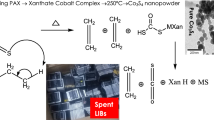
Abstract
A novel method for the fabrication of nanoengineered, mixed transition metal oxide anode active material is proposed based on implementing liquid nitrogen treatment during the chemical precipitation process, for the first time in open literature. Such interference in the precipitation is believed to change the surface energy of the nuclei leading to differentiation in the growth process. To exemplify this hypothesis with an environmentally friendly approach, kitchen scourer pads, an existing waste, are used as a starting material instead of using a mixture of primary quality metals’ salts. Therefore, in this study, firstly, an optimization is realized to leach the scouring pad with 100% efficiency. Then, by applying a conventional chemical precipitation to this leachate at pH 5.5, Sample 1-P is produced. Herein, innovatively liquid nitrogen treatment is carried out during the chemical precipitation to produce Sample 2-P. Lastly, these precipitates (Samples 1-P, 2-P) are calcinated in the air to form mixed transition metal oxide powders: Samples 1 and 2, respectively. Structural, chemical, and morphological characterizations are carried out to examine the effect of liquid nitrogen treatment on the powders’ properties. To discuss the effect of nitrogen treatment on the electrochemical performances of the anode active materials (Sample 1 and Sample 2), galvanostatic tests are realized. The results show that Sample 2 demonstrates a higher 1st discharge capacity (1352 mAh/g) and retains 62% of its performance after 200 cycles when 50 mA/g current load is applied. Moreover, this electrode delivers around 500 mAh/g at 1 A/g current load. The remarkable cycle performance of Sample 2 is believed to be related to the superior chemical, structural, and physical properties of the electrode active material.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The significant advantages of lithium-ion batteries (LIBs) over other secondary batteries, including their high energy density, long lifespan, low self-discharge, and environmental friendliness, make them one of the most widely used battery types. Numerous industries, such as energy storage, electric vehicles, and portable electronic, heavily rely on LIBs. [1, 2]. A through market research predicts that the rising demand for LIB would result in a 300 billion USD market expansion by 2030 [3]. According to the US Energy Department, by the year 2030, the total amount of energy that can be stored using LIB will be roughly 2500 GWh [4]. Therefore, a yearly production of ~ 50 metric tons of electrode active material (including anode and cathode) is required to meet this level of energy demand [3].
Today, most commercially available LIBs use graphite as an anode material. Graphite-based LIBs give long cycle life but there are few concerns about them which include their low energy and power densities, safety issues (over charging, thermal runaway, catching fire etc.) [5], and high carbon footprint behavior (per ton production of graphite anode material 5315.9 kg of CO2-eq is emitted from coal mines) [6]. This condition endangers the commitment of LIBs toward being environmentally friendly. Moreover, the restrictions in the geographical locations of coal mines/reserves are another concern of investors for the sustainability of their business. So, scientists have been working to design alternative green anode active materials with larger energy and power densities. Pertaining to this, considerable attention has been paid to transition metal oxides (TMO) due to their high theoretical capacities (~ 1000 mAh/g) [7,8,9,10,11]. The general lithium storage mechanism for most of TMOs is represented by the following Eq. 1 [12,13,14,15]:
McNulty et al. [16] claim that while designing an anode material (especially TMO based anode) the particle size, shape, and morphology are the fundamental factors to be considered. It has been reported by Liang et al. [17] that the critical particle size of an ideal metal oxide anode material should be around 150 nm. TMO anodes with the relevant characteristics have so far been developed using a variety of techniques. Along with hydrothermal [18, 19] and solvothermal [20, 21] techniques, plasma-assisted oxidation [7, 22], hard template method [23, 24], and rapid thermal chemical vapor deposition (RTCVD) [25], which operate at high temperatures and require extremely pure expensive precursors, have been also utilized. The production of metallic oxide nanoparticles from an aqueous solution by chemical precipitation is the most advantageous of all the reported synthesis techniques because it not only allows for precise control over particle morphology and composition but also results in high yield production with minimal energy and investment in equipment. The major drawback of this method is the formation of large sized particles since nucleation and growth occur very fast. Several techniques have been used to restrict the formation of large sized particles, including the addition of chelating agents, matrix, and templates into the electrolyte. Unfortunately, all these techniques may cause some amount of impurity formation which would affect the performance when it is used as electrode active material for LIBs, eventually.
Agglomeration is a well-known problem for powder fabrication via coprecipitation. Van der Giessen [26, 27] et al. proposed a method to inhibit the particle growth. They applied liquid nitrogen to the gel, to prevent further particle growth by dehydration which eventually results in smaller-sized particle formation in micron-scale. This approach was also implemented by other scientists who disclosed the same outcomes that freezing through liquid nitrogen helps in particle growth prevention in micron-scale [28,29,30,31]. But none of these works adopt this knowledge in anode design and study the effect of this cryo-assisted nitrogen treatment on the electrode active materials’ physical, structural, and electrochemical properties.
In this study, following the green aspect of the electrode design, our research group innovatively fabricated low carbon footprint electrode material by recovering values from wastes and/or by-products. Similarly, Yao et al. [32] formed α-Fe2O3 nanoparticles through a leaching solution of tin ore, then Wu et al. [33] processed spent pickled wastewater to fabricate iron oxide-based anode, and Kang et al. [34] used pickled waste liquor (PWL) as the starting material to fabricate spherical-shaped β-FeOOH as an anode material.
Therefore, in this study, kitchen scourers are used as the initial raw material because due to sanitary concerns, they are frequently discarded and replaced with new ones. This makes them abundantly and easily available as domestic waste. Knowing that hygienic concerns encourage people to renew scourers very frequently, an innovative solution for valorizing discarded scourers is also proposed for the first time in the open literature.
Moreover, as the kitchen scourers are made of steel and they have a very favorable physical aspect (i.e., they are in the form of thin wire like strips), valorizing this waste as the raw material enables the fabrication of mixed transition metal oxides in one step.
At this point, it is important to recall that mixed transition metal oxide performs better than pure, single metal oxide when it is used as electrode active material in LIBs [35,36,37]. Zhao et al. [38] emphasized the superior performance of the mixed transition metal oxide electrode (over single metal oxide electrode) and claimed that not only the cycle life and the capacity retention but also the rate performance is better due to structural characteristics of the powder. Furthermore, Pan et al. [39] stated that by synthesizing a combination of iron oxide and chromium a higher electrical conductivity, hence better electrochemical properties are achieved because of the imperfections caused by the presence of chromium in iron oxide. Further they explained that chromium shrank the particle size slightly and reduced the band gap of iron oxide meanwhile creating higher number of electron–hole pair which imparted in the elevation of electrochemical properties.
Thus, without using expensive metal salts, a scourer being easily accessible inorganic waste is utilized as the starting material during the production of a mixed transition metal oxide anode material for lithium-ion batteries. In the experiments firstly, an optimization in the leaching efficiency of scourer by sulfuric acid is realized (online supplementary Table T1). Secondly, the leachate is treated with a basic solution to adjust pH at 5.5 for a conventional chemical precipitation (sample 1-P). Herein pH is selected for recovering both iron and chromium at the same time [40,41,42]. And, to avoid the growth of hydroxide nuclei in micron-scale, liquid nitrogen treatment is applied to the precipitate (sample 2-P). Finally, obtained powders (sample 1-P (reference sample with no nitrogen treatment and sample 2-P with nitrogen treated sample)) are subjected to heat treatment in the air and then Sample 1 and Sample 2 are characterized and tested as anode materials for lithium-ion batteries.
Experimental
Synthesis Procedure
The kitchen scourer used in this study was a product purchased from the local market. Sulfuric acid (H2SO4) being used as reactive agent in the leaching was from Tekkim Kimya Sanayi Ltd. Şti. (94% purity). An optimization in the leaching process parameters was realized to get 100% leaching efficiency (online supplementary Table T1). 4g of the steel scourer was completely dissolved in 200 ml of 1M H2SO4 in a reflux experimental system at 75 °C for 2 h. During leaching, the solid-to-liquid ratio was kept at 1:50, and vigorous magnetic stirring was applied. Afterward, mixed transition metal oxide powder (sample 1) was obtained through conventional chemical precipitation followed by calcination. For that, the pH of the leachate was adjusted to 5.5 via dropwise addition of 14M NH4OH solution (25% sol, EMSURE ®) followed by stirring for one hour. Then, the solution was aged naturally for phase separation in 24 h, and the liquid phase was removed to obtain a slurry. To get Sample 1-P, half of the precipitates were washed and dried, and to obtain Sample 2-P, liquid nitrogen was poured on to the rest half of precipitates. Then, in both cases, the precipitates (Samples 1-P and 2-P) were centrifuged, washed (several times with deionized water), and dried (at 90 °C, overnight). Finally, both samples were heat treated at 750 °C with 10 °C/min for 5 h in air to get Sample 1 and 2, respectively (see online supplementary Fig. S1: flow chart for the fabrication of the electrodes).
Chemical, Morphological, and Structural Characterization
The chemical compositions of the scourer, sample 1, and sample 2 were measured by X-ray fluorescence (XRF, Hitachi X-MET8000). A morphological study was held with the aid of a scanning electron microscope (SEM, Zeiss Gemini 500). The structural phases were determined via X-ray diffraction (XRD, Brucker). The typical parameters including 2θ = 10–90°, Cu Kα X-rays, and scanning rate of 0.02°/sec were utilized during the characterization. The phase quantification and in-depth crystalline examination were accomplished through Rietveld refinement in DIFFRAC.TOPAS V6 (BRUKER) software tool. To get further insight of microstructural changes occurring in the material during charge–discharge, ex situ SEM was carried out. For ex situ SEM analysis, the half cells were opened (after 200 cycles) in an argon environment, anodes were washed with dimethylcarbonate (DMC), and dried, then subjected to analysis. Surface chemistry of the produced powders was explored by of X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-alpha X-ray Photoelectron Spectrometer, 0–1300 eV by Al Ka with step size 1 eV and spot size 400 µm). Raman spectrometry (Renishaw Raman spectrometer 532 nm laser source, 0.45 Watt laser power, 100–3000 cm−1 range, 10 n sec exposure time) and FTIR (Fourier-transform infrared spectroscopy-Pelkin-Elmer Spectrum Two, between 650 and 4000 cm−1) analysis were conducted to get in-depth knowledge of the bonds present in the samples 1 and 2 to discuss the effect of treatment on the powder chemistry. The UV–vis measurement was carried out using spectrophotometer (SpectraMax i3 Multi-Mode Platform, Molecular Devices).
Electrochemical Characterization
The working electrodes were prepared by coating copper foil (10 μm thick, Battery Grade) with slurry via Doctor Blade approach. Typically, a slurry was prepared by homogenously mixing the prepared active material, polyvinylidene difluoride (PVDF as binder, Gelon LIB Group), and Timcal-C64 (as conductive agent) in 60:15:25 wt% with an appropriate amount of n-methyl-pyrrolidone (NMP as solvent, EMPLURA®) in Thinky ARE-250. Subsequently, two-step drying of electrodes was realized, first at 70 °C in a vacuum for 12 h then at 120 °C for 48 h. Afterward, half cells were assembled using CR2032 type coin cells, MTI Corp. In a typical coin cell, an electrode (of diameter 12 mm) punched from the coated copper foil was used as an anode, microporous polypropylene film (Celgard 2400) was used as a separator, lithium foil (Battery Grade) was used as a counter electrode, and Merck quality standard solution made of 1M solution of LiPF6 (Dongguan Shanshan Battery Materials Co. Ltd.) dissolved in EC: DMC (1:1) was utilized as the electrolyte (Merck, 1.00028.1000). The coin cells were assembled in an Argon-filled glovebox (LABmaster Pro, MBRAUN, where the oxygen (O2) and humidity (H2O) levels were lower than 0.1 ppm).
The galvanostatic tests were conducted on the Neware battery analyzer system. The anodes (sample 1, sample 2) were cycled between 1 mV and 3 V (vs. Li/Li+) under a current load of 50 mA/g. For the rate test, anodes were cycled under different current loads i.e., 100 mA/g (10 cycles), 200 mA/g (10 cycles), 400 mA/g (10 cycles), 800 mA/g (10 cycles), 400 mA/g (10 cycles), 200 mA/g (10 cycles), 100 mA/g (20 cycles), 900 mA/g (40 cycles), 1000 mA/g (40 cycles), and 100 mA/g (65 cycles). Moreover, cycling voltammetry (CV) was applied on each electrode at a scan rate of 0.2 V/s between cutoff voltages of 1 mV–3 V (vs. Li/Li+) by Gamry Interface 1000E. Furthermore, to calculate the dominant mechanism (capacitance or diffusion) on the capacity of Sample 2, a potentiostatic test at different scan rates was applied by using Gamry Interface 1000E. In addition, electrochemical impedance spectroscopy (EIS), of 200 cycled half cells, was also carried out to analyze the difference between both electrode’s impedance after the cycle test (Gamry Interface1000E). The EIS test of half-cell was executed at 1 mV, from 0.01 Hz to 100 kHz in symmetric cells. The EIS parameters were fitted using Gamry software.
Results and Discussion
Chemical, Morphological, and Structural Characterization
An optimization in the leaching parameters is realized (given in supplementary table: Table T1 Optimization of leaching conditions) and 100% leaching efficiency is accomplished when 4 g scourer is leached out with 200 ml of 1 M H2SO4, with 1/50 solid liquid ratio, at 75 °C for 2 h. Then, samples 1 and 2 are produced following the procedure given in “Experimental: Synthesis Procedure” section (see online supplementary Fig S1: Flowchart for synthesis of both samples 1 and 2).
The chemical compositions of the scourer as well as samples 1 and 2 are obtained using XRF analysis. 95% Fe is found in the scourer along with Cr, Mn, Ni, Cu, and Mo. Then, with successful leaching and pH adjustment processes, both iron and chromium are precipitated together in both samples [43]. Results show that samples 1 and 2 contain approximately 50 ± 3% Fe and ≈16 ± 4% Cr with traces amount of Mn, Ni, Cu, Mo, and the rest is oxygen.
Figure 1a–d give SEM images of Samples 1 and 2 at different magnifications. The nucleation and growth mechanisms are studied in detail. As the leaching of scourer is achieved with 100% efficiency, all metals are found in the form of sulfate salts in the solution. Upon leaching iron, it may form both \({Fe}^{+3}/{Fe}^{+2}\) ions. When 14M NH4OH is introduced into this leachate, hydroxide forms by the completion of the reactions given by Eqs. 2 [44] and 3 [45]. Here, it is worth emphasizing that the iron form ferric hydroxide (even if it contains \({Fe}^{+2}\)) due to pH 5.5 at which \({Fe}^{+2}\) gets oxides to form Fe (OH)3 [44, 46, 47].
After scrutiny on Pourbaix diagrams, pH 5.5 is chosen to coprecipitate Fe and Cr together in hydroxide forms [48, 49]. Herein, sample 1 represents the reference showing the formation of precipitate under conventional conditions. And sample 2 reveals the effect of liquid nitrogen treatment under the defined precipitation conditions.
In both samples after pH adjustment to 5.5, first the nucleation begins in conventional manner which can be illuminated by the classical nucleation theory (CNT). According to CNT, the homogeneous nucleation from the solution is thermodynamically dependent on the total free energy of clusters. The free energy is sum of surface energy and bulk free energy, this free energy for a sphere-shaped cluster (with radius r) can be expressed in form of Eq. 4 [50, 51]:
where \(\Delta {G}_{v}\) is a free energy of cluster and is given by Eq. 5:
Here, r denotes the radius of the cluster, \({\varvec{\gamma}}\) the surface energy, T temperature, kB Boltzmann’s constant, S is the supersaturation of the solution, and v its molar volume. The free energy (\(\Delta G\)) increases initially and molecules in the solution form cluster. Once radius of cluster attains the specific radius then \(\Delta G\) decreases. This radius is termed as critical radius (\({{\varvec{r}}}_{{\varvec{c}}}\)) and the cluster having radius smaller then \({{\varvec{r}}}_{{\varvec{c}}}\) are called embryo, while the clusters having radius equal or greater then \({{\varvec{r}}}_{{\varvec{c}}}\) are termed as nuclei. As soon as the cluster achieves a size equal or greater than that of the critical radius (\({{\varvec{r}}}_{{\varvec{c}}}\)), then it becomes stable enough to further grow, leading to nano particle formation.
In this study, the nucleation mechanism is the same for samples 1 and 2 since the procedure as well as the experimental conditions are same. Therefore, the variation in particles’ properties comes from the growth step. Sample 1 is fabricated following the conventional procedure to be taken as reference. It is believed that the growth in sample 1 follows the classical diffusion-based growth model [50]. Likewise, a modern approach LaMer theory [52] also explains precipitates formation where the nucleation and growth mechanisms are studied based on time in 3 successive stages, stage 1—a sudden increase in the concentration of nuclei forming molecules (also termed as free monomers). Stage 2—surged nucleation of most of cluster, stage 3—growth of the formed nuclei by diffusion of other molecule from the solution as time passes either the molecule gets attached to growing particles or growing particles may get attached to each other and form aggregates.
Innovatively, in the present study, the liquid nitrogen treatment causes the nuclei’s surface energy to rise, and as a result, the nuclei tend to grow together following the progression called Ostwald ripening effect. According to the Ostwald ripening theory, small-sized embryos have a high surface energy. That high surface energy could cause them to dissolve again while preventing the production of new nuclei. This fact encourages diffusion-based growth promoting larger sized primary particles formation. Thus, in sample 2, nitrogen treatment raises the surface energy of the embryo resulting in a greater positive Gibbs energy (∆G). This leads to unstable embryo development, which in turn causes the Ostwald ripening action to dissolve the embryo. This ultimately explains why the nuclei in sample 2 are larger and more tightly packed together at nanoscale (compared to sample 1). On the other hand, thanks to nitrogen treatment that also alters the drying behavior of the precipitates. Bai et al. [53] have discussed that in the conventional precipitation—drying process (sample 1), continuous evaporation of the liquid phase between particles results in the generation of attractive forces among neighboring particles. The surface tension of the particles, coupled with the removal of liquid, leads to complete drying. This in turn creates pressure pushing the particles toward each other leading to the formation of irregular plate-like aggregates (made of tightly attached particles in sample 1, Fig. 1a, c). In contrast, nitrogen treatment (prior to drying) in sample 2 introduces freezing and supersaturation, leading to more globular shaped agglomerate formation (Fig. 2a). Due to which the liquid phase between growing particles solidifies into ice, preventing further growth of embryo and enhancing Ostwald ripening by increasing surface energy. That is why during the subsequent drying process, the force of attraction caused by surface tension is either nonexistent or minimal [53]. Thus, the formation of aggregates is not prominent, as the driving force for particle coalescence is absent or negligible [54, 55].
Figure 2b shows the XRD results of Samples 1 and 2. All the peaks match with those of hematite Fe2O3 (PDF 04-003-2900) and there is no additional peak belonging to/showing any other compound or phase presence. This implies the successful recovery of ions from the leachate solution in their oxide form. Herein, no chromium oxide peak appears on the XRD of both powders which is in well agreement with literature [56]. As explained by Benny et al. [57] due to similar ionic radii of chromium with iron (Fe+3 0.67 Å; Cr+3 0.64 Å) and its ability to form solid solution at all concentration ranges, in both samples, substitutional solid solution is present.
To further validate the result of XRD analysis and determination of crystallite size and lattice parameters, Rietveld analysis is also carried out using DIFFRAC.TOPAS 64 V.6 (BRUKER) software interface (online supplementary Fig. S2) with a goodness of fit of 1.04 for sample 1 and 1.26 for sample 2. Table 1 indicates the lattice parameters of both samples. Sample 2 demonstrates smaller lattice parameters (i.e., a and c) which could be related to the presence of slightly higher amount of Cr to replace Fe atoms in the crystal structure, as discussed by Riaz et al. [56]. This decrease in the lattice parameters generates compressive stress on crystal causing higher strain in sample 2 [58].
Figure 3a shows the FTIR curve of both samples’ peaks appearing at 439.9 cm−1 (for sample 1), 441.03 cm−1 (for sample 2); 531 cm−1 (for sample 1) and 541 cm−1 (for sample 2) are due to Fe–O bond [59]. There is peak shift in the FTIR spectrum due to increased atomic vibrations with decreasing lattice constant, as agreed with XRD pattern. Possible replacement of Cr+3 in Fe+3 may explain this shift, as mentioned in the earlier studies [60]. The minor peaks between 1000 and 1100 cm−1 can also be assigned to the Fe–O stretching [18]. The several noisy peaks at around 2000 cm−1 and between 3500 and 4000 cm−1 are due to H–OH and O–H bond stretching [59, 61]. Figure 3b represents the Raman spectrum of both samples; it contains the characteristics peaks of hematite in proximity of 220 cm−1, 285 cm−1, and 645 cm−1 attribute the Fe–O bond, these peaks positions are harmonized well with existing literature [58, 62]. Though a little peak shift occurs for both samples (642 cm−1 sample 2 and 655 cm−1 sample 1), this shift is ascribed to increased substitutionally contained Cr [58].
For further investigation of the nitrogen treatment effect on the chemical bond energies, XPS is carried out on both samples (Fig. 3c). Both XPS results are the same and no extra peak related to nitrogen is noted. As reported by Aalim et al. [60], the Fe2p3/2 and Fe2p1/2 peaks of pure hematite appear at 710 eV and 714 eV, respectively, but due to incorporation of Cr, there exists the peak shift in iron peaks toward high binding energy. In current study, the said peaks appear at 711 eV and 724 eV, indicating the substitution of Cr in hematite as the peaks are shifted toward high binding energy comparing to pure hematite. The band gap energies of the two samples (Fig. 3d) are also calculated in UV–Vis region using Tauc’s equation (Eq. 6) [63,64,65].
Here, \(\boldsymbol{\alpha }\) is absorption coefficient, h is planks constant, v is frequency of incident light, A is coefficient related to effective mass of electron, r is 0.5 for direct band gap and 2 for indirect band gap transition, and Eg is band gap energy. Upon extra plotting the linear region, the band gap energies of both sample are found to be 2.77 eV and 2.74 eV for sample 1 and sample 2, respectively. The monotonic reduction in band gap energy of hematite in sample 2 is due to relatively higher Cr content [58], which is beneficial to have, as chromium substitution eventually introduces reduction in lattice parameters, hence elevation in electrochemical performance.
Galvanostatic charge–discharge performances of both samples up to 200 cycles are given in Fig. 4a, b. 1st discharge capacities of Sample 1 and Sample 2 are found to be 848.8 mAh/g (0.371 mg/cm) and 1352 mAh/g (0.265 mg/cm), respectively. A decrease in capacity is observed for both samples, then the capacity tends to increase. After 200 cycles, the capacities delivered by sample 1 and sample 2 are noted to be 480 mAh/g and 847 mAh/g, respectively. The electrochemical performances of both samples are superior in comparison to the performance of the synthetically fabricated pure Fe2O3 powder [66, 67]. This superior performance can be attributed to the structural, chemical, and morphological properties of the electrode: Higher capacity of sample 2 can be attributed to its homogenous, and globular particles resulted by Ostwald ripening effect, and solid solutions rather than pure crystalline structure [68, 69]. Further, higher Cr solid solution reduced the band gap energy of the sample 2 and gave rise to imperfections corresponding to dislocations which induces the local stress fields because of reduced lattice parameters due to Cr doping [58], such introduced imperfections help to elevate the capacity.
Figure 4c, d shows the voltage–capacity curves which demonstrate the conversion reaction occurring in the electrodes. The lithiation mechanism (Eqs. 7–9) of iron oxide in cycling is explained by Larcher et al. [70] and other researchers [71,72,73]. Figure 4c, d agrees with those reports: For sample 2, at around 1.48 V, a change in slope is noted which shows the Li reaction with iron oxide. At this potential, a very limited amount of Li (1.8 mol of Li per mole of Fe2O3) is intercalated into iron oxide by formation of intermediate Li-intercalated phase (Li2(Fe2O3)) [70, 71]. Then, the occurrences of two peaks at around 0.95 V and 0.7 V versus Li/Li+ are ascribed to phase transformation of lithium iron oxide (α-LiFeO2) [74] along with SEI (solid electrolyte interface) formation (see Eqs. 7–9) [75, 76]:
The trend seen in the capacity-cycle performances of the electrodes (first decreasing then increasing) is discussed by Guo et al. [77]. They claim that there are two possible justifications for this initial decrease and then subsequent increase in the discharged capacity. According to them, (i) The initial drop in capacity is because of irreversible reactions that produce Fe nanoparticles. This metallic Fe increases the conductivity of the anode. This eventually improves the charge transfer kinetics ending up with increasing capacity in the next cycles. (ii) Another reason could be the decomposition of the electrolyte that causes formation of a polymer-based SEI layer over the electrode surface. This SEI assists in mechanical cohesion of active material without any significant interference to ions transportation. Moreover, the SEI layer may also store additional lithium ions by exhibiting pseudocapacitive effect, notably in low voltages [7]. Alternatively, Xie et al. [78] report that such fluctuation in capacity over cycles may be ascribed to enhanced reoxidation kinetics of the electrode as the number of Li+ accessible sites is increased due to activation of the active crystallites in cycling.
Figure 5a, b show the CV results of both samples 1 and 2. The shape of the curve is changed from 1st to other cycles in both electrodes. Irreversible reaction related to phase transformation and SEI formation may explain this change. In the first cathodic scan of sample 1 (Fig. 5a), three peaks are appearing at around 0.7 V, 0.9 V, and 1.42 V, then in the 2nd discharge reaction, bumps related to 0.75 and 1.2 V are seen which can be due to the formation of lithia (Li2O) and Fe° [79]. In the anodic scan, the broad peak at 1.5 V is ascribed to the reversal of lithium intercalation and Fe related reactions [80,81,82]. In case of sample 2, a very minute and broad peak appeared at cathodic scan which are attributed to (Eqs. 7–9) [71] and SEI formation. This peak later gets shifted to 0.8 V and 1.1 V in subsequent cycles. Like sample 1, in anodic scan of sample 2 just a broad peak appears at 1.5V is noted [80,81,82].
Additionally, the assessment of reversible capacity delivered by material under different current densities is also investigated in sample 2 (Fig. 5c). The outcomes illustrate the excellent electrochemical performance of sample 2 even at higher current loads which shows its structural rigidity. The capacity delivered at 1 A/g load is above 500 mAh/g and upon decreasing current density to 100 mA/g sample 2 significantly recoveres its initial capacity. This indicates that the electrode structure has not been deteriorated and the electrode does not peel or decompose even under a high current load (Fig. 5c).
The electrochemical impedance of both electrodes’ half cells is taken after 200 cycles (Fig. 6). Sample 1 has higher SEI and charge transfer resistance than sample 2. The main reason is the more homogeneous, and globular morphology of sample 2 favors the lithiation kinetics.
Then to understand the prevalent mechanism on the capacity of sample 2, sweep CV test is performed on both samples [81]. Firstly, the electrode has been cycled 3 times at 50 mA/g between 1 mV and 3 V, for the stabilization of the electrode/electrolyte interface. Later, voltammetry is applied with different scan rates of 0.3 mVs−1, 0.5 mVs−1, 1 mVs−1, and 1.5 mVs−1 between the same cutoff voltage range 1 mV and 3 V (Fig. 7a and b). Then, log of peak current values has been taken and plotted versus scan rate and fitted with the linear model (Fig. 7c, d). In the literature, it is mentioned that the capacitive effect can be quantified using the Eq. 10 [83], where a and b are constants. When the value of b is considered: 0.5 signifies that the reaction is diffusion controlled; while when the value of b is 1, it signifies that the reaction has capacitive control. Figure 7c, d shows ln (i, peak current)—ln (v, sweep rate) of two peaks detected at the anodic and cathodic sides of CV. The fitted line’s slope gives b value. Regression analysis of each fitted line indicates that the fitting is perfectly done as R2 and R2-adj are close to 1. The value of b for sample 2 shows mixed control, while b value of sample 1 (close to 0.5) is more diffusion controlled. The mixed controlled mechanism of sample 2 is because of its homogeneous micron-sized morphology which favors the lithium reaction by diffusion, as well as some contribution of capacitive controlled reaction due to pseudocapacitive effect [84]. As explained by Yin et al. [85], the pseudocapacitive effect (in sample 2) is mainly attributed due to homogeneous morphology of nano-sized primary particles.
Figure 8 contains the ex situ image of the electrode containing both powders, cycled for 200 cycles. SEM images show that sample 2 retains the electrode integrity after being cycled for 200 cycles, while on sample 1 remarkable cracks are noted resulting in low-capacity retention in sample 1. This reveals that sample 2 does not possess smaller particles, compared to sample 1. However, having the smallest particle is not necessary in all cases but adequately small and homogeneous distribution is more important that imparts significant structural stability as well as performance.
Conclusion
This study exemplifies how with the right material selection and process design, waste materials can be employed as starting materials in electrode active material synthesis. Within the scope of this study, the effects of nitrogen treatment during the precipitation process and how that treatment affects the powder’s performance as an electrode material are examined for the first time in the literature. The morphological, structural, and chemical characterizations’ results show that by applying nitrogen treatment during the precipitation process, the new embryos formation is hindered by Ostwald ripening phenomenon as their surface energy is modified hence the formation of nuclei then particles is altered, resulting in difference electrochemical performance, eventually.
The output of this work can be summarized as follows:
-
Adaptation of nitrogen treatment in precipitation process controls the surface energy of nuclei, hence particle properties.
-
By adjusting pH in precipitation, some Cr are precipitated along with Fe to form a mixed transition metal oxide in the form of a solid solution.
-
First time in the literature, inexpensive household product, a steel scourer, is utilized in the production of anode materials for lithium-ion batteries. A three-step process is designed including leaching, precipitation, and calcination, to fabricate mixed transition metal oxide powders.
-
Sample 2 performs the best when it is used as anode active material and delivers the highest capacities after 1st (1358 mAhg−1 with 71% coulombic efficiency) and 200th (847 mAhg−1 with 97% coulombic efficiency) cycles. The superior performance of the sample 2 anode material is attributed to several factors: The homogeneity in morphology and particle size (~ 150 nm) enhances the cycle life. And the imperfections in solid solution and the dense characteristic promote its rate performance.
References
Barré A, Deguilhem B, Grolleau S, Gérard M, Suard F, Riu D (2013) A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J Power Sources 241:680–689. https://doi.org/10.1016/J.JPOWSOUR.2013.05.040
Lu L, Han X, Li J, Hua J, Ouyang M (2013) A review on the key issues for lithium-ion battery management in electric vehicles. J Power Sources 226:272–288. https://doi.org/10.1016/J.JPOWSOUR.2012.10.060
Maqelepo L, Williams N, Taneja J, Mohamed Omar Al-Guthmy F, Yan W, Zhang R et al (2022) Analysis of the Li-ion battery industry in light of the global transition to electric passenger light duty vehicles until 2050. Environ Res Infrastruct Sustain 2:011002. https://doi.org/10.1088/2634-4505/AC49A0
U.S. Department of Energy (2020) Energy storage grand challenge: energy storage market report.
Wen J, Yu Y, Chen C (2012) A review on lithium-ion batteries safety issues: existing problems and possible solutions. Mater Express 2:197–212. https://doi.org/10.1166/MEX.2012.1075
Gao SW, Gong XZ, Liu Y, Zhang QQ (2018) Energy consumption and carbon emission analysis of natural graphite anode material for lithium batteries. Mater Sci Forum 913:985–990. https://doi.org/10.4028/WWW.SCIENTIFIC.NET/MSF.913.985
Varghese B, Reddy MV, Yanwu Z, Lit CS, Hoong TC, Rao GVS et al (2008) Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chem Mater 20:3360–3367. https://doi.org/10.1021/CM703512K/SUPPL_FILE/CM703512K-FILE002.PDF
Kim H, Cho J (2008) Hard templating synthesis of mesoporous and nanowire SnO2 lithium battery anode materials. J Mater Chem 18:771–775. https://doi.org/10.1039/B714904B
Lee GH, Lee JW, Choi JIL, Kim SJ, Kim YH, Kang JK (2016) Ultrafast discharge/charge rate and robust cycle life for high-performance energy storage using ultrafine nanocrystals on the binder-free porous graphene foam. Adv Funct Mater 26:5139–5148. https://doi.org/10.1002/ADFM.201601355
Li WY, Xu LN, Chen J (2005) Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv Funct Mater 15:851–857. https://doi.org/10.1002/ADFM.200400429
Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ (2008) Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv Funct Mater 18:3941–3946. https://doi.org/10.1002/ADFM.200801386
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM (2000) Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407:496–499. https://doi.org/10.1038/35035045
Malini R, Uma U, Sheela T, Ganesan M, Renganathan NG (2009) Conversion reactions: a new pathway to realise energy in lithium-ion battery—review. Ionics 15:301–307. https://doi.org/10.1007/S11581-008-0236-X
Kraytsberg A, Ein-Eli Y (2017) A critical review-promises and barriers of conversion electrodes for Li-ion batteries. J Solid State Electrochem 21:1907–1923. https://doi.org/10.1007/S10008-017-3580-9
Zheng M, Tang H, Li L, Hu Q, Zhang L, Xue H et al (2018) Hierarchically nanostructured transition metal oxides for lithium-ion batteries. Adv Sci. https://doi.org/10.1002/ADVS.201700592
McNulty D, Geaney H, Carroll E, Garvey S, Lonergan A, O’Dwyer C (2017) The effect of particle size, morphology and C-rates on 3D structured Co3O4 inverse opal conversion mode anode materials. Mater Res Express 4:025011. https://doi.org/10.1088/2053-1591/AA5A26
Liang C, Gao M, Pan H, Liu Y, Yan M (2013) Lithium alloys and metal oxides as high-capacity anode materials for lithium-ion batteries. J Alloys Compd 575:246–256. https://doi.org/10.1016/J.JALLCOM.2013.04.001
Habibi MH, Fakhri F (2017) Hydrothermal synthesis of nickel iron oxide nano-composite and application as magnetically separable photocatalyst for degradation of Solar Blue G dye. J Mater Sci: Mater Electron 28:14091–14096. https://doi.org/10.1007/S10854-017-7261-3/METRICS
Courtel FM, Abu-Lebdeh Y, Davidson IJ (2012) ZnMn2O4 nanoparticles synthesized by a hydrothermal method as an anode material for Li-ion batteries. Electrochim Acta 71:123–127. https://doi.org/10.1016/J.ELECTACTA.2012.03.108
Zhao L, Chen H, Wang Y, Che H, Gunawan P, Zhong Z et al (2012) Facile solvothermal synthesis of phase-pure Cu4O3 microspheres and their lithium storage properties. Chem Mater 24:1136–1142. https://doi.org/10.1021/CM203589H/SUPPL_FILE/CM203589H_SI_001.PDF
Zhang Z, Li W, Ng TW, Kang W, Lee CS, Zhang W (2015) Iron(II) molybdate (FeMoO4) nanorods as a high-performance anode for lithium ion batteries: structural and chemical evolution upon cycling. J Mater Chem A Mater 3:20527–20534. https://doi.org/10.1039/C5TA05723J
Bigiani L, Barreca D, Gasparotto A, Andreu T, Verbeeck J, Sada C et al (2021) Selective anodes for seawater splitting via functionalization of manganese oxides by a plasma-assisted process. Appl Catal B 284:119684. https://doi.org/10.1016/J.APCATB.2020.119684
Lou XW, Yuan C, Archer LA (2007) Shell-by-shell synthesis of tin oxide hollow colloids with nanoarchitectured walls: cavity size tuning and functionalization. Small 3:261–265. https://doi.org/10.1002/SMLL.200600445
Lou XW, Li CM, Archer LA (2009) Designed synthesis of coaxial SnO2@carbon hollow nanospheres for highly reversible lithium storage. Adv Mater 21:2536–2539. https://doi.org/10.1002/ADMA.200803439
Chang JP, Lin Y-S, Chu K (2001) Rapid thermal chemical vapor deposition of zirconium oxide for metal-oxide-semiconductor field effect transistor application. J Vacuum Sci Technol B Microelectron Nanometer Struct Process Measurement Phenom 19:1782–1787. https://doi.org/10.1116/1.1396639
van der Giessen AA (1966) The structure of iron (III) oxide-hydrate gels. J Inorg Nucl Chem 28:2155–2159. https://doi.org/10.1016/0022-1902(66)80100-8
van der Giessen AA (1968) Chemical and physical properties of iron(III)-oxide hydrate. Eindhoven University of Technology, Eindhoven. https://doi.org/10.6100/IR23239
Ishikawa T, Ueno T, Yasukawa A, Kandori K, Nakayama T, Tsubota T (2002) Structures of nanosized Fe–Ti mixed oxide particles produced by freezing method. J Mater Chem 12:2416–2420. https://doi.org/10.1039/B202509B
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL (2013) Synthesis and electrochemical performance of Li1.131Mn0.504Ni0.243Co0.122O2 cathode materials for lithium ion batteries via freeze drying. J Power Sources 221:300–307. https://doi.org/10.1016/J.JPOWSOUR.2012.08.031
Surace Y, Simões M, Karvonen L, Yoon S, Pokrant S, Weidenkaff A (2015) Freeze drying synthesis of Li3MnO4 cathode material for Li-ion batteries: a physico-electrochemical study. J Alloys Compd 644:297–303. https://doi.org/10.1016/J.JALLCOM.2015.04.221
Qiao YQ, Wang XL, Mai YJ, Xia XH, Zhang J, Gu CD et al (2012) Freeze-drying synthesis of Li3V2(PO4)3/C cathode material for lithium-ion batteries. J Alloys Compd 536:132–137. https://doi.org/10.1016/J.JALLCOM.2012.04.118
Yao J, Yang Y, Li Y, Jiang J, Xiao S, Yang J (2021) Interconnected α-Fe2O3 nanoparticles prepared from leaching liquor of tin ore tailings as anode materials for lithium-ion batteries. J Alloys Compd 855:157288. https://doi.org/10.1016/J.JALLCOM.2020.157288
Wu N, Zhang X, Ma C, Shi YR, Zhou JM, Wang Z et al (2019) High performance isomeric Fe2O3 nanospheres anode materials derived from industrial wastewater for lithium ion batteries. Electrochim Acta 297:1028–1034. https://doi.org/10.1016/J.ELECTACTA.2018.12.059
Kang F, Wu M, Xiao B, Chen R, Wei Y, Liu H et al (2020) Facile synthesis of schwertmannite@akaganeite core/shell nanostructure from pickling waste liquor: formation mechanism and potential application. J Clean Prod 260:120961. https://doi.org/10.1016/J.JCLEPRO.2020.120961
Wei T, Zhang Z, Zhu Z, Zhou X, Wang Y, Wang Y et al (2019) Recycling of waste plastics and scalable preparation of Si/CNF/C composite as anode material for lithium-ion batteries. Ionics 25:1523–1529. https://doi.org/10.1007/S11581-019-02892-Y/FIGURES/5
Zhu X, Xiao J, Chen Y, Tang L, Hou H, Yao Z et al (2022) A high-performance nano-Sn/G@C composite anode prepared by waste carbon residue from spent Lithium-ion batteries. Chem Eng J 450:138113. https://doi.org/10.1016/J.CEJ.2022.138113
Sharma I, Deshan ADK, Pham HD, Padwal C, Doherty WOS, Dubal D (2022) Zero-waste: carbon and SiO2 composite materials from the solid residue of the hydrothermal liquefaction of anaerobic digestion digestate for Li-ion batteries. Sustain Mater Technol 31:e00364. https://doi.org/10.1016/J.SUSMAT.2021.E00364
Zhao Y, Li X, Yan B, Xiong D, Li D, Lawes S et al (2016) Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv Energy Mater 6:1502175. https://doi.org/10.1002/AENM.201502175
Pan X, Duan X, Lin X, Zong F, Tong X, Li Q et al (2018) Rapid synthesis of Cr-doped γ-Fe2O3/reduced graphene oxide nanocomposites as high performance anode materials for lithium ion batteries. J Alloys Compd 732:270–279. https://doi.org/10.1016/J.JALLCOM.2017.10.222
Yu W, Lin PY, Jie ZY (2016) Effect of purification pretreatment on the recovery of magnetite from waste ferrous sulfate. Int J Miner Metall Mater 23:891–897. https://doi.org/10.1007/S12613-016-1304-2/METRICS
Mbedzi N, Ibana D, Dyer L, Browner R (2017) The effect of oxidant addition on ferrous iron removal from multi-element acidic sulphate solutions. AIP Conf Proc. https://doi.org/10.1063/1.4974413
Köse CH, Topkaya YA (2011) Hydrometallurgical processing of nontronite type lateritic nickel ores by MHP process. Miner Eng 24:396–415. https://doi.org/10.1016/J.MINENG.2010.11.010
Papassiopi N, Vaxevanidou K, Christou C, Karagianni E, Antipas GSE (2014) Synthesis, characterization and stability of Cr(III) and Fe(III) hydroxides. J Hazard Mater 264:490–497. https://doi.org/10.1016/J.JHAZMAT.2013.09.058
Das RP, Anand S (1995) Precipitation of iron oxides from ammonia-ammonium sulphate solutions. Hydrometallurgy 38:161–173. https://doi.org/10.1016/0304-386X(94)00052-5
Bell A, Matijević E (1975) Complex chemistry of hydrous chromium(III) oxide sol formation. J Inorg Nucl Chem 37:907–912. https://doi.org/10.1016/0022-1902(75)80671-3
Wermink WN, Versteeg GF (2017) The oxidation of Fe(II) in acidic sulfate solutions with air at elevated pressures. Part 2. Influence of H2SO4 and Fe(III). Ind Eng Chem Res 56:3789–3796. https://doi.org/10.1021/ACS.IECR.6B04641/ASSET/IMAGES/MEDIUM/IE-2016-04641S_0008.GIF
Flynn CM (1990) Dense hydrolysis products from iron (III) nitrate and sulfate solutions. Hydrometallurgy 25:257–270. https://doi.org/10.1016/0304-386X(90)90042-Z
Chen J, Tian Y (2021) Hexavalent chromium reducing bacteria: mechanism of reduction and characteristics. Environ Sci Pollut Res 28:20981–20997. https://doi.org/10.1007/S11356-021-13325-7
Pogliani L, Ameta SC, Haghi AK (2020) Chemistry and industrial techniques for chemical engineers. Routledge, London. https://doi.org/10.1201/9780429286674
Thanh NTK, Maclean N, Mahiddine S (2014) Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev 114:7610–7630. https://doi.org/10.1021/CR400544S
Bosetti L, Ahn B, Mazzotti M (2022) Secondary nucleation by interparticle energies. I. Thermodynamics. Cryst Growth Des 22:87–97. https://doi.org/10.1021/ACS.CGD.1C00927/SUPPL_FILE/CG1C00927_SI_001.PDF
Wu KJ, Tse ECM, Shang C, Guo Z (2022) Nucleation and growth in solution synthesis of nanostructures—from fundamentals to advanced applications. Prog Mater Sci 123:100821. https://doi.org/10.1016/J.PMATSCI.2021.100821
Bai Y, Li Y, Wu C, Lu J, Li H, Liu Z et al (2015) Lithium-rich nanoscale Li1.2Mn0.54Ni0.13Co0.13O2 cathode material prepared by co-precipitation combined freeze drying (CP–FD) for lithium-ion batteries. Energy Technol 3:843–850. https://doi.org/10.1002/ENTE.201500030
Egerton TA, Tooley IR (2014) Physical characterization of titanium dioxide nanoparticles. Int J Cosmet Sci 36:195–206. https://doi.org/10.1111/ICS.12113
Eggersdorfer ML, Pratsinis SE (2014) Agglomerates and aggregates of nanoparticles made in the gas phase. Adv Powder Technol 25:71–90. https://doi.org/10.1016/J.APT.2013.10.010
Riaz S, Akbar A, Naseem S (2014) Ferromagnetic effects in Cr-doped Fe2O3 thin films. IEEE Trans Magn. https://doi.org/10.1109/TMAG.2014.2312977
Benny S, Grau-Crespo R, De Leeuw NH (2009) A theoretical investigation of α-Fe2O3–Cr2O3 solid solutions. Phys Chem Chem Phys 11:808–815. https://doi.org/10.1039/B815907F
Irshad I, Lone AG, Want B (2024) Exploring the magnetic, optical and dielectric properties of Cr- doped hematite (α-Fe(2–x)CrxO3): a comprehensive study. J Alloys Compd 971:172696. https://doi.org/10.1016/J.JALLCOM.2023.172696
Adegoke HI, AmooAdekola F, Fatoki OS, Ximba BJ (2014) Adsorption of Cr (VI) on synthetic hematite (α-Fe2O3) nanoparticles of different morphologies. Korean J Chem Eng 31:142–154. https://doi.org/10.1007/S11814-013-0204-7/METRICS
Aalim M, Irshad I, Tantray AM, Sohail A, Want B, Shah MA (2023) Effect of chromium (Cr)-doping on electrochemical performance of microwave synthesized hematite (α-CrxFe2−xO3) nanosheets for supercapacitor application. J Mater Sci: Mater Electron 34:1–18. https://doi.org/10.1007/S10854-023-10825-9/FIGURES/13
Mohapatra M, Layek S, Anand S, Verma HC, Mishra BK (2013) Structural and magnetic properties of Mg-doped nano-α-Fe2O3 particles synthesized by surfactant mediation–precipitation technique. Phys Status Solidi 250:65–72. https://doi.org/10.1002/PSSB.201248151
Kilias SP, Chatzitheodoridis E, Lyon I (2017) Molecular, chemical and morphological evidence for hematite biogenicity at the quaternary cape Vani Mn-(Ba-Fe) deposit, Milos, Greece. Bull Geol Soc Greece 47:834. https://doi.org/10.12681/BGSG.11122
Sunny A, Prasad N, Subbaiyan D, Dillibabu S, Balasubramanian K (2018) Optical, vibrational and fiber optic gas-sensing properties of hematite microparticles. Appl Phys A Mater Sci Process 124:1–8. https://doi.org/10.1007/S00339-018-2077-4/FIGURES/9
(PDF) Band gap determination using Tauc’s plot for LiNbO 3 thin films n.d. https://www.researchgate.net/publication/279203048_Band_gap_determination_using_Tauc’s_plot_for_LiNbO_3_thin_films. Accessed 18 Feb 2024
Rahman MM, Hussain MM, Asiri AM (2017) Fabrication of 3-methoxyphenol sensor based on Fe3O4 decorated carbon nanotube nanocomposites for environmental safety: real sample analyses. PLoS ONE 12:e0177817. https://doi.org/10.1371/JOURNAL.PONE.0177817
Yan N, Zhou X, Li Y, Wang F, Zhong H, Wang H et al (2013) Fe2O3 nanoparticles wrapped in multi-walled carbon nanotubes with enhanced lithium storage capability. Sci Rep 3:1–6. https://doi.org/10.1038/srep03392
Li W, Yang F, Rui Y, Tang B (2019) Strong covalent interaction Fe2O3/nitrogen-doped porous carbon fiber hybrids as free-standing anodes for lithium-ion batteries. J Mater Sci 54:6500–6514. https://doi.org/10.1007/S10853-019-03330-0
Jain G, Balasubramanian M, Xu JJ (2006) Structural studies of lithium intercalation in a nanocrystalline α-Fe2O3 compound. Chem Mater 18:423–434. https://doi.org/10.1021/CM052014F/ASSET/IMAGES/MEDIUM/CM052014FN00001.GIF
Capone I, Hurlbutt K, Naylor AJ, Xiao AW, Pasta M (2019) Effect of the particle-size distribution on the electrochemical performance of a red phosphorus-carbon composite anode for sodium-ion batteries. Energy Fuels 33:4651–4658. https://doi.org/10.1021/ACS.ENERGYFUELS.9B00385/ASSET/IMAGES/LARGE/EF-2019-00385D_0005.JPEG
Larcher D, Bonnin D, Cortes R, Rivals I, Personnaz L, Tarascon J-M (2003) Combined XRD, EXAFS, and Mössbauer studies of the reduction by lithium of α-Fe[sub 2]O[sub 3] with various particle sizes. J Electrochem Soc 150:A1643. https://doi.org/10.1149/1.1622959/XML
Reddy MV, Yu T, Sow CH, Shen ZX, Lim CT, Rao GVS et al (2007) α-Fe2O3 nanoflakes as an anode material for li-ion batteries. Adv Funct Mater 17:2792–2799. https://doi.org/10.1002/ADFM.200601186
Morales J, Sánchez L, Martín F, Berry F, Ren X (2005) Synthesis and characterization of nanometric iron and iron-titanium oxides by mechanical milling. J Electrochem Soc 152:A1748. https://doi.org/10.1149/1.1972812
Chen J, Xu L, Li W, Gou X (2005) α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv Mater 17:582–586. https://doi.org/10.1002/ADMA.200401101
Fang S, Bresser D, Passerini S (2020) Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries. Adv Energy Mater. https://doi.org/10.1002/AENM.201902485
Yang T, Liu Y, Huang Z, Yang Q, Chen Y, Hu M et al (2014) Porous peony-like α-Fe2O3 hierarchical micro/nanostructures: synthesis, characterization and its lithium storage properties. RSC Adv 4:41578–41583. https://doi.org/10.1039/C4RA06709F
Kong F, Kostecki R, Nadeau G, Song X, Zaghib K, Kinoshita K et al (2001) In situ studies of SEI formation. J Power Sources 97–98:58–66. https://doi.org/10.1016/S0378-7753(01)00588-2
Guo J, Yang Y, Yu W, Dong X, Wang J, Liu G et al (2016) Synthesis of α-Fe2O3, Fe3O4 and Fe2N magnetic hollow nanofibers as anode materials for Li-ion batteries. RSC Adv 6:111447–111456. https://doi.org/10.1039/C6RA23601D
Xie Q, Zhao P, Xie D, Zhao Y, Zhang S, Huang W (2024) A multi-strategic exploration towards significantly enhanced electrochemical performance of Co3O4-based anodes for lithium-ion batteries. J Phys Chem Solids 193:112152. https://doi.org/10.1016/J.JPCS.2024.112152
Hassan MF, Guo Z, Chen Z, Liu H (2011) α-Fe2O3 as an anode material with capacity rise and high rate capability for lithium-ion batteries. Mater Res Bull 46:858–864. https://doi.org/10.1016/J.MATERRESBULL.2011.02.011
He C, Wu S, Zhao N, Shi C, Liu E, Li J (2013) Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 7:4459–4469. https://doi.org/10.1021/NN401059H
Liu H, Wang G, Park J, Wang J, Liu H, Zhang C (2009) Electrochemical performance of α-Fe2O3 nanorods as anode material for lithium-ion cells. Electrochim Acta 54:1733–1736. https://doi.org/10.1016/J.ELECTACTA.2008.09.071
Liu H, Wexler D, Wang G (2009) One-pot facile synthesis of iron oxide nanowires as high capacity anode materials for lithium ion batteries. J Alloys Compd 487:L24–L27. https://doi.org/10.1016/J.JALLCOM.2009.08.043
Duan H, Du L, Zhang S, Chen Z, Wu S (2019) Superior lithium-storage properties derived from a high pseudocapacitance behavior for a peony-like holey Co3O4 anode. J Mater Chem A Mater. https://doi.org/10.1039/c9ta00294d
Wang Q, Wu Y, Pan N, Yang C, Wu S, Li D et al (2022) Preparation of rambutan-like Co0.5Ni0.5Fe2O4 as anode for high–performance lithium–ion batteries. Front Chem 10:1052560. https://doi.org/10.3389/FCHEM.2022.1052560/BIBTEX
Yin H, Song C, Wang Y, Li S, Zeng M, Zhang Z et al (2013) Influence of morphologies and pseudocapacitive contributions for charge storage in V2O5 micro/nano-structures. Electrochim Acta 111:762–770. https://doi.org/10.1016/J.ELECTACTA.2013.08.005
Acknowledgements
The authors acknowledge gratefully REMER (Istanbul Medipol University)- for XRD, SEM, EDS Analyses. Moreover, thank Dr Alper Yeşilçubuk (from Arçelik, for XRF), Mr. Baris (from KOÇ University for XPS analysis), Dr. Hassan KURT, and Assoc Prof Dr Meral Yüce (from Sabancı University for Raman analysis).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Zhi Sun.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashraf, H., Karahan, B.D. Cryo-Assisted Nitrogen Treatment for the Fabrication of Nanoengineered, Mixed Transition Metal Oxide Anode from Inorganic Domestic Waste, for Lithium-Ion Batteries. J. Sustain. Metall. (2024). https://doi.org/10.1007/s40831-024-00891-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40831-024-00891-6