Abstract
Purpose
The soaring demand for cobalt for lithium-ion batteries has increased interest in the utilization of non-conventional cobalt sources. Such raw materials include complex ores containing minerals such as cobaltite and skutterudite, which, while rare, occur around the world, including in Finland, Canada, and the USA. The goal of this study was to evaluate the cradle-to-gate impacts of cobalt sulfate recovery from unutilized cobalt- and gold-bearing ores with the use of process simulation.
Methods
A literature analysis was conducted to establish the state-of-the-art processing methods for complex cobalt ores containing significant amounts of gold. The drafted process was simulated using HSC Sim software to obtain a mass and energy balance, which was compiled into a life cycle inventory (LCI). The environmental impact categories (global warming, acidification, eutrophication, ozone depletion, photochemical smog creation, water use) were calculated in GaBi software. Uncertainty regarding the possible future raw material composition was studied, and the simulation was used to investigate process performance and to evaluate the effect of variation in the process parameters on the environmental impact indicators.
Results and discussion
The results indicated that the main cobalt mineral type (cobaltite, linnaeite) had only minor effects on the evaluated impact categories. With cobaltite-dominated ores (High As case), the global warming potential (GWP) was estimated to be 20.9 kg CO2-eq, of which 12.7 kg CO2-eq was attributed to the hydrometallurgical process. With linnaeite-dominated ores, the equivalent values were 20.4 kg CO2-eq and 11.0 kg CO2-eq. The production of a high grade concentrate was observed to greatly decrease the impacts of the hydrometallurgical process, but the cobalt losses in the beneficiation stage and the mineral processing impacts would likely increase. The simulation showed that there is still potential to improve the cobalt recovery (to approximately 96%), which would also affect the indicator values.
Conclusions
The impacts were estimated prior to intensive metallurgical testing to determine the possible high impact areas in the process. Based on this, it is suggested that, during hydrometallurgical processing, improved treatment of cobalt-containing wash waters and the optimization of oxygen utilization efficiency in pressure leaching are the most significant ways to decrease the environmental impacts. Optimal solutions for the concentrate could be found when experimental data on the minerals processing steps becomes available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ongoing transition to electric mobility is projected to massively increase the demand for battery metals, such as cobalt, nickel, and lithium, many of which are considered critical raw materials in the EU (European Commission 2020). In ores, cobalt is usually extracted as a by- or co-product of nickel and copper, which makes the metal vulnerable to fluctuations in the demand for copper and nickel (Olivetti et al. 2017). Over 50% of the world’s cobalt is mined together with copper in the Democratic Republic of Congo (DRC), where small scale artisanal mining is estimated to cover approximately 20% of the country’s mining output and is associated with human rights violations, corruption, and environmental damage (Nkulu et al. 2018). Although end-of-life (EOL) batteries are estimated to become a significant source of secondary cobalt in the future, recycled cobalt alone will not be nearly enough to satisfy the increasing demand during the next decade (Alves Dias et al. 2018). Increasing and sustainable mine production of battery metals is necessary for widespread electrification.
Cobalt has historically also been produced as the main product from some complex arsenical deposits featuring minerals such as cobaltite (CoAsS), skutterudite (CoAs3), and smaltite (CoAs2). Although arsenical ores are rare, they can be found around the world, including in Norway (Groru 1997), Morocco, and Canada (Johnson et al. 2020), which could make them a potentially attractive raw material with the current development. The Blackbird mine in Idaho, USA, is perhaps the best-known example of the utilization of cobaltite ores, but the mine was closed in 1967 despite still containing considerable amounts of copper and cobalt (Gray et al. 2012). The increase in demand for cobalt has inspired several new projects in places such as Idaho and Finland (Anderson 2006; Witt et al. 2020). In addition to cobalt, many of these ores contain copper, gold, and silver, which may further drive the project economics (Slack 2010). Most gold is refined independently of other metals from primary gold ores, but gold is a significant by-product of the base metal industry due to its close association in deposits with chalcopyrite (CuFeS2) in particular. Most of the gold by-production thus originates from the copper industry, but it is also recovered in nickel, lead, and platinum group metal operations (Ferron 2005; Kyriakis 2005). Past examples of gold-bearing cobalt ores are also known from Canada, although these are rare (Ferron 2005).
Although process development in the field of base metal recovery from ores has been rapid, research literature on the environmental impacts of cobalt production is scarce despite the stringent environmental regulation. Published estimates exist for typical cobalt sources, such as the processing of DRC’s ores, but not for prospective raw materials or deposits currently being investigated. Dai et al. (2018) presented the life cycle inventory (LCI) data for cobalt and cobalt chemicals production at Chinese refineries from DRC’s Cu-Co ores. Cobalt was also one of the metals that was investigated on the study by Nuss and Eckelman (2014) based on the data from ecoinvent 2.2 database. Eckelman (2010) studied the energy footprint of different nickel products and considered the co-production of cobalt from Class I nickel production by using a mass-based allocation procedure. This methodology credited cobalt with 4% of the total output and therefore slightly decreased the intensity of Class I nickel refining. Khoo et al. (2017) addressed the burdens of laterite processing by both state-of-the-art and hypothetical routes, mainly to stainless steel. The state-of-the-art process in the treatment of limonite ores utilizing high pressure acid leaching (HPAL) is capable of recovering cobalt, and it was calculated that 13.5% of the burdens could be assigned to cobalt by combining mass allocation with economic allocation, which would correspond to 12.5 kg CO2-eq for 1 kg cobalt by calculation (Khoo et al. 2017).
Life cycle assessment (LCA) is a methodology for calculating the environmental footprint of a product or a process. While the methodology itself is standardized (ISO 14040:2006; ISO 14044:2006), discrepancies between system boundaries, assumptions, and calculation methods prevent the comparison of results between different studies, particularly in the metallurgical and mining sector. The reduction of metallurgical production systems into average black-box models in LCA studies has also been criticized, as it may be useful in presenting the results for a non-metallurgist audience but provides no insights into project development (Segura-Salazar 2019; Pell et al. 2019; Reuter et al. 2015). Recognizing the hotspots of a process and being aware of the risks associated with the disposal of waste streams is crucial in minimizing the impacts of mining and metal refining. Process simulation enables technical advancements and process updates to be investigated in the model throughout the project (Pell et al. 2019), which makes it an invaluable tool in evaluating the environmental footprint of a production process alongside LCA.
In this study, predictive simulation and life cycle models were built for the hydrometallurgical recovery of gold and cobalt sulfate from prospective complex cobalt- and gold-bearing concentrates. The process was modeled with the simulation module of the Metso Outotec’s (2021) HSC Chemistry software and the environmental indicators were calculated with GaBi (Sphera 2020). The goal of the study was to select the most suitable process routes for further investigation based on the environmental performance while also demonstrating the usefulness of the methodology in the scoping of possible process routes already during the pre-feasibility stage and for prospective processes. The use of process simulation and LCA could be used this way to predict different processing scenarios and their potential environmental impacts even prior to cost- and labor-intensive metallurgical testing and support development towards the most environmentally competitive processing options.
2 Materials and methods
The simulation-based LCA was conducted in three steps. First, a literature review was conducted to determine the model parameters, chemistry, and exractions at each process step. Second, process models were built based on the literature values and metallurgical know-how to compile LCI from mass and energy balances using HSC Sim 9. Lastly, an impact assessment was conducted using the GaBi software.
2.1 Raw material characterization
The concentrate was provided by Mawson Resources and originates from the Rajapalot gold-cobalt prospect in Finland. The sample was characterized by the SEM–EDS and XRD methods. Chemical analyses were conducted using both ICP-OES and ICP-MS and the gold content in the concentrate was analyzed after pre-treatment with the fire assay method. The chemical composition of the samples is presented in Table 1, as an average of the analyses.
Based on the mineral characterizations, iron was mainly present as pyrrhotite (Fe1-xS) and to a lesser extent as pyrite (FeS2). Earlier analyses of the concentrate support the notion that cobalt is found in cobaltite (CoAsS), or possibly cobalt pentlandite ((Co,Fe)9S8) and linnaeite (Co3S4) (AMC Consultants 2020). The concentrate also contained a small amount of chalcopyrite (CuFeS2). The gangue minerals were analyzed and consisted mainly of quartz, chlorite, and feldspar minerals with minor amounts of other minerals, such as rutile and uraninite. For a reasonably predictive mass and heat balance for the process, the minerology of the material was estimated based on the analyses (Table 2). It must be emphasized that the concentrate of this prospective mine is not in commercial use yet; therefore, the minerology must be considered rather an estimation based on a limited number of samples, analyses, and characterizations.
It should be acknowledged that the high content of chlorite minerals, mainly clinochlore but also chamosite, that are present in the concentrate may cause problems during flotation and in hydrometallurgical circuits especially in full scale operation (Tan et al. 2012). These minerals are acid dissolvable, which may result in excessive acid consumption and cause increased pulp viscosity in hydrometallurgical processing. This may disturb solid/liquid separation and the behavior of these gangue minerals can therefore have a drastic effect on the process (Tan et al. 2012; Chen 1998).
2.2 Life cycle assessment
The life cycle assessment was conducted using simulation-based methodology, which has previously been used to study the impacts of gold production (Elomaa et al. 2020a, b), copper (Abadìas Llamas et al. 2019), rare earths (Pell et al. 2019), and some secondary raw materials (Ghodrat et al 2017; Reuter 2015; Rinne et al. 2021). In the current study, material and energy flows in the process were calculated using the HSC Sim software and compiled into an LCI. The LCI was then exported to the GaBi LCA software and the impacts were calculated.
2.2.1 Goal, scope, and functional unit
The goal of the analysis was to perform a prospective cradle-to-gate environmental impact analysis for the hydrometallurgical processing of selected Co-Au concentrates to battery-grade cobalt sulfate, and to provide LCA practitioners process-level data on cobalt extraction from new raw materials. This can provide invaluable information for future mining projects and decision makers regarding the treatment of similar ores. The analysis was conducted in the GaBi LCA software with ecoinvent 3.5 (Ecoinvent 2018) and GaBi Professional databases 2020 version (Sphera 2020). The time scope was assumed to be potentially 2040–2050 based on the presumed approximate length of mining and refining projects, although this is uncertain.
The functional unit was defined as 1 kg cobalt sulfate heptahydrate (0.21 kg cobalt). Co-products were considered in the sensitivity analyses by different allocation methods.
2.2.2 System boundary and description
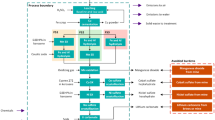
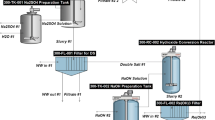
The analysis was performed cradle-to-gate, as shown in Fig. 1. All of the operations were assumed to occur in Finland, meaning that the Finnish electricity supply mix was used to represent electricity production. Transport of the concentrates and precipitates was thus excluded from the boundary. In addition to the units shown in the schematic diagram (Fig. 1), the process has additional solid/liquid separation and neutralization steps along with other ancillary units. The detailed flowsheets are available in the Supplementary Materials (Section S1).
The current Finnish electricity mix is not representative of the energy used in the assumed 2040–2050 time frame, which presumably affects the results. The use of heavy fuel oil in the heating of the process and diesel in mining may also change over the coming decades with the decarbonization of energy production. In 2018, the share of renewables in final energy consumption was 41%, while the use of nuclear power accounted to 18% (Statistics Finland 2019). For 2030, the goal is for 54% of total energy consumption to be from renewable sources (Ministry of Economic Affairs and Employment 2019). The effect of this could be assumed to be comparably small considering the already relatively clean electricity mix, and the decarbonization might be expected to affect the upstream processes more.
Mining and beneficiation were not modeled in detail, and instead proxy data from Norgate and Haque (2012) was used by adapting the data for the ore grades. Data for the open-pit mining of refractory gold concentrates was considered more representative of the ore than the available data for cobalt mining, which is why gold mining was used. The beneficiation referred to in Fig. 1 consists of ore comminution by grinding, and the upgrading of the ore by unit operations such as flotation, magnetic separation, and gravity separation based on the current state-of-the-art for gold and base metal ores. The actual flowsheet will be determined with more accurate details along project development based on metallurgical test work series and modeling.
A prospective process flowsheet was drafted based both on the available literature and the state-of-the-art practices in both complex sulfide ore and cobalt processing. The cobalt-gold-copper-containing concentrate was leached in sulfuric acid solutions at elevated temperature and pressure (base metal refining in Fig. 1), leaving gold in the leaching residue to be recovered by standard cyanidation and carbon adsorption (Au refining). Iron and arsenic (impurities) were first removed from the pressure leaching solution, after which copper was precipitated as copper sulfide that could potentially be used as raw material at existing copper smelters. Cobalt was separated from the dissolved magnesium by precipitation as cobalt sulfide—an intermediate which was refined further into battery-grade cobalt sulfate by re-leaching, solvent extraction, and crystallization (Co refining). The term “mining” refers to both mining and beneficiation hereinafter. A detailed description of the process units and the process phenomena is available in the Supplementary Material.
2.2.3 Impact categories
The investigated impact categories were global warming, acidification, freshwater eutrophication, photochemical ozone formation, stratospheric ozone depletion, gross energy requirement, freshwater aquatic ecotoxicity, human toxicity, and freshwater use. Awuah-Offei and Adekpedjou (2011) suggested that global warming, acidification, ozone depletion, acidification, eutrophication, human toxicity, and freshwater aquatic toxicity are well-suited for assessing the impacts of mining together with land, water, and energy use, and the categories were selected based on that.
2.3 Process simulation
Process simulation was used to study the effect of some process parameters on the performance and the environmental impacts of the modeled ore leaching process.
2.3.1 Simulated cases and model throughput
Two cases (High As and Low As) were simulated in the flowsheet to study the effect of raw material characteristics on the environmental performance of the process under high uncertainty. Variation in cobalt grade was also studied for both of the cases. The cobalt content in the concentrate was varied in the model from 0.5 to 10.0% by adjusting the content of cobalt minerals and quartz respectively while keeping the other minerals constant. The 1.35% Co grade was obtained from the analyses and was thus used as the fixed point, or “base” in the study. The limits for the concentrate (0.5% and 10%) were selected to study the behavior of the model over a wide range of concentrate compositions. In the past, cobaltite concentrates with grades averaging 17.5% Co have been utilized in Idaho (Fassell 1962), but the ore grades have fallen rapidly over the recent decades, which is also seen in the concentrate grades. The actual grades will be determined by thorough metallurgical test work on both cobalt and gold recovery, and thus, a wide scale was applied.
High As
The main cobalt mineral is cobaltite (CoAsS) and the As content of the concentrate consequently increases when the cobalt content does. Changing the cobalt content therefore also affected the percentage of arsenic (0.6–12.7%) and sulfur (13.9–16.3%) in the concentrate. The quartz content also varied between 0.1 and 18.8%.
Low As
Cobalt is present in linnaeite (Co3S4), leading to lower arsenic contents than in the High As case. Small amounts of arsenic (0.03%) were assumed to be found in the other minerals. Increasing the cobalt content also increased the sulfur content in the concentrate (13.7–17.7%) and the quartz content varied between 3.0 and 19.4%.
The throughput to the simulation model was also determined in this stage. Total global refinery production of cobalt was approximately 98 kt in 2016 (Alves Dias et al. 2018). While Finland is a significant producer of cobalt within the EU and a globally important supplier of refined cobalt, the country’s mine production (approximately 3000 t/a) accounts for less than 2% of global production (Alves Dias et al. 2018). The current estimation for the cobalt resources in the deposits concerned is approximately 5100 metric tons (AMC Consultants 2020). The throughput in the model was calculated assuming that the mine is operated for 10 years with 1.35% Co grade in the concentrate, giving approximately 5.4 metric tons of concentrate feed per hour. In the case of the industrial refining of the studied ore body, the annual Finnish cobalt mine production would also increase by approximately 15%, and approximately 2% in European (Finland, Norway, Belgium, France) refinery production (Alves Dias et al. 2018).
2.3.2 Assumptions in the simulation
The assumptions made in the process simulation affect the inputs and outputs of the model and are thus also transferred to the LCA step. The chemistry and the design parameters of the model are described in detail in the online Supplementary Material, and the effect of some of these assumptions was tested by sensitivity analysis in the current study.
The recovery rates of cobalt during pressure oxidation (base metal refining) were based on the earlier research work of Dannenberg et al. (1987), who studied the behavior of high-grade cobaltite-copper (5.3% Co, 3.6% Cu) concentrates in high pressure acid leaching. Based on the results, over 99% of both cobalt and copper were leached after 4 h at T = 195 °C and 100 psi (700 kPa) oxygen overpressure. Similar results were obtained in larger scale experiments, but the behavior of iron and arsenic was distinctly different depending on the reactor sizes. Approximately 26% of arsenic and 41% of iron were left in solution after four-hour leaching in the described conditions, which was a steep increase from the small batch experiments. The extraction percentages were used to control the precipitation of arsenic and iron in the model. Based on the same experiments, it was also assumed that 1% of dissolved cobalt and 7% of dissolved copper would co-precipitate during the rejection of iron and arsenic in base metal refining.
Iron behavior in pressure leaching depends on several factors, such as acidity, temperature, and the presence of alkali ions, with possible precipitates being hematite (Fe2O3), ferric arsenate (FeAsO4), basic ferric sulfate Fe(OH)SO4, and jarosites (MeFe3(SO4)2(OH)6, where Me is usually K or Na). Although this has little effect on the results of the model, it could be critical for the filterability of the residues and therefore essential for industrial scale operations. Furthermore, hematite is stable in the alkaline conditions of Au refining, whereas jarosite and basic ferric sulfate are not (Fleming 2010). In the absence of alkali, hematite and basic ferric sulfate were predicted to be the dominant iron compounds and form at equal rates in the pressure leaching steps, which was considered a reasonable compromise. All of the basic ferric sulfate was assumed to react with lime during the subsequent Au refining step.
In Au refining, gold was presumed to be almost completely leached (99%) after 24 h of cyanidation and effectively recovered by carbon adsorption (99%), using the conventional process parameters also assumed by Elomaa et al. (2020a). The gold in the ore is known to be amenable to cyanidation with high extraction rates and no preg-robbing is expected to occur. The pressure leaching treatment (during base metal refining) causes some uncertainty; however, it is possible that some of the gold becomes locked inside the forming iron precipitates, a phenomenon known to occur especially in the roasting of refractory gold ores (Wang et al. 2018). This is also likely to occur during pressure oxidation. The extraction and final recovery of gold was expected to have no meaningful effect on the results due to the functional unit in the LCA study.
It is important to acknowledge that the microstructure of the raw material and the gangue minerals have a profound effect on leaching extraction and behavior during the solid/liquid separation steps. More accurate information on the reaction rates, optimal conditions, and chemistry can only be obtained by thorough extensive and labor intensive metallurgical test work. Industrially proven and reliable process options were selected to counter this problem to the best extent, and the sensitivity of the environmental indicators to several process parameters was assessed to study the data gaps and the validity of the model.
3 Results and discussion
Results were obtained for both the process model and the environmental impacts for the High As and Low As cases. Further uncertainty in the life-cycle model was also addressed by conducting a sensitivity analysis for some of the parameters in the process simulation.
3.1 Life cycle inventory
The inputs and outputs from the process simulation were normalized for 1 kg cobalt sulfate (0.21 kg cobalt). The LCI data for the sub-systems described in Fig. 1—mining, base metal refining, Co refining, and Au refining—are presented in Table 3. The Finnish electricity grid mix was used to represent electricity and heavy oil burning was used for heat generation.
The analysis was conducted cradle-to-gate. The impact of mining and beneficiation was estimated based on the inventory data of Norgate and Haque (2012) for the open-pit mining of gold and the data was adapted to account for the ore grades. The mine operation step was the largest single source of uncertainty in the study due to the lack of any very representative literature data.
The electricity consumption of the process was calculated based on the mass balance obtained from the simulation. Reactors, filters, thickeners, crystallizers, and electrowinning were included, while the rest of the equipment, such as pumps and lights, were estimated to consume 1000 kWh based on the model throughput (5.4 t/h). The methodology used for calculating the energy consumption has previously been presented by Elomaa et al. (2020a) and Rinne et al. (2021). The heat balance of the process was used to estimate the need for cooling water or heating, and the heat was assumed to come from steam generated with heavy oil in the case of the hydrometallurgical units and natural gas for the furnaces in gold refining.
The aqueous emissions were estimated based on metal and sulfate contents in the effluents and the moisture remaining in solid residue cakes. The most significant ions either in terms of their impact (Huijbregts 1999) or their levels in the wastewaters were included in the inventory, while some (Fe(II,III), Na, Ca, Mg) were excluded. Sulfate-contaminated wastewaters are a typical problem for minerals processing and refining, which is why sulfate was included in the LCI. All of the ions were collectively inventoried under base metal processing due to their low levels in the individual flowsheets. Cyanide was presumed to break down completely to non-toxic cyanate, which was not included.
3.2 Impact analysis
The results are shown in Fig. 2 for each of the process steps (mining, base metal refining, Co refining, and Au refining). The overall GWP value was 20.9 kg CO2-eq for the High As case and 20.3 kg CO2-eq for the Low As case due to the small differences in the consumption of utilities and chemicals. Of this, the hydrometallurgical process accounted for 12.7 and 10.9 kg CO2-eq, respectively. Based on the analysis, the Co refining step is by far the least impactful, while mining and base metal refining appear the most significant steps in most of the categories. Mining was particularly overrepresented in terms of POCP, AP, EP, and HTP. The emissions from mining were mainly to air: the ozone-depleting substances were observed to be volatile organic compounds (VOCs), while NOx and ammonia contributed to both eutrophication and acidification. The cause for human toxicity was explained my indirect heavy metal emissions from the background processes. Minor gaseous H2S emissions may be expected from sulfide precipitation, and some gaseous emissions were assumed to occur during cyanide destruction, but it was seen that the emissions from the hydrometallurgical process were largely either soluble compounds to freshwater or indirectly from upstream processes.
The difference between the impacts of mining between the investigated cases was due to the difference in cobalt recovery, causing higher impacts in the Low As case. Chemical and heat consumption, on the other hand, were higher in the High As case, particularly in base metal refining, which increased the impacts of the hydrometallurgical process. The formation of ferric arsenates in the High As case also increased the amount of gold-bearing leach residues subjected to Au refining, which was seen in the consumption of water and some of the chemicals and thus in the environmental impacts. In terms of FAETP, the two cases were nearly equal, while High As case produced more emissions that were toxic to humans.
The differences between the investigated Low and High As cases were extremely small (e.g., AP: High As 0.30 kg SO2-eq, Low As 0.28 kg SO2-eq; ODP: High As 3.8E-6 kg R11-eq, Low As 3.4E-6 kg R11-eq). The slightly lower cobalt recovery in the Low As case was particularly reflected in mining, leading to higher environmental impacts particularly in the mining-dominated EP, POCP, and ODP indicators. In the other evaluated categories, the High As case was shown to have higher impacts. The effect of the main cobalt minerals was so small that the other, so far unknown, process phenomena and the uncertainty in the recovery rates had a more profound effect on the footprint of the process than the main cobalt minerals. This includes factors such as acid and cyanide consumption in side reactions and the effect of various minerals and precipitates on the solid/liquid separation. Some variables, such as carbon consumption in the gold CIL circuit, also depend on circuit design, which affects the breakdown of carbon.
Toxic emissions to aquatic life (FAETP) were mainly from base metal refining, where all of the soluble metals (Cu, As, Co) had been mapped. Considering the large difference between the cases in terms of As(V) emissions in Table 3 and the small difference seen between the cases, it was determined that soluble cobalt and particularly copper contributed to the category. The toxic compounds resulting from mining were observed to be heavy metals and organic compounds to air, with the main cause being the wearing of the grinding media (assumed chrome steel balls). The large difference between the cases in HTP, on the other hand, appears to be due to the release of arsenic.
It is noteworthy that relatively low cobalt recoveries were obtained without the treatment of filter wash waters for cobalt recovery. In the case of the presumed cobalt grade (Co = 1.35%), 81.6% of Co was recovered in the High As case and 81.3% in the Low As case. It was also observed that the cobalt losses decreased significantly when the cobalt grade in the concentrate fed to the hydrometallurgical process increased. This may be attributed to the decreasing volumes of filter wash water.
Based on the process simulation, cobalt recovery could thus be significantly increased by further flowsheet optimization, which would be reflected positively in the environmental impacts. Detailed results on the simulation results (valuable metal recoveries, waste streams, chemical consumption, heat balances) are available in the Supplementary Material. Assuming that the effect of increasing the recoveries would be minimal on the other process variables and flows, the improved recovery rate would decrease the GWP from 20.9 to 17.7 kg CO2-eq for the High As case and from 20.3 to 17.2 kg CO2-eq for the Low As case.
3.2.1 Contribution breakdown
The process had no clear direct emissions in most of the impact categories aside from mining, so the estimated impacts were primarily from upstream processes: energy and chemicals production. A detailed contribution analysis of chemicals and utilities is provided in Fig. 3. The effects of mining were more uncertain due to the lack of models specific to the ore.
Detailed contribution of chemicals and utilities to the environmental indicators in the process steps, with BM refining standing for base metal refining, including both High and Low As cases. The “Others” group consists mainly of minor reagents, including organics in solvent extraction, hydrogen sulfide, hydrochloric acid, and activated carbon. a Global warming. b Acidification. c Freshwater eutrophication. d Photochemical ozone creation. e Stratospheric ozone depletion. f Freshwater consumption. g Freshwater aquatic ecotoxicity. h Human toxicity
In mining, energy use and blasting accounted for most of the impacts, which was to be expected based on earlier studies on minerals and metals processing (Norgate et al. 2007; Norgate and Haque 2012). Diesel use explained most of the GWP, while blasting contributed the most significantly to most of the other categories. The direct consumption of water was also large in mining, as shown in Fig. 3f, while upstream processes explained most of the freshwater use in the hydrometallurgical process. Several earlier studies have concluded that clean energy is among the most impactful methods for reducing the emissions from mining and minerals processing (Farjana et al. 2019; Norgate et al. 2007). Since relatively low-carbon Finnish electricity was used, the share of electricity consumption was rather low in nearly all of the categories, regardless of the process stage. Switching from diesel to fully electric haul trucks could therefore be an attractive option to reduce emissions, but considering the time scope of the project (assuming 2040–2050), the electrification of mining may occur during that time frame, which would obviously reduce particularly greenhouse gases, ozone-generating substances (NOx).
In the hydrometallurgical process, oxygen production for the pressure leaching vessels (autoclaves) in both base metal processing and Co refining explained most of the impacts. Oxygen utilization can be optimized for large scale through impeller design and optimal oxygen partial pressure in leaching. Some of the oxygen will inevitably be lost to the pressure leaching off-gas. Although the recycling of the off-gas can reduce the oxygen consumption to nearly the stoichiometric amount, there are significant costs associated with this (Marsden and House 2006). Sulfuric acid production was also a noticeable source of acidifying emissions, and its consumption could be further decreased by circulating some of the acidic solutions back to leaching.
In Au refining, the environmental impacts arose primarily from the production of sodium sulfite, which was used as a cyanide destruction chemical. Given the importance of cyanide management from an environmental perspective, a technically robust and simultaneously cost-effective method for cyanide destruction is critical. Several methods have been suggested to recover free cyanide in particular from gold-barren pulps and solutions for reuse, thus also reducing the need for detoxification. The simplest way to achieve this is by thickening the tailings slurry and circulating the overflow back to leaching (Fleming 2005). More complex cyanide recovery schemes, such as AVR or the Cyanisorb process, can also recover cyanide from weak acid dissociable cyanide complexes, such as most Ag and Cu cyanide complexes. These methods rely on lowering the pH and converting cyanide to HCN gas, which is scrubbed with caustic solution to form a strong cyanide solution for reuse (Fleming 2005). The effects of cyanide recovery on the results would likely be limited, but it could have practical benefit in reducing the cyanide content in the tailings and reducing costs. The chemical recovery of cyanide through HCN also poses a massive risk for human health and the environment if not handled correctly, due to the high toxicity of the gas.
The hydrometallurgical process was assumed to leave trace amounts of heavy metals to wastewaters, which were presumed to be released to freshwater. Despite the assumption that the aqueous emissions would be small, particularly the minute cobalt and copper amounts were significant in terms of freshwater aquatic ecotoxicity, as seen in Fig. 3g, while arsenic from High As case had a more noticeable effect in human toxicity. Optimal treatment of the wastewaters is necessary, but the complete prevention of heavy metal mobilization to the environment is likely not possible.
3.2.2 Effect of the concentrate grade
The effect of the cobalt concentrate grade on greenhouse gas emissions was studied and the results are presented in Fig. 4, with mining excluded due to the uncertainty associated with the beneficiation step when the concentrate grade is manipulated. It should be observed that the ore grade was presumed to be constant, and the differences between the concentrate grades are due to the amount of processed raw materials, varying cobalt recoveries, and possible chemical consumption.
An inverse dependence was observed between the concentrate cobalt grade and GWP, and a sharp decrease in GWP occurred between 0 and 3% Co grades: from 28.0 to 6.4 kg CO2-eq in High As and 22.4 to 5.7 kg CO2-eq in Low As. The differences between the cases also decreased when the concentrate grade increased, as shown in Fig. 4a. At 0.5% Co, the GWPs of High and Low As cases were 28.0 and 22.4 kg CO2-eq, respectively, while at 10% Co, they were 2.9 and 2.5 kg CO2-eq.
It can also be seen (Fig. 4b) that the decreased impacts when the Co grade increases are due to the improved environmental performance in base metal refining and Au refining: the GWP of base metal refining fell from 18.7 to 1.7 kg CO2-eq in the studied interval (0.5–10% Co), and Au refining from 8.4 to 0.4 kg CO2-eq. The footprint of refining was nearly constant in the process, decreasing only from 1.0 to 0.9 kg CO2-eq with the increasingly valuable grade in the concentrate. It was thus observed that the impacts of Co refining were largely independent of the other processes and that the variation was mainly caused by overall cobalt recovery. Nevertheless, reducing the impacts from the hydrometallurgical process by producing higher grade concentrates has implications on the impacts of minerals processing, such as energy consumption in comminution and the final yields. The optimization of the whole process should therefore be done with experimental data when test work has been conducted. The current study did not consider the parameters of the mineral processing steps, which are their own dedicated entity.
3.3 Sensitivity analysis
The significance of uncertainty related to the process parameters was investigated by conducting a sensitivity analysis with respect to the hydrometallurgical process. The effects of changing the solid/liquid ratio, initial slurry H2SO4 concentration, oxygen utilization efficiency, iron behavior, and thickener and filter parameters were studied by changing the base values by ± 20% in the simulation and calculating the impacts for the new set of inputs and outputs. Iron behavior was studied by changing the concentration of dissolved iron in the pressure leach discharge and the ratio of hematite and basic ferric sulfate in the pressure leaching precipitates (Fe2O3/Fe(OH)SO4). The underflow solid content was selected for the thickeners and moisture content in the cake for the filters. The results of the analysis are presented in Fig. 5 with respect to the hydrometallurgical process (base metal refining, Co refining, Au refining), excluding mining.
The selected process parameters mostly affected the energy and chemical consumptions of the process, which was reflected in the estimated environmental impacts. In the case of filter cake moisture, the recovery of cobalt was also affected, which can be seen in the impacts. This is because the washing efficiency can never be 100%, meaning that a small amount of solution is left in the filter cake.
The analyses showed that a 20% disturbance in the values changed the environmental indicators (GWP, AP, EP, POCP, ODP, Water) by approx. 0–2% for the most part. The major exceptions were the oxygen utilization efficiency in pressure leaching, solid content in the feed slurry, and cake moisture, which had more profound effects on the impacts. A lower solid content in the leach slurry increased the consumption of electricity, and pH- or concentration-controlled chemicals (neutralization chemicals, sulfuric acid, cyanide). Aside from POCP, increasing the slurry solid content and thus decreasing the volume of treated solution led to a decrease in the categories: a + 20% increase in solids loading decreased GWP by 0.7%, while a 20% decrease increased it by 4.8%. The cause of the response seen in POCP, where + 20% and − 20% both increased the indicator (approx. ± 1.6%), is unknown.
Of the studied indicators, AP was observed to be affected mainly by changes in acid consumption caused by varying the process variables: namely the slurry solid content, thickener UF solids, and cake moisture. Decreasing the initial slurry acidity from 30 g/L H2SO4 to 24 g/L decreased the AP by 2.8%, whereas a 20% increase in slurry acidity (36 g/L H2SO4) caused a much lower increase of 0.5%. The asymmetrical response was due to the circulation of some of the acidic effluents from the process back to leaching, which decreased acid consumption, particularly at higher acidities. The effect of the thickener UF slurry solid content (+ 20% causing an increase of 2.5%, − 20% causing a decrease of 3.8%) and filter cake moisture (+ 20%: + 2.8%, while − 20%: − 2.2%) was similar: the more solution circulated back, the less acid consumed and thus the AP decreased.
Fe3+ concentration in pressure leaching discharge and the formed iron precipitates (Fe2O3 and Fe(OH)SO4) had little practical effect on the model. The largest disturbance was seen in POCP, when the ratio of hematite and basic ferric sulfate in the leaching residues was manipulated: − 20% less hematite decreased the impacts by 1.9%, whereas + 20% hematite increased the POCP value by 0.8%. This was despite the fact that basic ferric sulfate was presumed to react in Au refining, thus increasing the consumption of lime, while hematite was thought to be inert, and thus, the response was possibly due to the increased acid formation in the hematite formation reaction or other side reaction. The observed effect of hematite formation increasing chemical consumption is unrealistic on a larger scale due to the differences in precipitate characteristics affecting solid/liquid separation, and the effect was largely minimal in the other categories (GWP, AP, EP, ODP, water).
Oxygen utilization efficiency in the pressure leaching vessels was observed to be the main source of uncertainty contributing to all of the investigated categories: improving oxygen utilization and thus decreasing oxygen consumption by 20% (from 80 to 96%) decreased the impacts by over 5% in all of the categories other than AP (− 3.9%), with the largest effect seen in EP, − 10.3%. Decreasing oxygen utilization to 64% likewise increased the categories in excess of 5%, far more than any of the other studied parameters. In commercial operations, impeller design has an important role in ensuring efficient oxygen mass transfer and the even distribution of gas in the vessel, but oxygen utilization is difficult to measure and optimize on a laboratory scale (Brewer 2004). High oxygen utilization is also desirable from an economic standpoint due to the cost intensity of oxygen production on site (Green et al. 2018).
The effect of the process parameters on toxicity (FAETP, HTP) was more unclear. These categories were more sensitive to the aqueous heavy metal ions than process inputs, and small random variation in the outputs would thus see a relatively large response. Changes in the water balance would be expected to have the most significant effect on the heavy metal flows. Increasing UF solid content, for instance, lead to lower FAETP (− 3.0%), but higher HTP (+ 1.0%), which implies that increasing UF solid content may have decreased copper in the outflows but slightly increased the amount of soluble arsenic leaving the process. Decreasing UF solid content had an opposite response, but less significant in terms of FAETP (FAETP + 1.3%, HTP − 1.5%) Cake moisture appeared the most important parameter affecting toxicity categories, which is intuitive considering that a large amount of the metals were presumed to mobilize from the moisture contained in solid residues: leach residue, jarosite-ferric arsenate precipitates, cyanidation tailings, neutralization tailings. Oxygen efficiency affected these categories less than the others (FAETP ± 3.1%, HTP ± 3.6%).
A sensitivity analysis was also conducted to see what effect the allocation of co-products has on the results by several means, see Fig. 6. Although economic allocation is usually not recommended, the method is noted by Santero et al. (2016) to capture the driving force of operations better where base and precious metals are extracted together: it can be economic to recover high-value precious metals, such as gold, in minute amounts. Due to price fluctuations, economic allocation was conducted using the average prices for gold, cobalt, and copper during two reference years, 2018 and 2019 (statista 2020, 2021a, b).
The sensitivity of the environmental impact categories to process parameters and variables in the High As case (Co 1.35%). The initial values were 25% cake moisture, 40% UF solids, Fe2O3/Fe(OH)SO4 = 1, 10 g/L Fe(III) in the discharge, 30 g/L H2SO4 in the slurry, 80% oxygen utilization efficiency, and 20% slurry solid content. a Global warming. b Acidification. c Freshwater eutrophication. d Photochemical ozone creation. e Stratospheric ozone depletion. f Freshwater consumption. g Freshwater aquatic ecotoxicity. h Human toxicity
By mass basis, 93–94% of the burden was allocated to cobalt sulfate (19.5 and 19.1 kg CO2-eq High and Low As) and less than 1% to gold. Massive fluctuations in cobalt prices were seen between 2018 and 2019, due to which the GHGs allocated to cobalt sulfate were nearly twice as high in 2018 than in 2019: for the 2018 reference, the GWP of the High As case was 10.3, whereas the value was 6.0 kg CO2-eq for 2019. The shares seen for economic allocation (Fig. 6b) would suggest that gold, while certainly a significant product, is not necessarily the main product of the operations based on the value of cobalt. The situation would consequently be better described as gold and cobalt being co-products and copper a potential by-product, rather than one being the by-product of another.
4 Discussion
Although the use of process simulation enables the generation of highly precise LCI data and the evaluation of individual process parameters and their effect on the results, there is still uncertainty related to the results. A combination of pilot-scale and laboratory-scale experimental results was used to predict the environmental impacts of processing cobalt-gold concentrates with certain variations. There is a limited amount of information on the processing of such raw materials available in the literature, and proven process options were selected to decrease this inherent uncertainty. Improving the simulation model with experimental data on leaching or solution purification steps would also improve the life cycle assessment (LCA) of prospective Co refining from these ores.
Errors in the simulation are also carried over to the LCA stage, which means that the uncertainties in the simulation also affect the impact assessment. The heat balance of the process is particularly uncertain due to the steady-state simulation and the fact that heat losses were not considered, and it should be presumed that the model underpredicts the heating and cooling requirements in the process. The consumption of H2S gas in the precipitation of Cu and Co was also presumed to be stoichiometric, which is why the consumption of H2S is underestimated in the model. Excess H2S would be scrubbed from the off-gas using caustic soda solution, which was not implemented in the model, but which could be a significant factor due to the intensity of NaOH production.
It was assumed that the ore would be both mined and refined in Finland due to the strong metallurgical infrastructure in the country, and the transport impacts of concentrates and precipitates were thus presumed insignificant and left outside of the boundary. If we assume that some part of the processing and refining, such as the cyanidation of gold, occurs elsewhere, the importance of electricity could increase in addition to the transport impacts. This is particularly true for the cyanidation of gold due to the long retention times in the leaching reactors and the large amount of slurry, which leads to comparably high energy consumption. The process is prospective, however, and the commissioning of the possible plant is not expected to occur in the near future (assumed in this study 2040–2050 for reference). The share of renewable and nuclear energy is expected to grow in the switch to low-CO2 energy, which would further decrease the significance of energy in the process. On the other hand, low-fossil energy sources would also affect the impacts of the upstream processes. The largest effect could, based on the contribution analyses, be obtained with clean oxygen production and the electrification of mines, which affects the GWP. The impacts from blasting, acidic emissions from sulfuric acid production, the wearing of the grinding medium, and the release heavy metals to freshwater are not likely affected by decarbonization, however.
In addition to challenges with data quality, uncertainty arises from the scaling of the process to industrial operations. Carbon-in-leach (CIL), for instance, was applied in cyanidation instead of carbon-in-pulp (CIP) technology due to the presence of fines in the raw material, which tends to make CIL the preferable method (Staunton 2005). While CIL is also increasingly used for non-preg-robbing ores due to the lower capital costs from fewer reactors, it is not without its problems, and CIP could potentially be a more viable option on industrial scale since the ore was not preg-robbing. The implementation of CIP would further increase the electricity consumption of the gold refining operation, but the consumption of carbon could decrease. Also, the model did not consider the effect of the buffer tanks needed due to the long residence times in some of the units, particularly in gold cyanidation and elution from carbon. Furthermore, solid/liquid separation is scarcely ever considered in experimental work despite its importance on industrial scale. Simple settling and residue washing experiments could be conducted to improve the accuracy of the models.
Some uncertainty remains with the human toxicity and freshwater aqueous ecotoxicity categories. The release of heavy metals, such as arsenic and cobalt, from the process residues is unknown at this point in the project. The mobilization of arsenic from wastes can be minimized but not completely prevented. Small amounts of toxic components also remain in the wastewaters even after the final neutralization stages. The current model only accounts for some of the metals that remain soluble in the neutralized effluents based on approximate estimates and could be improved with leachability tests on the solid residues and experimental work on the neutralization of process effluents.
5 Conclusions
The hydrometallurgical simulation model was built based on experimental parameters reported for similar raw materials and solutions. The simulation should therefore be updated to obtain more precise results when experimental data becomes available in the future. The largest data gap in the study was the effect of mining and beneficiation. It was seen that increasing cobalt content in the concentrate greatly reduced the impacts from hydrometallurgical processing, but the impacts from beneficiation would also be expected to increase through lower recovery rates. The simulation also showed that there is potential for improvement in the cobalt recovery rates during the hydrometallurgical process: the recovery of cobalt varied from 80.1–87.6% in the High As and 77.9–84.5% in the Low As case, mainly due to metal losses to filter wash waters, which showed recoverable cobalt concentrations. The recovery of cobalt could thus be improved to approximately 96%. Experimental work is, however, necessary to validate the findings in the hydrometallurgical model.
Cobalt refining was observed to have little effect on the impacts, whereas particularly mining and the pressure leaching of base metals, and the production of intermediate cobalt and copper products (base metal refining) produced most of the emissions. The impacts from gold refining were due to the large amount of pressure leaching residues subjected to cyanide leaching and the detoxification of the tailings. With the estimated composition (Co grade 1.35%), the global warming potential was determined to be approximately 20.6 kg CO2-eq for 1 kg of cobalt sulfate produced. Whether cobalt was found in cobaltite or linnaeite had very little effect on the results, and the focus should be placed on optimizing the minerals processing step to concentrate cobalt while securing high recovery rates.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abadías Llamas A, Valero Deldago A, Valero Capilla A, Torres Cuadra C, Hultgren M, Peltomäki M, Roine A, Stelter M, Reuter MA (2019) Simulation-based exergy, thermo-economic and environmental footprint analysis of primary copper production. Min Eng 131:51–65. https://doi.org/10.1016/j.mineng.2018.11.007
Alves Dias P, Blagoeva D, Pavel C, Arvanitidis N (2018) Cobalt: demand-supply balances in the transition to electric mobility. EUR 29381 EN, Publications Office of the European Union, Luxembourg. https://doi.org/10.2760/97710
AMC Consultants (2020) Rajapalot property mineral resource estimate NI 43–101 Technical Report, Ylitornio – Rovaniemi, Finland
Anderson CG (2006) The Design-of-Experiment optimization and development of cobaltite ore mineral processing. JOM 58:43–46. https://doi.org/10.1007/s11837-006-0200-z
Awuah-Offei K, Adekedjou A (2011) Application of life cycle assessment in the mining industry. Int J Life Cycle Assess 16:82–89. https://doi.org/10.1007/s11367-010-0246-6
Brewer RE (2004) Copper concentrate pressure leaching - plant scale-up from continuous laboratory testing. Mining Metall Explor 21:202–266. https://doi.org/10.1007/BF03403186
Chen G (1998) Pressure acid leaching of nickel laterite. Master’s thesis, The University of British Columbia, Department of Metals and Materials Engineering
Dai Q, Kelly JC, Elgowainy A (2018) Cobalt life cycle analysis update for the GREET model. Argonne National Laboratory. Available at: https://greet.es.anl.gov/publication-update_cobalt [Accessed 09.09.2020]
Dannenberg RO, Gardner PC, Crane SR, Seidel DC (1987) Recovery of cobalt and copper from complex sulfide concentrates. Pittsburgh, PA: U.S. Department of the Interior, Bureau of Mines, Report of Investigations 9138
Eckelman MJ (2010) Facility-level energy and greenhouse gas life-cycle assessment of the global nickel industry. Res Con Rec 54:256–266. https://doi.org/10.1016/j.resconrec.2009.08.008
Ecoinvent 3.5 (2018) ecoinvent 3.5. Available at: https://www.ecoinvent.org/database/older-versions/ecoinvent-35/ecoinvent-35.html [Accessed 28.06.2021]
Elomaa H, Sinisalo P, Rintala L, Aromaa J, Lundström M (2020) Process simulation and gate-to-gate life cycle assessment of hydrometallurgical refractory gold concentrate processing. Int. J. Life Cycle Assess 25:456–477.https://doi.org/10.1007/s11367-019-01723-6
Elomaa H, Rintala L, Aromaa J, Lundström M (2020b) Process simulation based life cycle assessment of cyanide-free refractory gold concentrate processing – case study: cupric chloride leaching. Min Eng 15.https://doi.org/10.1016/j.mineng.2020.106559
European Commission (2020) Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions - critical raw materials resilience: charting a path towards greater security and sustainability. Brussels, 3.9.2020, COM(2020) 474 final
Farjana SH, Huda N, Parvez Mahmud MA, Saidur R (2019) A review on the impact of mining and mineral processing industries through life cycle assessment. J Clean Prod 231:1200–1217. https://doi.org/10.1016/j.jclepro.2019.05.264
Fassell WM (1962) Hyperatmospheric extractive metallurgy: its past, present and future. Pure Appl Chem 5:683–700. https://doi.org/10.1351/pac196205030683
Ferron CJ (2005) Recovery of gold as by-product from the base-metal industries. In: Adams MD, Wills BA (eds) Advances in Gold Ore Processing, vol 15. Elsevier, Amsterdam, pp 831–856
Fleming CA (2005) Cyanide recovery. In: Adams MD, Wills BA (eds) Advances in Gold Ore Processing, vol 15. Elsevier, Amsterdam, pp 703–727
Fleming CA (2010) Basic iron sulfate – a potential killer in the processing of refractory gold concentrates by pressure oxidation. Miner Metall Process 27:81–88. https://doi.org/10.1007/bf03402383
Ghodrat M, Rhamdhani MA, Brooks G, Rashidi M, Samali B (2017) A thermodynamic-based life cycle assessment of precious metal recycling out of waste printed circuit board through secondary copper smelting. Environ Dev 24:36–49. https://doi.org/10.1016/j.envdev.2017.07.001
Gray JE, Eppinger RG (2012) Distribution of Cu Co, As, and Fe in mine waste, sediment, soil, and water in and around mineral deposits and mines of the Idaho Cobalt Belt. USA J Appl Geochem 27:1053–1062. https://doi.org/10.1016/j.apgeochem.2012.02.001
Green C, Robertson J, Marsden JO (2018) Pressure leaching of copper concentrates at Morenci, Arizona – 10 years of experience. Miner Metall Process 35:109–116. https://doi.org/10.19150/mmp.8459
Grorud HF (1997) Textural and compositional characteristics of cobalt ores from the Skuterud Mines of Modum. Norway, Norsk Geologisk Tidsskrift 77:31–39
Huijbregts MAJ (1999) Priority assessment of toxic substances in the frame of LCA: development and application of the multi-media fate, exposure and effect model USES-LCA, Leiden University
ISO 14040 (2006) Environmental management, Life Cycle Assessment, Principles and Framework. Finnish Standards Association, SFS, Helsinki
ISO 14044 (2006) Environmental management, Life Cycle Assessment, Requirements and Guidelines. Finnish Standards Association, SFS, Helsinki
Johnson DB, Dybowska A, Schofield PF, Herrington RJ, Smith SL, Santos AL (2020) Bioleaching of arsenic-rich cobalt mineral resources, and evidence of concurrent biomineralization of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy 195. https://doi.org/10.1016/j.hydromet.2020.105395
Khoo JZ, Haque N, Woodbridge G, McDonald R, Bhattacharya S (2017) A life cycle assessment of a new laterite processing technology. J Clean Prod 142:1765–1777. https://doi.org/10.1016/j.jclepro.2016.11.111
Kyriakis G (2005) Extraction of gold from platinum group metal ores. In: Adams MD, Wills BA (eds) Advances in Gold Ore Processing, vol 15. Elsevier, Amsterdam, pp 857–870
Marsden J, House I (2006) The chemistry of gold extraction. Society for Mining, Metallurgy, and Exploration, Littleton, Colorado
Ministry of Economic Affairs and Employment of Finland (2019) Finland’s Integratd Energy and Climate Plan, Publications of the Ministry of Economic Affairs and Employment. Energy 2019:66
Nkulu CBL, Casas L, Haufroid V, De Putter T, Saenen ND, Kayembe-Kitenger T, Obadia PM, Wa Mukoma DK, Ilunga JL, Nawrot TS, Luboya Numbi O, Smolders E, Nemery B (2018) Sustainability of artisanal mining of cobalt in DR Congo. Nat Sustain 1:495–504. https://doi.org/10.1038/s41893-018-0139-4
Norgate TE, Jahanshahi S, Rankin WJ (2007) Assessing the environmental impact of metal production processes. J Clean Prod 15:838–848. https://doi.org/10.1016/j.jclepro.2006.06.018
Norgate T, Haque N (2012) Using life cycle assessment to evaluate some environmental impacts of gold production. J Clean Prod 29–30:53–63. https://doi.org/10.1016/j.jclepro.2012.01.042
Nuss P, Eckelman MJ (2014) Life cycle assessment of metals: a scientific synthesis, PloS One 9. https://doi.org/10.1371/journal.pone.0101298
Olivetti EA, Ceder G, Gaustad GG, Fu X (2017) Lithium-Ion battery supply chain considerations: analysis of potential bottlenecks in critical metals. Joule 1:229–243. https://doi.org/10.1016/j.joule.2017.08.019
Outotec M (2021) HSC Chemistry 10. Available at: https://www.mogroup.com/portfolio/hsc-chemistry/ [Accessed 13.4.2021]
Pell R, Wall F, Yan X, Li J, Zeng X (2019) Mineral processing simulation based-environmental life cycle assessment for rare earth project development: a case study on the Songwe Hill project. J Environ Manage 241. https://doi.org/10.1016/j.envman.2019.109353
Reuter MA, van Schaik A, Gediga J (2015) Simulation-based design for resource efficiency of metal production and recycling systems: cases – copper production and recycling, e-waste (LED lamps) and nickel pig iron. Int J Life Cycle Assess 20:671–693. https://doi.org/10.1007/s11367-015-0860-4
Rinne M, Elomaa H, Porvali A, Lundström M (2021) Simulation-based life cycle assessment for hydrometallurgical recycling of mixed LIB and NiMH waste. Res Con Rec 170. https://doi.org/10.1016/j.resconrec.2021.105586
Santero N, Hendry J (2016) Harmonization of LCA methodologies for the metal and mining industry. Int J Life Cycle Assess 21:1543–1553. https://doi.org/10.1007/s11367-015-1022-4
Segura-Salazar J, Lima FM, Tavares LM (2019) Life cycle assessment in the minerals industry: current practice, harmonization efforts, and potential improvement through the integration with process simulation. J Clean Prod 232:174–192. https://doi.org/10.1016/j.jclepro.2019.05.318
Slack JF (2010) Descriptive and geoenvironmental model for cobalt-copper-gold deposits in metasedimentary rocks – chapter G of mineral deposit models for resource assessment. U.S. Department of the Interior, U.S. Geological Survey. Sci Invest Rep 2010–5070-G.
Sphera (2020) GaBi Solutions. Available at: https://gabi.sphera.com [Accessed 29.06.2021]
statista (2021) Annual average gold price from 1900 to 2020 (in U.S. dollars per troy ounce). Available at: https://www.statista.com/statistics/268027/change-in-gold-price-since-1990/ [Accessed 11.3.2021]
statista (2021) Average cobalt spot price in the United States from 2008 to 2020 (in U.S. dollars per pound). Available at: https://www.statista.com/statistics/339743/average-spot-price-of-cobalt-in-the-us/ [Accessed 11.3.2021]
statista (2020) Average annual market price of copper from 2009 to 2019 (in U.S. dollars per metric ton). Available at: https://www.statista.com/statistics/533292/average-price-of-copper/ [Accessed 11.3.2021]
Statistics Finland (2019) Use of fossil fuels and renewable energy increased in Finland in 2018. Available at: https://www.tilastokeskus.fi/til/ehk/2018/ehk_2018_2019-12-12_tie_001_en.html [Accessed 29.06.2021]
Staunton WP (2005) Carbon-in-pulp. In: Adams MD, Wills BA (eds) Advances in Gold Ore Processing, vol 15. Elsevier, Amsterdam, pp 562–587
Tan H, Skinner W, Addai-Mensah J (2012) Leaching behaviour of low and high Fe-substituted chlorite clay minerals at low pH. Hydrometallurgy 125–126:100–108. https://doi.org/10.1016/j.hydromet.2012.05.015
Wang Y, Xiao L, Liu H, Qian P, Ye S, Chen Y (2018) Acid leaching pretreatment on two-stage roasting pyrite cinder for gold extraction and co-precipitation of arsenic with iron. Hydrometallurgy 179:192–197. https://doi.org/10.1016/j.hydromet.2018.06.008
Witt WK, Hagemann SG, Roberts M, Davies A (2020) Cobalt enrichment at the Juomasuo and Hangaslampi polymetallic deposits, Kuusamo Schist Belt, Finland: a role for an orogenic gold fluid? Miner. Deposita 55:381–388. https://doi.org/10.1007/s00126-019-00943-y
Acknowledgements
The authors wish to thank Petteri Halli D.Sc. (Tech) for the SEM-EDS and XRD analyses and the Geological Survey of Finland for valuable discussions.
Funding
Open access funding provided by Aalto University. This study was supported by the School of Chemical Engineering (Aalto University); the BATCircle project, grant number 4853/31/2018, funded by Business Finland; and the Academy of Finland-funded “RawMatTERS Finland Infrastructure” (RAMI). The raw material was provided by Mawson Gold Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Matthias Finkbeiner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rinne, M., Elomaa, H. & Lundström, M. Life cycle assessment and process simulation of prospective battery-grade cobalt sulfate production from Co-Au ores in Finland. Int J Life Cycle Assess 26, 2127–2142 (2021). https://doi.org/10.1007/s11367-021-01965-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-021-01965-3