Abstract
Purpose
The study aimed to identify the environmental hotspots of lab-scale preparation of high purity porous Al2O3 pellets with suitable feature to work properly as metal layer-based deposition substrates for hydrogen separation membranes. The work intention was providing hints that may help the designing of upscaled systems, fundamental for the development of a possible future industrial production of hydrogen separation metal layer-based membranes technology.
Methods
The goal of this study was achieved assessing and analyzing environmental impacts of Al2O3 pellet production at lab scale. Primary data were collected in Padua laboratories of National Research Council of Italy. Secondary data were retrieved from Ecoinvent 3.7 database. Life cycle assessment (LCA) was performed using Environmental Footprint 3.0 method employing SimaPro 9.3 as software. Moreover, the CML LCIA method v. 4.7 was used to verify the robustness analysis of characterized results.
Results
Life cycle impact assessment highlighted as the main driver of environmental impacts was mainly associated to the pellet consolidation process and their morphological characterization stage. In particular, the impact of the first energy consuming process resulted strictly related to the peculiar energy mix used (linked to the laboratory geographical location). Conversely, morphological characterization stage was found to affect mainly the mineral resource depletion category due to the Au coating used for performing scanning electron microscope (SEM) analyses.
Conclusions
The study identified the environmental hotspots related to lab-scale preparation of porous alumina pellets as substrate for hydrogen separation metal layer-based membranes. The optimization strategies evaluated in this work were addressed to improve the environmental profile of experimental activities considering several scenarios, in view of a possible industrial scale-up.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The strategy outlined in the European Green Deal considers the hydrogen technology as a key technology for the energy transition towards a low-carbon economy. Nowadays, hydrogen is mainly obtained by steam reforming of natural gas (Dincer and Acar 2014), although the biomass gasification seems to be gradually a more forthcoming prospective (Demirbas 2008; Hosseini et al. 2015). In both cases, the H2-rich gas mixture contains several by-products (carbon oxides, sulfides, and hydrides), which must be removed through purification and separation steps.
At industrial scale, separation of gas mixtures present in the syn gas is usually carried out by doing multiple consecutive cycles of steps, which involves gas absorption and adsorption, cryogenics, and membrane technologies (Scott 1995; Bernardo et al. 2009). For instance, pressure swing adsorption or cryogenic separation systems can be coupled with molecular sieves, exploiting the different physical behavior of gasses at different pressures and temperatures. The continuous alternation of conditions allows to increase gas purity (Scott 1995).
Between the several continuous hydrogen separation strategies, metal-based membrane technology is considered one of the most promising, permitting to simplify the whole process, limiting working steps, and decreasing energy consumption and costs (Gallucci et al. 2013).
Nowadays, metal membranes are mainly composed by palladium alloys, as, for example, Pd–Ag, Pd-Cu, and Pd-Au (Hatlevik et al. 2010; Yun and Ted Oyama 2011; Barison et al. 2018; Lee et al. 2021). Palladium is the preferred metal for this application, due to its capability to catalyze molecular hydrogen breaking and dissolution in its lattice. Dense metal membranes can allow to achieve infinite hydrogen selectivity with respect to helium or nitrogen (Tong et al. 2005; Huang and Dittmeyer 2006; Kim et al. 2022).
The drawback of this metal is related to its criticality. In fact, the high content of critical raw materials represents one of the main problems for a large-scale employment of such membranes. The two main strategies to decrease the critical raw material use are the substitution of the critical elements (Fasolin et al. 2022) and the membrane designing based on a thin selective metallic layer on top of a supporting porous inert substrate. This latter configuration is depicted in Fig. 1: several molecules of the syn gas are physically blocked by the membrane. The adsorbed hydrogen molecules on the metallic surface can be dissociated by Pd. Thus, the absorbed hydrogen atoms can diffuse through the entire membrane, desorbing and recombining inside the alumina pores. The hydrogen molecules can be finally released by the membrane driven by the pressure difference.
In this context, porous supports play an important role on the development of these kind of membranes. In fact, the metallic membrane density is strictly related to substrate surface morphology, and the substrate porosity strongly affects the hydrogen permeation. Among the tested substrates for hydrogen separation membranes (Xomeritakis and Lin 1997; Yun and Ted Oyama 2011; Li et al. 2019; Lu et al. 2020), alumina is considered an excellent material being mechanically and chemically stable in operating conditions. Moreover, using alumina as substrate, the interdiffusion phenomena typical in the case of steel substrate at high temperatures are avoided, thus ensuring the hydrogen permeation (Ayturk et al. 2007). A previous work on membrane substrate showed the experimental procedure to achieve alumina pellet disks with fine surface porosity, ideal for deposition of a dense micrometric metallic layer with no need of any interlayer, that would increase the processing time, costs, and complexity (Barison et al. 2018). In order to evaluate the environmental impacts of the membrane substrate production, one of the most appropriate methods is life cycle assessment (LCA), a structured and internationally standardized method to quantify all relevant emissions and consumed resources (ISO 14040–14,044). LCA analysis may assess all the related environmental, health impacts, and resource depletion issues related to products or processes. LCA may cover the whole chain (from the extraction of resources, production, use, recycling, up to the disposal of the remaining waste), or only a part of it: “cradle to grave” or “cradle to gate” approach, respectively.
In the last years, life cycle studies of laboratory activities have been assuming an important role in scientific literature, focusing both on emerging technologies (Pallas et al. 2020) and on consolidated synthesis routes (Bauer et al. 2008; Fiameni et al. 2020). The main aim of a life cycle analysis at lab scale is to identify the possible environmental hotspots of new technologies which are going to be developed at industrial scale (Walser et al. 2011; Villares et al. 2016; Pallas et al. 2020). Indeed, previous works showed that 70% of the total environmental impact can be related to the early development phases of a new process (Jeswiet and Hauschild 2005). In particular, for emerging technologies at low technology readiness levels (TRL), lab-scale production was considered to be a crucial step to identify the most relevant environmental hotspots that may strongly affect pilot and industrial processes (Pérez-López et al. 2014; Elginoz et al. 2020).
The goal of this work was to evaluate the environmental footprint of high purity porous alumina pellets as suitable for supporting metallic selective film of hydrogen separation membranes. Optimal synthesis conditions which can minimize impacts were identified by analyzing several scenarios which provided useful information in view of a possible industrial scale-up of the process.
To the best of our knowledge, similar studies discussing the LCA of porous alumina pellet preparation are not reported in literature, neither at lab nor industrial scale. For these reasons, the results of this work may represent the basis for planning environmentally sustainable large-scale production of alumina-based devices for metallic membranes for hydrogen production.
2 Methods
2.1 System description: preparation of alumina porous pellets
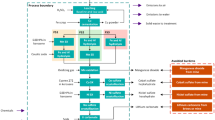
The main steps and the system boundaries of the whole process for obtaining optimized porous alumina pellets are depicted in Fig. 2.
It can be divided into two main phases: the “experimental trials” and the “pellet production.” The first phase (described in the inventory paragraph 3.7 “Experimental trials phase”) is aimed at identifying the optimized experimental conditions appropriate for carrying out the second one (treated in Sect. 3.8 inventory paragraph and showed in Fig. 3).
Main steps with related inputs and outputs of the Sect. 3.8
In particular, the “pellet production” was addressed to prepare a certain number of pellets suitable for being used as substrate for deposition process of the active metal layer (Fasolin et al. 2018, 2022; Barison et al. 2018). This latter stage was not considered in this work, being out of the system boundaries.
In the “experimental trials” phase, α-Al2O3 and polymethyl methacrylate powders (PMMA) were ball milled testing deionized water, ethanol, and isopropanol as milling medium. Zirconia jars of 45 mL and zirconia balls with diameter of 5 and 10 mm were used for the process. Later, increased powder volumes were treated using of 250 mL jar, while the use of isopropanol was excluded, since this solvent behavior seemed not so different from the ethanol one (for further details, see the inventory paragraph 3.1 “Milling and drying”). The experimental set-ups and conditions of this stage are listed in Table 1.
Milled powders were then dried and uniaxially pressed and sintered in furnace obtaining consolidated pellets. After the free cooling process, the sintered disks were polished and cleaned in an ultrasonic bath of acetone (for further details, see the inventory paragraphs Sect. 3.2 and Sect. 3.3).
Field emission secondary electron microscopy (FE-SEM) analysis permitted to verify the fine and homogenous pore size along the samples (Fasolin et al. 2018), supporting the identification of experimental conditions for producing pellets. For increasing the electrical conductivity of the pellet surfaces, samples were coated with an Au thin film (20 nm) by physical vapor deposition (PVD) direct current magnetron sputtering (for further details, see the inventory paragraph Sect. 3.5).
Figure 4 depicts a secondary electron image of a pellet with the typical porosity suitable for hydrogen permeation.
The identified optimized synthesis conditions to be used in the successive “pellet production” phase for obtaining porous alumina pellets were finally confirmed performing gas permeability tests at room temperature, 300, and 350 °C (for further details, see the inventory paragraph Sect. 3.6).
2.2 Functional units
A first life cycle impact assessment (LCIA) was carried out with a functional unit (FU) of 7.6 g of dried powder (obtained using a 45 mL jar), aiming to identify the milling medium with lower environmental impact.
The main FU, used for the LCIA of the whole process addressed to the development and fabrication of porous alumina pellets with features suitable to work properly as substrate for hydrogen separation membrane deposition, was set as the production of 40 polished disk-shaped high purity alumina pellets possessing 20 mm of diameter, thickness of 0.9 mm, and a weight of 0.9 g. It is worth noting that FU unit was chosen since, to authors experience, it is the minimum number necessary for carrying out a small experimental deposition campaign, even though this process was not considered in the boundary system of this work.
2.3 Modelling assumptions
The following assumptions were taken to model the system:
-
The infrastructures, the labor, and the instrumentations (and their maintenance) were not considered since they have a lifetime of decades and are used for many other purposes and projects. Previous literature works reporting life cycle studies similar to that proposed in this article showed that at laboratory scale, the type of instruments used is not comparable to the equipment and machineries of an industrial plant. For this reason, a LCA study at lab scale is reasonable to neglect the contribution to the impact of the infrastructures and the instrumentations manufacturing (Bauer et al. 2008; Piccinno et al. 2016, 2018; Ang et al. 2021).
-
The electrical energy consumption of weighting devices was not considered, because its contribution can be considered negligible.
-
Based on operator experience on milling processes, it was reasonable to consider the performing of the jars cleaning only at the end of the “experimental trials” phase.
-
The cleaning of jars after the “pellet production” phase was not considered due to its negligible environmental burden.
-
Unused powder obtained by the optimized milling conditions was not discarded as waste but kept for any future possible laboratory activities.
-
The wear of zirconia jars and zirconia balls were not considered relevant, due to the high long lifetime of such kind of ceramic items.
-
The mortar and sieve wear used after the milling step was considered negligible due to their long lifetime. The same assumption was utilized for the alumina plates used in sintering process.
-
The transportation of gasses used in the characterization processes (N2, Ar, and H2) and the transportation of Al2O3 for jar cleaning were also excluded, due to the very low quantities used in this process (few grams) and to the proximity of the production plant to the laboratory (less than 1 km).
-
The sample end-of-life stages were not taken into account because they are used in a successive experimental phase (deposition of hydrogen separation membranes), outside of the system boundaries considered in this work.
2.4 Data sources
Activity data about milling and sintering processes (i.e., electricity consumption), consumables (gasses, solvents, etc.), characterization analyses, wastes, and emissions were directly collected during the Al2O3 pellet production carried out at the National Research Council of Italy laboratories in Padua (Italy) between 2020 and 2021.
Distances between supplier locations of raw materials and solvents were calculated using Google Maps and Sea-Distances.org.
Life cycle inventory data on electricity generation, gas production, transport, etc., were retrieved from Ecoinvent 3.7 database, with a good temporal representativeness (data from 2004 to 2020). A detailed description of life cycle inventory data is provided in Section 4.
3 Inventory analysis
The system boundary of the Al2O3 pellet production process is shown in Fig. 2. The discussion on inventory data used for modelling the system is presented for each activity separately.
3.1 Powder milling and drying
Corundum powders (α-Al2O3, 99%, size < 1 µm) were purchased by Alfa Aesar (Germany), while PMMA in beads form was furnished by Soken Chemical & Engineering (average size 1.5 µm, Shanghai product MX-150). Transport from Shanghai (China) to Padua (Italy) was calculated to be 19,500 km to reach Rotterdam by container ship and 1300 km up to Padua (Italy).
Solvents employed as milling medium were deionized water (Milli-Q Millipore apparatus at Padua CNR Labs, 18 MΩ), ethanol (Carlo Erba Reagents, 99.9%), and isopropanol (Honeywell, 99.8%). Transport for organic solvents from the production plant to the laboratory was estimated by an average distance of 1000 km by lorry (Euro 5 emission profile). This assumption was made considering an average of the distances between the plant production and Padua CNR laboratory.
The electricity consumption (from low-voltage Italian grid mix) was directly measured in CNR labs. In the case of using 45 mL zirconia jars, planetary micro mill (Fritsch Pulverisette 7) was employed and the electricity consumption for a milling process of 12 cycles (20 min with 15 min pause for every cycle) was 236 Wh, independently from the solvent used. Using the 250 mL and a planetary mono mill (Fritsch Pulverisette 5), the electricity consumption for a milling process of 12 cycles (20 min with 15 min pause for every cycle) was 580 Wh as average.
Drying processes were performed by an oven (Memmert UFE 500) for 2 h at 35 °C in the case of ethanol and isopropanol and 50 °C in the case of H2O. The process gave rise to “emissions to air” as a function of the solvent used. The electricity consumption varied from roughly 160 to 985 Wh. The powder residues stitched onto jar walls produced during the milling and drying processes were estimated of about 3% of the precursor weights; these residues were treated as “municipal solid waste.” At the end of dried process, 7.6 g of dried powder was obtained for each milling batch using 45 mL jar and from 48.5 to 67.9 g using 250 mL jar as a function of the different solvent and precursor quantities. For each solvent and for each different jar volume, a certain number of milling batches were produced to optimize the process. Detailed activity data tables are listed in the supplementary information (Tables S1-6).
3.2 Pellet pressing and sintering
The inputs of this process involved energy consumption (from low-voltage Italian grid mix) related to dried powder uniaxial pressing (performed by Nannetti Mignon SS/EA apparatus) and to the successive sintering process at 1500 °C in a furnace (Nabertherm HT 04/17), performing an isotherm step for 1 h at 386 °C. This latter temperature corresponds to the PMMA burning temperature. The weight difference between the pressed pellet (1.3 g) and the consolidated one after sintering process (0.9 g) was attributed to the PMMA burning, with consequent emission in air of water and carbon dioxide (Zeng et al. 2002). Finally, very slow heating rate (0.5 °C min−1) was carried out up to 1000 °C to avoid pellet bending, and then, free cooling was performed.
Detailed activity data table is listed in the supplementary information (Table S7).
3.3 Pellet polishing and cleaning
The inventory of these processes considered consumable materials and energy consumption used for the polishing and the ultrasonic cleaning of pellets. The polishing residues were considered as “municipal waste.” Similarly, acetone used for ultrasonic cleaning was considered as “spent solvent mixture.” All detailed data are listed in Table S9 in the supplementary information.
The polishing process was carried out by Struers Tegramin 20 employing a P1200 grit polishing SiC papers. It lasted for 5 cycles of 4 samples (0.3 g of paper per pellet), and it was associated to the database “Silicon Carbide {GLO} market for.” The SiC paper was purchased from Denmark, and the transport up to Padua was included in the analysis (by lorry, Euro 5 emission profile).
Cyanoacrylate glue was used for fixing the samples at the polishing apparatus, but its environmental burden was considered negligible due to the low quantities used (10−3 g per pellet). The electricity consumption (from low-voltage Italian grid mix) was directly measured for a single cycle of 15 N lasting 15 s, considering the simultaneous processing of 4 samples. The polishing instrumentation needed 300 mL of tap water, namely, 75 mL for each sample.
The polishing process produced water considered as “wastewater,” while solid alumina and SiC residues as “municipal waste.”
Cyanoacrylate glue used for fixing samples during polishing process was removed treating simultaneously 4 samples with 20.0 g of acetone (5.0 g per sample). Ultrasonic cleaning (CEIA CP104 tank) was performed for 10 min using 25.0 g of acetone (99.9%, P.Q.R. Prodotti Chimici Riuniti) for simultaneous treatment of 16 samples (1.6 g per pellet). The energy consumption, from low-voltage Italian grid mix, was directly measured.
Transport of organic solvents from the production plant to the laboratory was estimated by an average distance of 1000 km by lorry (Euro 5 emission profile). This assumption was made considering an average distance between plant production and CNR Padua laboratory.
Overall, the used solvent mixture (6.6 g per pellet) was discarded as “spent solvent mixture,” considering 0.1 g of acetone as “emission to air.”
All detailed activity data are listed in Table S8 in the supplementary information.
3.4 Jar cleaning
The jar cleaning process involved a milling step carried out with corundum powder (with grain size of 80 µm) and water. Their amounts varied as a function of jar volume (45 mL or 250 mL). Powders and water were then treated as “municipal waste.” As previously stated, the transport of the cleaning powder was not included in this process, due to the geographical proximity of the supplier. All detailed activity data are listed in Table S9-10 in the supplementary information.
3.5 FE-SEM analysis
The morphological characterization of pellets by FE-SEM (Sigma Zeiss) involved high electric energy consumption, mainly due to the vacuum system. Before the analyses, samples were typically coated with a 20 nm gold (Au) thin film by an Emitech K575X Turbo Sputter. This practice aims to reduce bias phenomena and permitting the collecting of electron images with optimal resolution. Au target was purchased by a local dental goldsmith company, and for this reason, the transport was not considered. Argon and nitrogen gasses were also used in coating process and in FE-SEM characterization, respectively. As previously reported, the transportation of gasses used in the characterization was excluded, due to the proximity of the production plant to the laboratory (less than 1 km). The used gasses were considered as “emission to air.” All detailed inventory data are listed in Table S11 in the supplementary information.
Chromium (Cr) target was supposed to be employed as substitution of gold as metal coating material in the LCIA of Sect. 5. Au deposition conditions were hypothesized to be maintained considering the ratio between the two element masses (7.9 g/cm3 and 19.3 g/cm3 for Cr and Au, respectively). Cr target was supposed to be purchased from a supplier located in Milan (Italy), and the transportation (by lorry, Euro 5 emission profile) was calculated up to Padua. All detailed activity data are listed in Table S12 in the supplementary information.
3.6 Pellet permeability measurements
Gas permeabilities of N2 (99.999% purity) and H2 (produced onsite by an electrolyzer Perkin Elmer PGX Plus H2 160 mL/min) were measured by a custom-built stainless steel test station (Barison et al. 2018). Membranes were inserted in a stainless steel module with graphite gaskets and then placed in a furnace (Nabertherm N11/HR) with K-type thermocouple for temperature control. The gas flows were set by independent mass flow controllers (1179A, 1179B, and 647C, MKS), while pressure at the feed side was controlled by a Baratron pressure transducer (722B, MKS). During permeability tests performed at room temperature, 300 °C, and 350 °C, the pressure of the feed side was set at 400 kPa, while it was maintained at the atmospheric pressure at the permeate side. The process implicated electricity consumption, use of gasses (treated as “emission to air”), and two graphite gaskets to seal off the test cell. The latter were purchased from a supplier located in Milan (Italy), and the transportation (by lorry, Euro 5 emission profile) was calculated up to Padua. After the measure, the graphite gaskets were treated as “municipal solid waste” (for detailed activity data, see Table S13 in the supplementary information.
3.7 “Experimental trials” phase
The “experimental trials” process contained all the laboratory procedures and analyses necessary to identify the optimal experimental conditions for obtaining pellets suitable as substrate for metallic film-based membranes for hydrogen separation. In particular, the “trial pellet preparation” included the “milling and drying” step with different conditions and jar volumes” (Table 1) which were successively divided in the “unused trial powder” (i.e., the quantity of exceeding dried powder not used in consolidation process), and the pellets obtained by the powder subjected to the “pressing and sintering” and “polishing and cleaning” steps. Some of those pellets were then employed for morphological FE-SEM characterization and permeability tests. Table S14 of the supplementary information lists all detailed activity data of this process.
3.8 “Pellet production” phase
The optimized milling conditions obtained as result of the “experimental trials” phase were used for the consequent “pellet production” phase. They were identified as 38.7 g of α-Al2O3 and 21.3 g of PPMA, milled using the 250 mL jar at 280 rpm in deionized water (120.0 g). Table S15 of the supplementary information lists all detailed activity data of this process.
4 Results and discussion
LCIA characterization, normalization, and weighting were carried out using the Environmental Footprint (EF) 3.0 LCIA method, as recommended by the European Commission (Zampori and Pant 2019; European Commission 2021). The analyses were performed by SimaPro 9.3 software. Moreover, the analysis was performed also with CML LCIA method v. 4.7 (CML-Leiden University 2016) to verify the robustness of the results.
4.1 Impact assessment results and interpretation
4.1.1 Environmental impact evaluation of milling medium solvents
With the aim of identifying the milling medium solvent (water, ethanol, or isopropanol) with lower environmental impact, a LCIA was carried out considering a FU (already stated in paragraph 2.2) of 7.6 g of dried powders obtained by milling and drying processes (Sect. 3.1) with 45 mL jars (Tables S1-3 of the supplementary information). It is worth noting that this FU considered not only the milling process but also the drying stage, in which the solvents evaporated at different temperatures (see Sect. 3.1), entailing different electrical energy consumptions, higher in the case of water. The LCIA characterization results for the three solvents are reported in the supplementary information (Figure S1 and Table S16), while the comparison of weighted potential impacts (Fig. 5) showed that the total potential environmental impact of water-based process was lower than the ethanol and isopropanol ones (a decrease of 53% and 40%, respectively), despite it required much more energy for the drying stage (158 Wh against 104 Wh of organic solvents). This behavior was mainly affected by the impact of the “photochemical ozone formation” impact category, which depended on the emission of the volatile organic compounds (VOCs) originated by the organic solvents used (Fig. 5).
Comparison of weighted potential impacts of powders obtained by milling and dry processes with different solvents (FU: 7.6 g of powders) using 45 mL jars (Tables S1-3 in the supplementary information document). The dimensionless unit mPt stands for millipoints (one thousandth of the yearly environmental load of an average citizen in Europe), unit of the weighted results
In order to test how an organic solvent could affect the pellet porosity, powder with ethanol as milling medium was prepared using a higher volume jar (250 mL). Nevertheless, the use of organic solvents did not demonstrate to give any advantage in terms of material features. All these considerations and evidence justified the choice of water as milling medium for the optimized condition for the “pellet production” phase.
4.1.2 Life cycle impact assessments of development and production of porous alumina pellets
LCIA characterization of the whole process for obtaining 40 polished disk-shaped porous alumina pellets was performed with a FU of 40 polished disk-shaped high purity alumina pellet production (as stated in paragraph 2.2). The analysis showed that the environmental burden was almost divided equally between “pellet production” and “experimental trials” phases on average (56% and 44%, respectively) (Fig. 6 and the respective results listed in Table S17 of the supplementary information document). Nevertheless, it is worth noting that the “pellet production” phase was the most impacting process for more than half impact categories. Conversely, the “experimental trials” phase was the most impacting process only for “photochemical ozone formation” (due to the VOC emission, as depicted above) and “resource use, minerals, and metals” (as it will be discussed further on).
The impact assessment highlighted as one of the main environmental critical issues was related to the use of mineral resources. A deeper analysis showed that the “resource use, mineral, and metals” in EF method was mainly related to the employment of Au for the sample coating prior the FE-SEM characterization, as it is possible to observe in comparison of weighted impacts of the different process phases in Fig. 7; in this case, results of jar cleaning processes were omitted due to their very low values (76% lower than the pellet permeability measurements, for instance).
The precious metal is a fundamental material in several technological fields for its peculiar stable electrical conduction features, and its use was already identified as one of the main hotspots of lab-scale processes analyzed by LCA (Gentile et al. 2014; Pallas et al. 2020).
In general, Au coating of specimens prior of FE-SEM characterization is a very common practice in research analysis laboratories due its mechanical processability, high sputtering rate, and resistance to corrosion and because it was rarely present in the composition of insulating samples. Nevertheless, the high potential environmental impact revealed led to the necessity gold with a less precious impacting metal, such as chromium (Stokroos et al. 1998), generally avoided because it was present in the composition of many materials, such as ceramics or steels. Alternatively, the coating could be even avoided completely by virtue of the specific apparatus used in this work (see paragraph Sect. 3.5), which was able to obtain micrographs with sufficient resolution even in absence of conductive coatings.
Figure 8 showed a comparison between the weighted impacts of the “pellet production” and those related to “experimental trials” phase with Au coating, Cr coating, and without coating deposition. Using Au as coating material, the “experimental trials” phase presented higher potential impact respect to “pellet production” one (3.34 mPt and 3.14 mPt, respectively). The use of Cr coating gave rise to the same potential impact detected avoiding the coating process (1.82 mPt), with a decrease of 54% of the potential impact of the “experimental trials” phase, resulting lower than the “pellet production” phase one. These results suggested that the environmental burden can be attributable completely to the use of Au, while the deposition process resulted substantially negligible.
Results of characterization, normalization, and weighting with EF 3.0 method of the “pellet production” phase and “experimental trials” phase Au coating, Cr coating, and without coating deposition. The dimensionless unit mPt stands for millipoints (one thousandth of the yearly environmental load of an average citizen in Europe), unit of the weighted results
Section 3.8 potential impact depended mainly by the energy consuming “pressing and sintering” step which resulted as the most critical impact of the whole process, even higher than “pellet FE-SEM analysis” one (2.96 and 1.82 mPt, respectively) (Fig. 9). The “pressing and sintering” step involved electricity as inputs and carbon dioxide and water as emission on air (Table S7 in the Supplementary section). The high environmental impact is mainly due to the electrical mix typical of the laboratory location (Italy).
At variance to the “pellet production” step, “experimental trials” phase did not depend on the number of pellets prepared and possessed a constant potential environmental impact. For this reason, its environmental burden attributable to each pellet decreased with the increasing of the prepared pellets.
In particular, the weighted impacts of “experimental trials” phase resulted equal to the “pellet production” values in the case of 43 pellets. Conversely, avoiding the Au coating, the “experimental trials” phase impacts counted more than the “pellet production” phase when prepared substate number was 23. For this reason, the Au coating can be considered a hotspot of the whole process.
In a perspective of a scaled-up pellet production, it would be interesting evaluating the case in which the potential environmental impact of “experimental trials” phase may be considered negligible. Based on authors’ experience, such cutoff value can be assessed at 3%, which corresponds to the production of 1473 pellets. This number is not common for an experimental work at lab scale, but it would absolutely be consistent with a small-scale industrial production following similar production route.
Although the deposition process was set outside of the system boundaries, its properties and parameters affected the substrate feature requests and the number of samples needed for completing the experimental campaign. In this context, it is important to consider other two cases: first, in which the metallic alloy does not work as an efficient membrane, the deposition process could be stopped in the middle of the campaign (roughly 20 pellets). In the second case, in which the deposited coatings work efficiently, it would be possible to plan a higher number of samples. For instance, 100 pellets overall could be a reasonable sample number for an expanded experimental campaign.
The environmental impacts (referred to one pellet) of the two cases above mentioned are depicted in Fig. 10, in addition to the functional unit identified previously (40 pellets). Evidently, the environmental burden of one pellet was a function of number of pellets produced, due to the constant weight of the “experimental trials” phase: the higher the pellet number, the lower the resulting potential impact ascribable to each pellet.
In order to evaluate the representativeness and the robustness of LCIA results obtained in this work with EF 3.0 method, the LCIA was carried out using CML 4.7 method. This kind of analysis, in particular, can give a more complete view of the considered system and highlighting the parameters which affect more the model (Wei et al. 2015).
The contributions of impact categories are depicted in Fig. 11, while the results are listed in Table S18 in the supplementary information.
The contribution of “experimental trials” process to the “resource use, minerals, and metals” impact category of the EF 3.0 method was compared to “abiotic depletion” impact category of the CML method, obtaining substantially the same results (79%). Similarly, the process impact contributions of the CML “global warming” resulted substantially the same of EF “climate change” ones (65% and 35% for “pellet production” and “experimental trials,” respectively). Comparing EF “photochemical ozone formation” with CML “photochemical oxidation” impact categories of “experimental trials” process, a slight difference between the two methods can be observed (69% and 92%, respectively).
On the contrary, the difference between the two methods can be appreciated focusing onto the “human toxicity” impact category: using EF 3.0 method, “pellet production” phase showed higher potential impact of “human toxicity” (cancer and non-cancer summed) than the “experimental trial” phase (2.69 × 10−7 and 2.19 × 10−7 CTUh, respectively) while trend was reversed (15.4 and 20.2 kg 1,4-dichlorobenzene eq, respectively) using CML 4.7 method.
5 Conclusions
The main aim of this study was to improve the sustainability of laboratory activities by identifying the environmental hotspots in the preparation of porous alumina pellets, suitable as substrates for hydrogen separation metal layer-based membranes. Several process phases were considered, including the characterization steps, such as morphological and permeability analyses.
A “cradle to gate” LCA approach was used to study the environmental implications of the preparation of 40 pellets. The LCIA was performed employing the EF 3.0 method, testing the robustness of the results using the CML 4.7 method.
A preliminary environmental impact evaluation showed that the employment of water as milling media can considerably reduce the burden of the “photochemical ozone formation” (a decrease of 53% and 40% with respect to the use of ethanol and isopropanol), due to the avoided emission of VOCs originated from the use of organic solvents. On the other hand, the analysis highlighted as in the “pellet production” phase, the “pressing and sintering” step for obtaining consolidation of pellets was the major driver of potential environmental impacts. Indeed, the resulting impact value was 2.96 mPt, even higher than “pellet FE-SEM analysis” step (1.82 mPt) in the “experimental trials” phase. This energy consuming process is strictly related to peculiar electrical mix used by the laboratory, which is in Italy. It is plausible to argue that the introduction of green electrical sources may dramatically decrease the impact of such step and, as a consequence, the impact of the whole process.
Moreover, it is worth noting that the morphological characterization phase exploiting electronic microscopy resulted in a significant potential environmental impact on the mineral resource use due to the gold coating of samples. In this case, this burden could be easily decreased of the 54% by substituting the precious material or avoiding the coating process.
These important hints confirmed the crucial role of an environmental study of lab-scale processes which can be the seed for a sustainable design of a possible industrial scale-up of hydrogen separation metal layer-based membrane technology.
Data availability
All data will be made available upon reasonable request for academic use and within the limitations of the provided informed consent by the corresponding author upon acceptance. Every request for raw and analyzed data and materials will be evaluated by scientists of National Research Council of Italy.
References
Ang P, Mothe SR, Chennamaneni LR, et al (2021) Laboratory-scale life-cycle assessment: a comparison of existing and emerging methods of poly(ϵ-caprolactone) synthesis. ACS Sustain Chem Eng 9:669–683. https://doi.org/10.1021/acssuschemeng.0c06247
Ayturk ME, Engwall EE, Ma YH (2007) Microstructure analysis of the intermetallic diffusion-induced alloy phases in composite Pd/Ag/porous stainless steel membranes. Ind Eng Chem Res 46:4295–4306. https://doi.org/10.1021/ie061677j
Barison S, Fasolin S, Boldrini S et al (2018) PdAg/alumina membranes prepared by high power impulse magnetron sputtering for hydrogen separation. Int J Hydrogen Energy 43:7982–7989. https://doi.org/10.1016/j.ijhydene.2018.03.065
Bauer C, Buchgeister J, Hischier R et al (2008) Towards a framework for life cycle thinking in the assessment of nanotechnology. J Clean Prod 16:910–926. https://doi.org/10.1016/j.jclepro.2007.04.022
Bernardo P, Drioli E, Golemme G (2009) Membrane gas separation: A review/state of the art. Ind Eng Chem Res 48:4638–4663. https://doi.org/10.1021/ie8019032
CML-Leiden University (2016) CML 2016: CML-IA Characterisation Factors
Demirbas A (2008) Biohydrogen generation from organic waste. Energy Sources, Part A Recover Util Environ Eff 30:475–482. https://doi.org/10.1080/15567030600828909
Dincer I, Acar C (2014) Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrogen Energy 40:11094–11111. https://doi.org/10.1016/j.ijhydene.2014.12.035
Elginoz N, Atasoy M, Finnveden G, Cetecioglu Z (2020) Ex-ante life cycle assessment of volatile fatty acid production from dairy wastewater. J Clean Prod 269:122267. https://doi.org/10.1016/j.jclepro.2020.122267
European Commission (2021) Commission Recommendation on the use of the Environmental Footprint methods
Fasolin S, Barison S, Agresti F et al (2022) New sustainable multilayered membranes based on ZrVTi for hydrogen purification. Membranes 12. https://doi.org/10.3390/membranes12070722
Fasolin S, Barison S, Boldrini S et al (2018) Hydrogen separation by thin vanadium-based multi-layered membranes. Int J Hydrogen Energy 43:3235–3243. https://doi.org/10.1016/j.ijhydene.2017.12.148
Fiameni S, Battiston S, Castellani V et al (2020) Implementing sustainability in laboratory activities: a case study on aluminum titanium nitride based thin film magnetron sputtering deposition onto commercial laminated steel. J Clean Prod 285:124869. https://doi.org/10.1016/j.jclepro.2020.124869
Gallucci F, Fernandez E, Corengia P, van Sint Annaland M (2013) Recent advances on membranes and membrane reactors for hydrogen production. Chem Eng Sci 92:40–66. https://doi.org/10.1016/j.ces.2013.01.008
Gentile AA, Rocco C, Modeo S, Romano T (2014) Gold recovery from thin film deposition facilities: environmental aspects of a novel method. J Clean Prod 83:473–482. https://doi.org/10.1016/j.jclepro.2014.07.077
Hatlevik Ø, Gade SK, Keeling MK et al (2010) Palladium and palladium alloy membranes for hydrogen separation and production: history, fabrication strategies, and current performance. Sep Purif Technol 73:59–64. https://doi.org/10.1016/j.seppur.2009.10.020
Hosseini SE, Abdul Wahid M, Jamil MM, et al (2015) A review on biomass-based hydrogen production for renewable energy supply. Int J Energy Res 39:1597–1615. https://doi.org/10.1002/er.3381
Huang Y, Dittmeyer R (2006) Preparation and characterization of composite palladium membranes on sinter-metal supports with a ceramic barrier against intermetallic diffusion. J Memb Sci 282:296–310. https://doi.org/10.1016/j.memsci.2006.05.032
Jeswiet J, Hauschild M (2005) EcoDesign and future environmental impacts. Mater Des 26:629–634. https://doi.org/10.1016/j.matdes.2004.08.016
Kim TW, Lee EH, Byun S, et al (2022) Highly selective Pd composite membrane on porous metal support for high-purity hydrogen production through effective ammonia decomposition. Energy 260:125209. https://doi.org/10.1016/j.energy.2022.125209
Lee YH, Jang Y, Han DH et al (2021) Palladium-copper membrane prepared by electroless plating for hydrogen separation at low temperature. J Environ Chem Eng 9:106509. https://doi.org/10.1016/J.JECE.2021.106509
Li Y, Yang X, Liu D et al (2019) Permeability of the porous Al2O3 ceramic with bimodal pore size distribution. Ceram Int 45:5952–5957. https://doi.org/10.1016/J.CERAMINT.2018.12.064
Lu HT, Li W, Miandoab ES et al (2020) The opportunity of membrane technology for hydrogen purification in the power to hydrogen (P2H) roadmap: a review. Front Chem Sci Eng 15:3 15:464–482. https://doi.org/10.1007/S11705-020-1983-0
Pallas G, Vijver MG, Peijnenburg WJGM, Guinée J (2020) Life cycle assessment of emerging technologies at the lab scale: the case of nanowire-based solar cells. J Ind Ecol 24:193–204. https://doi.org/10.1111/jiec.12855
Pérez-López P, González-García S, Jeffryes C, et al (2014) Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J Clean Prod 64:332–344. https://doi.org/10.1016/j.jclepro.2013.07.011
Piccinno F, Hischier R, Seeger S, Som C (2016) From laboratory to industrial scale: a scale-up framework for chemical processes in life cycle assessment studies. J Clean Prod 135:1085–1097. https://doi.org/10.1016/j.jclepro.2016.06.164
Piccinno F, Hischier R, Seeger S, Som C (2018) Predicting the environmental impact of a future nanocellulose production at industrial scale: application of the life cycle assessment scale-up framework. J Clean Prod 174:283–295. https://doi.org/10.1016/j.jclepro.2017.10.226
Scott K (1995) Gas Separations. In: Scott K (ed) Handbook of Industrial Membranes. Elsevier Science, Amsterdam, pp 271–305
Stokroos I, Kalicharan D, Van Der Want JJL, Jongebloed WL (1998) A comparative study of thin coatings of Au/Pd, Pt and Cr produced by magnetron sputtering for FE-SEM. J Microsc 189:79–89. https://doi.org/10.1046/J.1365-2818.1998.00282.X
Tong J, Matsumura Y, Suda H, Haraya K (2005) Thin and dense Pd/CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane. Sep Purif Technol 46:1–10. https://doi.org/10.1016/j.seppur.2005.03.011
Villares M, Işildar A, Mendoza Beltran A, Guinee J (2016) Applying an ex-ante life cycle perspective to metal recovery from e-waste using bioleaching. J Clean Prod 129:315–328. https://doi.org/10.1016/J.JCLEPRO.2016.04.066
Walser T, Demou E, Lang DJ, Hellweg S (2011) Prospective environmental life cycle assessment of nanosilver T-shirts. Environ Sci Technol 45:4570–4578. https://doi.org/10.1021/ES2001248/SUPPL_FILE/ES2001248_SI_001.PDF
Wei W, Larrey-Lassalle P, Faure T, et al (2015) How to conduct a proper sensitivity analysis in life cycle assessment: taking into account correlations within LCI data and interactions within the LCA calculation model. Environ Sci Technol 49:377–385. https://doi.org/10.1021/es502128k
Xomeritakis G, Lin YS (1997) Fabrication of thin metallic membranes by MOCVD and sputtering. J Membr Sci 133:217–230. https://doi.org/10.1016/S0376-7388(97)00084-7
Yun S, Ted Oyama S (2011) Correlations in palladium membranes for hydrogen separation: a review. J Membr Sci 375:28–45
Zampori L, Pant R (2019) Suggestions for updating the product environmental footprint (PEF) method. JRC Technical Repports; Publications Office of the European Union: Luxembourg 76
Zeng WR, Li SF, Chow WK (2002) Review on chemical reactions of burning poly(methyl methacrylate) PMMA. J Fire Sci 20:401–433. https://doi.org/10.1177/0734904102020005482
Acknowledgements
The authors are grateful to Dr. Valentina Castellani for the support in LCA analysis and for the fruitful discussion about the results of this work.
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. This work has been funded by the project “Frontier materials for energy uses” within the Italian National Research Council—Italian Ministry of Economic Development Agreement 2019–2021 “Ricerca di Sistema Elettrico Nazionale.”
Author information
Authors and Affiliations
Contributions
Simone Battiston: conceptualization, methodology, investigation, writing—original draft, data curation, and visualization. Stefania Fiameni: conceptualization, methodology, investigation, writing—original draft, data curation, and visualization. Stefano Fasolin: data curation. Simona Barison: review and editing and supervision. Lidia Armelao: review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Chris Yuan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Battiston, S., Fiameni, S., Fasolin, S. et al. Life cycle environmental impact assessment of lab-scale preparation of porous alumina pellets as substrate for hydrogen separation metal layer-based membranes. Int J Life Cycle Assess 28, 1117–1131 (2023). https://doi.org/10.1007/s11367-023-02179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-023-02179-5