, Chen ZS, Wang HH, Yang H, Wen T, Wang SQ, Hu BW, Wang XK (2021) Removal of organic compounds by nanoscale zero-valent iron and its composites
Liang C, Bruell CJ, Marley MC, Sperry KL (2004a) Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 55:1213–1223
Liang C, Bruell CJ, Marley MC, Sperry KL (2004b) Persulfate oxidation for in situ remediation of TCE. II. Activated by Chelated Ferrous Ion Chemosphere 55:1225–1233
Liang C, Lai MC (2008) Trichloroethylene degradation by zero valent iron activated persulfate oxidation. Environ Eng Sci 25:1071–1078
Liang C, Lee IL, Hsu IY, Liang CP, Lin YL (2008) Persulfate oxidation of trichloroethylene with and without iron activation in porous media. Chemosphere 70:426–435
Liang C, Wang ZS, Bruell CJ (2007) Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 66:106–113
Liang LP, Xi FF, Tan WS, Meng X, Hu BW, Wang XK (2021) Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 3:255–281
Lin HF, Ravikrishna R, Valsaraj KT (2002) Reusable adsorbents for dilute solution separation. 6. Batch and continuous reactors for the adsorption and degradation of 1,2-dichlorobenzene from dilute wastewater streams using titania as a photocatalyst. Sep Purif Technol 28:87–102
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C (2015) Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ 533:76–81
Mondal P, Anweshan A, Purkait MK (2020) Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 259:127509
Nasiri J, Motamedi E, Naghavi MR, Ghafoori M (2019) Removal of crystal violet from water using β-cyclodextrin functionalized biogenic zero-valent iron nanoadsorbents synthesized via aqueous root extracts of Ferula persica. J Hazard Mater 367:325–338
Neta P, Madhavan V, Zemel H, Fessenden RW (1977) Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds. J Am Chem Soc 99:163–164
Oh SY, Kang SG, Chiu PC (2010) Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci Total Environ 408:3464–3468
Ouyang Q, Kou F, Tsang PE, Lian J, Xian J, Fang J, Fang Z (2019) Green synthesis of Fe-based material using tea polyphenols and its application as a heterogeneous Fenton-like catalyst for the degradation of lincomycin. J Clean Prod 232:1492–1498
Pasinszki T, Krebsz M (2020) Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 10:917
Pasinszki T, Krebsz M, Ko´tai L, Sajo´ IR, Homonnay Z, Kuzmann E, Kiss LF, Va´czi T, Kova´cs I (2015) Nanofurry magnetic carbon microspheres for separation processes and catalysis: synthesis, phase composition, and properties. J Mater Sci 50:7353–7363
Pei G, Sun C, Zhu Y, Shi W, Li H (2018) Biosurfactant-enhanced removal of o, p-dichlorobenzene from contaminated soil. Environ Sci Pollut R 25:18–26
Peluffo M, Pardo F, Santos A, Romero A (2016) Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil. Sci Total Environ 563–564:649–656
Qiu MQ, Hu BW, Chen ZS, Yang H, Zhuang L, Wang XK (2021) Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 3:117–123
Qu JH, Liu Y, Cheng L, Jiang Z, Zhang GS, Deng FX, Wang L, Han W, Zhang Y (2021) Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms. J Hazard Mater 403:123607
Saritha P, Raj DSS, Aparna C, Laxmi PNV, Himabindu V, Anjaneyulu Y (2009) Degradative oxidation of 2,4,6 trichlorophenol using advanced oxidation processes - A comparative study. Water, Air, Soil Pollut 200:169–179
Timmins GS, Liu KJ, Bechara EJH, Kotake Y, Swartz HM (1999) Trapping of free radicals with direct in vivo EPR detection: a comparison of 5,5-dimethyl-1-pyrroline-N-oxide and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide as spin traps for HO and SO4•−. Free Radical Biol Med 27:329–333
Trellu C, Mousset E, Pechaud Y, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2016) Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J Hazard Mater 306:149–174
Tsang DCW, Graham NJD, Lo IMC (2009) Humic acid aggregation in zero-valent iron systems and its effects on trichloroethylene removal. Chemosphere 75:1338–1343
Tsitonaki A, Smets BF, Bjerg PL (2008) Effects of heat-activated persulfate oxidation on soil microorganisms. Water Res 42:1013–1022
Voelker BM, Morel FMM, Sulzberger B (1997) Iron redox cycling in surface waters: Effects of humic substances and light. Environ Sci Technol 31:1004–1011
Wang CW, Liang C (2014) Oxidative degradation of TMAH solution with UV persulfate activation. Chem Eng J 254:472–478
Xiao CY, Li HY, Zhao Y, Zhang X, Wang XY (2020) Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J Environ Manage 275:111262
Xu MY, Deng J, Cai AH, Ma XY, Li J, Li QS, Li XY (2020) Comparison of UVC and UVC/persulfate processes for tetracycline removal in water. Chem Eng J 384:123320
Yan J, Gao W, Dong M, Han L, Qian L, Nathanail CP, Chen M (2016) Degradation of trichloroethylene by activated persulfate using a reduced graphene oxide supported magnetite nanoparticle. Chem Eng J 295:309–316
Yan J, Han L, Gao W, Xue S, Chen M (2015) Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour Technol 175:269–274
Yang J, Guo Y, Xu D, Cao L, Jia J (2012) A controllable Fe0-C permeable reactive barrier for 1,4-dichlorobenzene dechlorination. Chem Eng J 203:166–173
Yu SJ, Pang HW, Huang SY, Tang H, Wang SQ, Qiu MQ, Chen ZS, Yang H, Song G, Fu D, Hu BW, Wang XX (2021) Recent advances in metal-organic framework membranes for water treatment: A review. Sci Total Environ 800:149662
Zazou H, Oturan N, Sönmez Çelebi M, Hamdani M, Oturan MA (2019) Cold incineration of 1,2-dichlorobenzene in aqueous solution by electrochemical advanced oxidation using DSA/Carbon felt, Pt/Carbon felt and BDD/Carbon felt cells. Sep Purif Technol 208:184–193
Zhang H, Ji L, Wu F, Tan J (2005) In situ ozonation of anthracene in unsaturated porous media. J Hazard Mater 120:143–148
Zhang S, Wang JQ, Zhang Y, Ma JZ, Huang LTY, Yu SJ, Chen L, Song G, Qiu MQ, Wang XX (2021) Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ Pollut 291:118076
Zhu C, Fang G, Dionysiou DD, Liu C, Gao J, Qin W, Zhou D (2016) Efficient transformation of DDTs with persulfate activation by zero-valent iron nanoparticles: A mechanistic study. J Hazard Mater 316:232–241
Zhu C, Zhu F, Wang F, Gao J, Fan G, Zhou D, Fang G (2017) Comparison of persulfate activation and Fenton reaction in remediating an organophosphorus pesticides-polluted soil. Pedosphere 27:465–474
Acknowledgements
This work was financially supported by National Key Research and Development Programme (Grant Nos. 2019YFC1804002 and 2018YFC1803000) and National Natural Science Foundation of China (Grant No. 42077181).
Funding
National Key Research and Development Programme, 2019YFC1804002, Jinchun Yan, 2018YFC1803000, Mengfang Chen, National Natural Science Foundation of China, 42077181, Jinchun Yan
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Huijun Zhao
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• nZVI was synthesized through green processes using extracted tea polyphenol
• Efficient degradation of 1,2-DCB in soil was observed in the nZVI/PS system
• SO4• − and •OH radicals were accounted for 1,2-DCB degradation
• The intermediates and degradation pathways of 1,2-DCB were interpreted
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, J., Hu, L., Gao, W. et al. Remediation of 1,2-dichlorobenzene contaminated soil by activated persulfate using green synthesized nanoscale zero valent iron: activation mechanism and degradation pathways. J Soils Sediments 22, 1135–1144 (2022). https://doi.org/10.1007/s11368-021-03116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-021-03116-5