Abstract
Water is an indispensable resource for human activity and the environment. Industrial activities generate vast quantities of wastewater that may be heavily polluted or contain toxic contaminants, posing environmental and public health challenges. Different industries generate wastewater with widely varying characteristics, such as the quantity generated, concentration, and pollutant type. It is essential to understand these characteristics to select available treatment techniques for implementation in wastewater treatment facilities to promote sustainable water usage. This review article provides an overview of wastewaters generated by various industries and commonly applied treatment techniques. The characteristics, advantages, and disadvantages of physical, chemical, and biological treatment methods are presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background of wastewater treatment
Water is an indispensable resource that sustains ecosystems, supports human life, and drives industry and agriculture. The demand for freshwater has been continuously growing with the global population. Of the water on Earth, 97% is saline and only 3% is freshwater (Oki and Kanae 2006). Only 0.5% of this freshwater is available for human use and exists in liquid form in rivers, lakes, ponds, and groundwater (Sangamnere et al. 2023). The severity of water scarcity has been underscored by the United Nations World Water Assessment Programme (WWAP); that is, the number of people residing in regions that experience water scarcity for at least 1 month annually is currently 3.6 billion and could increase to 4.8–5.7 billion by 2050 (WWAP 2018).
A comprehensive approach is needed to address growing water scarcity that includes water management and wastewater treatment and reuse (Bauer et al. 2020). The global increase in freshwater usage and wastewater generation has resulted in persistent water scarcity. The quantity of wastewater generated globally each year is estimated at 380 billion m3 and is expected to increase by 51% by 2050 (Qadir et al. 2020). The discharge of untreated wastewater remains a global issue. According to a WWAP report (2017), 80% of global wastewater is untreated and discharged, and Jones et al. (2021) have reported that 48% of global wastewater is untreated. The direct discharge of wastewater leads to the pollution of water bodies and groundwater, which can result in eutrophication (Qadir et al. 2020) and endanger the health of plants and animals (Ahmed et al. 2021a). Natural water bodies have self-purification ability, whereby some pollutants can be removed through natural physical, biological, and chemical means. However, the excess discharge of untreated wastewater surpasses the self-purification ability of natural water bodies (Pratiwi et al. 2023).
The implementation of wastewater treatment is a necessary but challenging task to prevent water pollution and meet water demand, especially in low-income regions. In high-income regions, such as North America, Western Europe, and Japan, 74% of the wastewater is treated, whereas 4% of wastewater is treated in low-income regions (Jones et al. 2021). High-income countries enforce wastewater quality regulations and possess the technology and infrastructure needed to install wastewater treatment plants (WWTPs), whereas low-income countries lack these resources. Consequently, those living in low-income regions may be exposed to wastewater and have limited access to clean water (WWAP 2017). Thus, global efforts are needed to increase the quantity of wastewater treated.
The properties of influent wastewater, such as the pollutant concentration, must be analyzed to determine WWTP specifications. Wastewater is largely classified by its generation source into municipal, agricultural, and industrial wastewater (Samer 2015). Industrial wastewater can be further classified into cooling, washing, and process wastewater (Crini and Lichtfouse 2019). Wastewater can be discharged from point and nonpoint sources. Point sources discharge wastewater from easily identifiable outlets, such as WWTPs of industrial facilities and municipal WWTPs. Nonpoint sources cannot be easily identified and include multiple sources, such as agricultural and urban runoff. Thus, point sources can be monitored and regulated more easily and are often more concentrated than nonpoint sources (Jones et al. 2021). Differences in water usage result in a variety of pollutants in wastewater that may contain toxins or pathogens harmful to human health and the ecosystem. Some common pollutants include organic compounds, inorganic compounds, phosphorus, nitrogen, and heavy metals (Akpor et al. 2014).
Overview of wastewater treatment
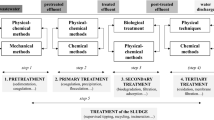
Typically, WWTPs are designed to treat wastewater cost-effectively while achieving a desired water quality (Crini and Lichtfouse 2019). Wastewater treatment often requires the sequential use of various wastewater treatment techniques, each of which is suitable for removing certain contaminants. A wastewater treatment process generally consists of preliminary, primary, secondary, and tertiary stages (Naidoo and Olaniran 2013; Quach-Cu et al. 2018). The flow of a general wastewater treatment process and corresponding treatment techniques used are shown in Fig. 1.
Preliminary treatment removes large debris, grit, and solids from wastewater through processes such as screening, comminution, and grit removal. Next, primary treatment removes suspended solids, grit, fats, and oils through processes such as sedimentation and dissolved air flotation (DAF). Secondary treatment consists of using biological techniques to reduce organic matter, nitrogen, and phosphorus in wastewater through biochemical reactions such as conversion into biomass (Samer 2015). Tertiary treatment, also known as advanced treatment, is applied to further upgrade the treated wastewater to meet specific standards for water reuse or discharge. Tertiary treatment includes methods such as membrane filtration, adsorption, and chemical oxidation.
Treatment techniques can be classified into physical, biological, and chemical methods. Physical methods include screening, comminution, grit removal, sedimentation, DAF, adsorption, ion exchange, and membrane filtration. Biological treatment can be subdivided into aerobic and anaerobic treatment depending on oxygen availability. Chemical treatment includes precipitation, coagulation, flocculation, evaporation, distillation, membrane distillation, solvent extraction, electrochemical methods, chemical oxidation, and advanced oxidation processes (AOPs). The characteristics, advantages, and disadvantages of each method are detailed in “Treatment of industrial wastewater” section.
Industrial wastewater is often more toxic than municipal wastewater (Häder 2018). Industrial wastewater contains various contaminants at different concentrations depending on the industry (Ahmed et al. 2021a). Thus, industrial wastewater may not be adequately treated by municipal WWTPs, which are designed mainly for the removal of biochemical oxygen demand (BOD) (Abu Shmeis 2018). Effective treatment of industrial wastewater requires an analysis of the wastewater properties, such as the type and concentration of pollutants, and implementation of a suitable treatment process. Considering the variation in wastewaters across industries, WWTPs are implemented on a case-by-case basis using available treatment techniques. Treatment methods are selected by considering many factors, such as the influent wastewater characteristics, regulatory standards, land availability, technological availability, and economic viability.
Objective of this review
The objective of this review paper is to examine the diverse types of contaminants found in wastewater generated across various industries and to evaluate the range of treatment techniques currently being implemented. An in-depth analysis is presented of the characteristics, advantages, and disadvantages of available physical, chemical, and biological wastewater treatment methods. Although numerous review papers have been written on singular treatments or specific industrial sectors, there is a limited number of holistic overviews covering the wide spectrum of wastewaters generated by multiple industries and corresponding treatment methods. This review bridges this gap by providing a comprehensive description of industrial wastewaters and the strengths and weaknesses of treatment technologies. This information is essential for selecting and integrating suitable treatment options that are cost-effective and sustainable as well as compliant with regulatory standards.
Methods
The selection of industries for this study was based on the thorough review of academic literature and reports. The primary criteria for inclusion were the volume of wastewater produced, the diversity and complexity of pollutants, the environmental and health impacts, and regulatory focus.
The reference search was conducted across multiple academic databases, including Google Scholar, PubMed, ScienceDirect, and Web of Science, using keywords related to the specific industry, wastewater, and wastewater treatment. Additionally, the search for references on the wastewater treatment technologies was conducted by using the academic databases and consulting recent reviews in the field and tracing the citations. This methodology offers a detailed and balanced review, highlighting both the pollutants present in the wastewater of the selected industries and the technologies available for their treatment.
Wastewater generated by various industries
Limited information is available on the overall volume of industrial wastewater generated and discharged globally. However, according to reports from the European Union (EU, which consists of developed countries), manufacturing generated the most wastewater among the industrial sectors (WWAP 2017). The major point sources of manufacturing industrial wastewater include industries such as petrochemicals, pharmaceuticals, pulp and paper, textiles, iron and steel metal manufacturing, and food.
The use of water for various purposes in industry generates wastewater with different characteristics, such as the types and concentrations of pollutants. Industrial wastewater may be heavily polluted and contain toxic pollutants. Industrial wastewater can be broadly categorized into cooling, washing, and process wastewater (Crini and Lichtfouse 2019). Wastewater generated from various industries and uses has diverse characteristics. The pollutants in industrial wastewater include solids, sediments, organic compounds, nutrients, and heavy metals. Considering the diversity and potential toxicity of industrial wastewater, a treatment technique that can sufficiently remove pollutants must be selected and implemented. The pollutants in wastewaters from the cement, chemical, food, iron and steel, pharmaceutical, pulp and paper, and textile industries are reviewed in this section. Wastewater treatment techniques used in each industry are summarized.
Cement, concrete, and ceramics industry
Cement is an important material for constructing buildings and infrastructure, which drive economic development and urbanization. Table 1 shows the three main phases of cement production. Water is used for cooling and washing equipment and, if wet scrubbers are used, for removing particulate matter (PM). In some cases, water is used in the preparation of raw materials for clinker production (Perera et al. 2020). The used cooling water is typically recycled and reused in the process (Sharma et al. 2012). Cement production generates air pollutants, such as nitrogen oxides (NOx), particulate matter (PM), carbon dioxide (CO2), and sulfur dioxide (SO2) (Chen et al. 2015). Wet scrubbers are used to remove PM from the exhaust gas by trapping particles in water droplets, generating wastewater (Zhu et al. 2022). Other methods used to prevent air pollution include the use of simple membrane filters made of fabric, electrostatic precipitators, and bag filters (which are considered the best removal option for PM) (Zhu et al. 2022). Cement industry effluents contain suspended solids (such as calcium carbonate), dissolved solids (such as potassium hydroxide), sodium hydroxide, chlorides, and sulfates, BOD (approximately 5 mg/L), chemical oxygen demand (COD, approximately 60 mg/L), nutrients, and heavy metals (such as iron, zinc, and manganese (Meme and Nwadukwe 2016; Ipeaiyeda and Obaje 2017).
In concrete production, manufactured cement is mixed with water and aggregates (such as sand and gravel). The washing of truck mixers in a ready-mix concrete plant generates wastewater with a high pH that contains dissolved solids, cement, and other pollutants (Ekolu and Dawneerangen 2010). Concrete production is characterized by high water usage, where 150 L of freshwater is used to produce a cubic meter of concrete. Thus, the utilization of wastewater has been considered as a possible solution to reduce water consumption and wastewater discharge (Azeem et al. 2023).
Ceramics are a broad category of inorganic, nonmetallic materials that are typically hard, brittle, and heat-resistant. Ceramics are used in various applications for properties such as electrical and thermal insulation, corrosion resistance, and decorative appeal. Ceramic products include construction materials (such as tiles and bricks), refractories (such as crucibles and molds), pottery products, and toilets.
Ceramic manufacturing techniques span a wide range from hand-building to advanced industrial techniques. Although the specific manufacturing steps depend on the type of ceramic and the product, several key steps are common to all processes. First, the raw materials, such as clay, alumina, and silica, are prepared through processes such as mixing and grinding. Binders (colloids or polymers, such as polyvinyl alcohol and polyethylene glycol) and plasticizers (such as water, ethylene glycol, and stearic acid) are added during the forming of dry powders and plastics, whereas deflocculants, surfactants, and antifoaming agents are added during slurry processing (U.S. EPA 1996). Water is the most used liquid during these processes (U.S. EPA 1996). Next, the prepared raw materials are formed into desired shapes using various methods, such as pressing and casting. The formed ceramics are dried carefully and subjected to bisque firing to remove remaining water and impurities. A glaze is optionally applied to the bisque-fired ceramics. The glazed ceramic is fired again using a temperature, duration, pressure, and atmosphere appropriate for the type of clay and glaze. Effluents are generated during various steps of ceramics production, including glaze and slurry preparation, mold preparation, casting, and glazing (de Almeida et al. 2016). These effluents have high concentrations of total suspended solids (TSS) (2,000–10,000 mg/L) and dissolved solids (300–1,000 mg/L), moderate COD (500–1200 mg/L), and low concentrations of heavy metals (such as lead, cadmium, iron, copper, and manganese) (Dinçer and Kargı 2000). Effluent recycling is one way of avoiding effluent handling and reducing usage of water and raw materials (de Almeida et al. 2016).
Physical and chemical treatment
Wastewater from the cement industry has a high pH and turbidity and is treated by neutralization followed by sedimentation (Freeda Gnana Rani et al. 2005; Zhu et al. 2022). Wastewater from the concrete industry is similarly treated by neutralizing the pH and removing TSS. Coagulation and flocculation may be used to increase the settleability of TSS. De Paula et al. (2014) proposed a coagulation–flocculation treatment process using aluminum sulfate, Moringa oleifera powder, and floc sedimentation. The process achieved 90% turbidity removal, such that the treated water could be used to wash vehicles and flush toilets. Physical and chemical treatments of wastewater from the ceramic industry include adsorption, screening, sedimentation, filtration, coagulation, flocculation, and filtration (Pujiastuti et al. 2021). Pujiastuti et al. (2021) used a polyaluminum chloride coagulant to treat wastewater from the ceramic industry, achieving removals of up to 99.9% TSS, 98.23% COD, and 99.1% lead.
Biological treatment
Biological treatment is not commonly used for wastewater from the cement industry because the typical pollutants are TSS, dissolved solids, and heavy metals and the pH is high. However, some studies have been conducted on applying biological treatment to wastewater from the cement industry. Ali et al. (2021) investigated the use of a pilot-scale process consisting of a primary sedimentation tank, integrated fixed-film activated sludge (AS), and a final settling tank. The removals of TSS, COD, BOD, total nitrogen (TN), and total phosphorus (TP) were 94.5%, 87.8%, 90.8%, 75.9%, and 69.4%, respectively. The proposed treatment was found to be effective, satisfying the regulation standards in Egypt to reuse treated wastewater in agriculture. Biological treatment is not commonly used for wastewater from the ceramic industry. However, Dinçer and Kargı (2000) studied using AS to treat and reduce the BOD and COD of wastewater from the ceramic industry after chemical precipitation, pH adjustment, and nutrient balancing.
Chemical industry
The chemical industry produces a broad range of chemicals, including commodity, specialty, and fine chemicals. These chemicals are used in various sectors, such as agriculture and manufacturing, to make final products. The basic chemicals produced by the petrochemical industry are particularly important because of subsequent use in the manufacturing of plastics, fibers, lubricants, and detergents. Petrochemicals are produced from fossil fuels, such as crude oil, coal, and natural gas or biomass (such as corn and sugarcane). The refining of crude oil provides fuels (such as liquefied petroleum gas, gasoline, and diesel) and chemical products (such as waxes, greases, asphalts, olefins, and aromatics) (Dincer and Zamfirescu 2014). The main petrochemical products are olefins (such as ethylene and propylene) and aromatics (such as benzene, toluene, and xylene) (Ren et al. 2009; Do et al. 2016).
Water usage in refineries for cooling, distillation, hydrotreating, and desalting generates wastewater (Ghimire and Wang 2019). Pollutants found in wastewater from the petrochemical industry include aromatics, hydrocarbons, sulfides, ammonia, and heavy metals (such as chromium, iron, nickel, and copper) (Radelyuk et al. 2019; Wake 2005; Yu et al. 2017). These compounds can be harmful to both the environment and human health. Petrochemical wastewater is treated by physical, biological, and chemical methods.
Physical treatment
Oily wastewater is commonly treated by using sedimentation and DAF to separate and remove oil (Abuhasel et al. 2021). Adsorption and ion exchange can be used to remove dissolved organics from petrochemical wastewater (Fakhru’l-Razi et al. 2009). Membrane separation can be used to remove oils, total organic carbon (TOC), and metal ions from oily wastewater (Yu et al. 2017). Some challenges encountered using membrane technology include cost, thermal stability, and corrosion resistance, which may be overcome by developing novel materials and combination with other treatment technologies (Yu et al. 2017).
Biological treatment
Aerobic and anaerobic treatment is commonly used to remove organics, ammonium, and sulfide from petrochemical wastewater because of advantages such as low cost and a high pollutant removal efficiency (Ghimire and Wang 2019). However, refractory organic compounds, such as aromatics, may be difficult to remove by traditional biological methods. Liu et al. (2014) found that wastewater from a petrochemical complex treated using AS still contained refractory organic compounds, such as alkanes, chloroalkanes, aromatics, and olefins. These compounds could be removed using a ponds-and-wetland system.
Chemical treatment
Coagulation, flocculation, and electrochemical technologies may be used to treat oily wastewater (Abuhasel et al. 2021). Advanced oxidation processes, such as heterogeneous photocatalysis, can be used to degrade refractory organic pollutants, such as phenolic compounds, in refinery wastewater (Diya’uddeen et al. 2011; Bustillo-Lecompte 2020).
Food industry
The industrial manufacture of food is essential for human life and to meet global challenges, such as hunger and environmental sustainability. The food supply chain involves the production of crops and livestock, food processing, and logistics (Sun et al. 2017). Water is used for various processing purposes across different food industries. This water is categorized mainly into process water (such as that used as a raw material) and nonprocess water (such as that used for washing, cooling, and heating) (Abdel-Fatah 2023). Water usage requirements, such as the water quality and volume, vary across different sectors and processes in the food industry. Consequently, the generated wastewater can have varying characteristics, including the contamination level and volume. The generated wastewater may contain high levels of COD, BOD, TSS, TN, and TP resulting from various processes and nonprocess water usage. Most of the water used in many food industry sectors is employed for washing foodstuff and equipment, generating wastewater containing organics and nutrients.
The food industry can be categorized into several key sectors. These sectors can be ranked in terms of decreasing water consumption as meat, dairy products, other foods, fruits and vegetables, bakery products, grain mill and starch products, edible oils and fats, and fish and shellfish (Mark and Strange 1993; Ranken et al. 1997; Asgharnejad et al. 2021; Eurostat 2023). “Other foods” include sugar, coffee, tea, cocoa, chocolate, confectionery, condiments and seasonings, prepared meals, homogenized food, and dietetic food (Eurostat 2023). Table 2 summarizes the products, water usage, wastewater characteristics, and wastewater treatment methods for key sectors of the food industry. Sugar, coffee, and tea are included because of being important internationally traded products that require large quantities of water for production (Asgharnejad et al. 2021). In 2021, the estimated global production of green coffee, tea (i.e., green, black, and partly fermented tea), and raw cane or beet sugar was 10.50, 6.81, and 176.95 million tons, respectively (FAO 2024). Various physicochemical and biological technologies can be used to treat wastewater from the food industry. The treatment method is selected considering factors such as the wastewater characteristics, quality demand for the treated water, cost, and energy requirements. Technologies can be combined to improve the overall treatment efficiency. Considering the high water consumption and wastewater generation of the food industry, direct or indirect use of treated water and resource recovery, such as biogas production, is encouraged to mitigate water scarcity (Shrivastava et al. 2022).
Iron and steel industry
Iron and steel are important drivers in modern society and economic development because of their use in infrastructure (such as roads and bridges, buildings, housing, machinery, and equipment in various industries), transportation (such as cars and trains), and consumer goods (such as tools and utensils). However, the production of iron and steel requires extensive usage of water and energy and generates wastewater containing toxic pollutants as well as CO2 emissions (Garg and Singh 2022).
The raw materials used to produce iron and steel include iron ore, coal for making coke, and limestone (Kumar et al. 2023). Iron and steel can also be produced from recycled scrap metal using electric arc furnaces (EAFs) (Yang et al. 2014). The steel industry uses a large quantity of water for operations, such as cooling, scrubbing, and descaling (Colla et al. 2017). Water consumption in a steel plant can range from 1 to 150 m3 per ton of steel depending on the location, plant configuration, and local regulations (Suvio et al. 2012). Either once-through cooling or recirculating cooling is used according to water availability, which depends on the plant location. Once-through cooling is used in coastal locations where seawater is abundant and accounts for approximately 80% on average of the water consumed in steel plants (Suvio et al. 2012).
Iron and steel manufacturing involves the processes described below (United States Environmental Protection Agency (EPA) 2008; Garg and Singh 2022).
-
1.
Coke production: Metallurgical coke is produced by heating coal in an oxygen-free environment.
-
2.
Sintering: Fine raw materials, including iron ore, limestone, and coke, are agglomerated at high temperatures. The product (which is called sinter) is fed to a blast furnace (BF).
-
3.
Ironmaking: Materials containing iron, such as sinter, are reduced in the BF twice using hot gas. Alternatively, direct reduced iron can be produced from iron ore using natural gas, coal gas containing hydrogen, or CO, which does not require the production and use of coke.
-
4.
Desulfurization of iron: Reagents, such as CaC2 and CaCO3, are injected into the molten iron. The reagents react with sulfur to produce slag, which is removed.
-
5.
Steelmaking: Steel is produced in basic oxygen furnaces (BOFs), EAFs, or open hearth furnaces (OHFs). In a BOF, oxygen is injected into molten metal to remove impurities. In an EAF, current is run through scrap metal using carbon electrodes, and the scrap metal is melted and refined. An OHF is a shallow basin in which metal scrap and molten metal from the BF are heated, melted, and refined.
-
6.
Product preparation: Processes, such as pouring the molten steel into ingots, reheating, casting, shaping, and rolling, are used to finish the product.
Water is used in most processes of steel production, including coke production, sintering, the BF and BOF stages, and rolling, for purposes such as cooling, quenching, and gas-cleaning (Suvio et al. 2012). In addition to these main steel manufacturing processes, a large quantity of water is used in supporting processes, such as power generation and equipment cooling (Suvio et al. 2012). These complex iron and steel production processes generate wastewater containing a large variety of pollutants. The main pollutants in wastewater from the steel industry include COD, NH3–N, volatile phenols, cyanide, TSS, heavy metals, and petroleum (Tong et al. 2018; Choudhury et al. 2023). The largest water consumption occurs in ironmaking and steelmaking, whereas most of the pollution in iron and steel industry wastewater results from coking (Tong et al. 2018).
Reducing the water intake and wastewater discharge of the iron and steel industry requires the implementation of wastewater treatment and water recycling or reuse. Wastewater from the iron and steel industry is treated by physical, biological, and chemical methods. A suitable wastewater treatment method can be selected based on the wastewater pollutants, concentration, quantity, and characteristics, which vary across plants and processes, such as coking, ironmaking, and steelmaking (Lawal and Anaun 2022). Although conventional primary, secondary, and tertiary treatments are used in industry, emerging processes, such as hybrid biological processes, AOPs, and membrane filtration, have been effectively used for pollutant removal (Rawat et al. 2023).
Physical treatment
Adsorption is a simple, low-cost, and effective method for removing a variety of pollutants, including heavy metals in wastewater (such as that from the iron and steel industry) (Feng et al. 2022b). Activated carbon is widely used as an adsorbent because of its effectiveness but can be relatively costly (De Gisi et al. 2016). The iron- and steelmaking industry is unique in that byproducts, such as metallurgical slag, can be used as low-cost alternative adsorbents (Manchisi et al. 2020). Nguyen et al. (2018) reported that the byproducts of coal fly ash and blast furnace slag can be used as low-cost adsorbents to effectively remove heavy metals, such as Pb, Cu, Cd, Cr, and Zn. Unmodified raw coal fly ash has been demonstrated as an adsorbent for treating coking wastewater (Wang et al. 2018). The raw coal fly ash achieved 90% COD removal and could be regenerated by the Fenton process.
Membrane filtration is sufficiently effective for treating wastewater from the iron and steel industry that the water can be reused at the industrial scale. However, membrane fouling is a major problem that decreases the permeate flux and increases energy usage (Liang et al. 2023; Lin et al. 2023). The presence of salts in wastewater can cause problems, such as membrane fouling, and recycled water containing salt can cause salt deposition or corrosion of equipment (Colla et al. 2016). Lin et al. (2023) reported that deposits of Fe and Mn ions and oxides in integrated steelwork wastewater may cause fouling of ultrafiltration (UF) membranes and should be removed before UF. Liang et al. (2023) suggested that further investigation of reverse osmosis (RO) membranes is needed to prevent fouling because these membranes are used for recycling wastewater from the iron and steel industry. Huang et al. (2011) reported that constructed wetlands are an effective pretreatment before UF and RO for reducing iron and manganese concentrations in wastewater from the iron and steel industry and to improve the quality of the treated water for reuse. The constructed wetland, UF, and RO system achieved 98% desalination. An RO system can effectively reduce the concentration of salts, electrical conductivity, and total dissolved solids (TDS), enabling the treated water to be reused and increasing the equipment lifespan (Colla et al. 2016).
Biological treatment
Aerobic and anaerobic treatment, such as AS, is used to treat wastewater from the iron and steel industry. Considering that the complexity and toxicity of this wastewater may reduce the performance of conventional biological treatment, hybrid biological processes, such as anoxic–oxic–anoxic–oxic (AOAO), anaerobic–anoxic–oxic (AAO), and anaerobic–anoxic–oxic–oxic (AAOO), have been developed for the efficient removal of pollutants (Rawat et al. 2023). Biological treatment plants can use various microorganisms (e.g., bacteria, algae, yeast, and fungi) that have different metabolic pathways to remove organic and inorganic pollutants in wastewater from the iron and steel industry (Kajla et al. 2021). Hybrid biological processes employing diverse microbial communities have been used at the industrial scale to meet effluent standards. Ma et al. (2015) collected sludge from coking WWTPs in China that employ different processes, including anaerobic–oxic (AO), AAO, anaerobic–oxic–oxic (AOO), AAOO, and AOAO. An analysis of the composition of the microbial community in the sludge showed that most sludge contained Thiobacillus, Comamonas, Thauera, Azoarcus, and Rhodoplanes. The key parameters for the biological treatment, such as the operation mode, flow rate, and temperature, were found to affect the makeup of the microbial community and thereby, the pollutant removal performance.
Chemical treatment
Chemical methods, such as coagulation–flocculation, AOPs, and electrochemical techniques, have been used to treat wastewater from the iron and steel industry (Garg and Singh 2022). Coagulation–flocculation has been used in conventional integrated wastewater treatment systems as a primary treatment to remove pollutants, such as oils and heavy metals (Das et al. 2018). Coagulation–flocculation can also be used as a pretreatment to prevent filtration membranes from being fouled by effluent (Lin et al. 2023). Coking wastewater may be treated by AOPs, such as ozonation (Wang et al. 2019), catalytic ozonation (Feng et al. 2022a), Fenton oxidation (Chu et al. 2012; Kwarciak-Kozłowska and Włodarczyk 2020), photolysis (Włodarczyk-Makuła et al. 2016), and photocatalysis (Sharma and Philip 2016), to degrade pollutants and increase the biodegradability of the effluent in subsequent biological treatment. Studies have been performed on using electrochemical methods, such as electrocoagulation and electrochemical oxidation, to treat coking wastewater. Wang et al. (2022) developed Ti/SnO2RuO2–Yb electrodes for the electrochemical oxidation of coking wastewater, achieving 85.06% COD removal and 60.59% TOC removal. Ozyonar and Karagozoglu (2015) studied how pretreated coking wastewater was affected by electrocoagulation and electrochemical peroxidation using a direct pulse current. Electrochemical peroxidation was found to be more effective than electrochemical oxidation in removing COD, TOC, phenol, CN–, and SCN–. Mierzwiński et al. (2021) investigated the electrocoagulation of coking wastewater using floc characterization, mathematical modeling, and designing an industrial-scale electrocoagulation reactor. Overall, chemical treatment methods can be integrated with physical or biological methods to improve pollutant removal and promote wastewater reuse in the iron and steel industry. Emerging chemical methods are effective but present challenges, such as a high cost and difficulty in scale-up for industrial use.
Pharmaceutical industry
Pharmaceutical products are crucial to society for the prevention, treatment, and management of various medical conditions, enhancing overall public health and well-being. A large quantity of water is used as a raw material, ingredient, and solvent in the industrial production of pharmaceuticals and separation processes, such as extraction and washing (Gadipelly et al. 2014). Chemical synthesis and fermentation processes are the major processes that generate wastewater during pharmaceutical production (Gadipelly et al. 2014). Pharmaceuticals used in households and agriculture can appear in wastewater. The variety of pharmaceutical products, intermediates, and raw materials results in a diversity of wastewater contaminants. Wastewater from the pharmaceutical industry contains organics that may not be biodegraded, such as antibiotics, anti-inflammatories, steroids, hormones, antidepressants, and spent solvents (Rana et al. 2017; Samal et al. 2022). Discharge of and exposure to pharmaceuticals may have detrimental effects on plants and animals (Gadipelly et al. 2014; Samal et al. 2022).
Physical treatment
Membrane technologies, such as those based on polymer membranes, are low-energy simple strategies for removing pharmaceutical active compounds from wastewater. However, membrane fouling is an important limitation and has been mitigated by membrane modification (Ratnasari 2023). The use of activated carbon for the removal of pharmaceuticals has been studied, but further research needs to be conducted on the cost-effectiveness and application of this technology to real wastewater (Rasras et al. 2021).
Biological treatment
Aerobic and anaerobic biological treatments, such as AS, membrane bioreactors (MBRs), moving bed biofilm reactors (MBBRs), and constructed wetlands, may be used to remove pharmaceuticals from wastewater (Moghaddam et al. 2023). The conventional activated sludge (CAS) treatment often used in municipal WWTPs cannot sufficiently remove persistent micropollutants. However, MBRs are a promising technique for removing these micropollutants because the longer sludge retention time and higher sludge concentration used results in a higher efficiency (Tiwari et al. 2017).
Chemical treatment
Electrochemical coagulation has been studied at the lab scale for the removal of pharmaceuticals from wastewater, but further research needs to be carried out on this technology using real wastewater and performing a cost analysis (Alam et al. 2021). Refractory pharmaceuticals can be effectively degraded using AOPs, such as ozone (O3), hydrogen peroxide (H2O2), Fenton oxidation, and photocatalysis, which use highly reactive radical species (Gadipelly et al. 2014).
Pulp and paper industry
Pulp and paper products, such as newspapers, books, packaging materials, and tissue products, play a vital role in society. Wastewater is generated by the pulp and paper industry through processes such as wood preparation, pulp manufacturing, pulp bleaching, and papermaking (Ashrafi et al. 2015). The use of different processes and raw materials results in diverse wastewater characteristics, such as the quantity generated and the pollutant concentration. The quantity of generated wastewater can be as high as 60 m3/ton of paper produced (Thompson et al. 2001). Preparing wood to produce chips includes harvesting, debarking, chipping, and screening (Amândio et al. 2022). The generated wastewater contains TSS, BOD, dirt, grit, and fibers (Pokhrel and Viraraghavan 2004). Wood pulp can be manufactured from the prepared wood by mechanical, chemical, or hybrid methods (Toczyłowska-Mamińska 2017). Mechanical pulp production involves grinding or refining wood. In hybrid processes, wood pulp is produced by cooking with alkali and milling (Nong et al. 2020). Four common chemical pulping processes are kraft, sulfite, neutral sulfite semichemical, and soda (Cheremisinoff and Rosenfeld 2010). The kraft process accounts for 90% of chemical pulp production because of a high product quality and low production cost (Argyropoulos et al. 2023). During this process, an alkaline solution of sodium hydroxide and sodium sulfide, also known as white liquor, is used to dissolve lignin from cellulose fibers under high temperature and pressure. The dilute spent liquor is concentrated using evaporators to approximately 60%–65% solids (Young et al. 2003; Cheremisinoff and Rosenfeld 2010). The resulting “black liquor” contains organics, such as lignin and hemicellulose, and inorganics, such as salts and sulfur compounds, which are generated as byproducts (Valderrama et al. 2021). The black liquor is burned for energy and to recover chemicals (Young et al. 2003). The wood pulp is then bleached with compounds containing chlorine or oxygen (Bajpai 2018), which generates bleaching wastewater with a high COD and TSS as well as low biodegradability (Eskelinen et al. 2010) and refractory organic compounds, such as adsorbable organic halides (AOX) (Patel et al. 2021). In the final papermaking process, additives, such as dyes, may be used to make colored paper, whereby the generated wastewater may contain particulate waste as well as organic and inorganic compounds, such as the dyes used (Patel et al. 2021). Wastewater generated from the pulp and paper industry is treated physically, biologically, or chemically to reduce water pollution and recover energy and materials.
Physical treatment
Sedimentation and flotation are used to remove TSS in wastewater from pulp and paper mills. Primary clarifiers can effectively remove more than 80% TSS (Thompson et al. 2001). Another commonly used technique, DAF, can remove 80%–98% TSS (Miranda et al. 2009). Manago et al. (2018) investigated the removal of fibers using DAF with polyaluminum chloride as a coagulant, where more than 81.7% TSS was removed. Filtration using membranes of various pore sizes can be used to remove pollutants in wastewater from the pulp and paper industry, such as organics, ions, and AOX (Esmaeeli et al. 2023). Membranes used for wastewater treatment in the pulp and paper industry must be able to withstand extreme conditions, such as high temperatures and pHs, as well as antifouling measures for organic and inorganic foulants (Esmaeeli et al. 2023). Valderrama et al. (2021) developed nanofiltration (NF) membranes to treat black liquor. These membranes have a high rejection of organics with a TOC removal of 92.5% and high salt removals, such as 88.7% sulfate, 73.21% Na+, and 99.99% Mg2+. Adsorption methods, such as the use of activated carbon and zeolite, have been investigated for the treatment of wastewater from the pulp and paper industry by removing heavy metals, such as Cd, Ba, and Cu (Aprianti et al. 2018), as well as COD and color (Kapatel et al. 2022).
Biological treatment
In the pulp and paper industry, aerobic and anaerobic treatment is used to reduce the high organic content of wastewater. Aerobic treatment, such as AS, aerated lagoons, and stabilization ponds, have traditionally been used, whereas anaerobic treatment, such as the upflow anaerobic sludge blanket (UASB) reactor, is promising because of advantages such as lower sludge generation, lower energy consumption, and biogas generation (Patel et al. 2021; Esmaeeli et al. 2023). Aerobic treatment is more suitable for low- and medium-strength effluents, whereas anaerobic treatment is better for treating high-strength effluents (Buyukkamaci and Koken 2010; Esmaeeli et al. 2023). Buyukkamaci and Koken (2010) performed a cost analysis of 96 treatment plants with 12 flow schemes, including physical, chemical, and biological treatment, and reported that biological processes are most economical for treating wastewater from the pulp and paper industry.
Chemical treatment
Chemical methods for treating wastewater from the pulp and industry wastewater include chemical precipitation, coagulation and flocculation, and AOPs, such as ozonation, ozone/H2O2, photocatalysis, and Fenton oxidation (Ashrafi et al. 2015; Esmaeeli et al. 2023). Kaur et al. (2020) reported that using a conventional alum coagulant and chitosan flocculant to treat wastewater from pulp and paper mills removed 81% TSS and 78% COD. Eskelinen et al. (2010) compared the removals of COD from simulated wastewater containing model compounds of wood extractives (abietic acid, linoleic acid, and β-sitosterol) using various chemical treatment methods, including ultrasonic (US) irradiation combined with Fenton-like oxidation (Fe3+/H2O2), photo-Fenton degradation (Fe3+/H2O2/UV), chemical precipitation using CaO, and electrooxidation. Although the highest COD removal was obtained using chemical precipitation with CaO, combination with subsequent biological treatment is needed to meet the legislative COD limit of 200 mg/L. Ribeiro et al. (2020) reported maximum AOX removals of 85% and 95% by using the Fenton and photo-Fenton processes, respectively, to treat bleaching wastewater from a kraft pulp mill over a 10-min period. Studies were performed on increasing the COD removal efficiency by combining biological treatment with AOPs, such as O3, O3/UV, UV, UV/H2O2, heterogeneous photocatalysis (TiO2/UV and ZnO/UV), Fenton oxidation, and photo-Fenton oxidation. Specifically, O3 treatment has been implemented at an industrial scale in pulp and paper mills (Hermosilla et al. 2014). Combining AOPs with biological treatment may improve the wastewater treatment efficiency and reduce costs (Hermosilla et al. 2014).
Textile industry
The textile industry provides essential goods to society, such as clothing and fabrics. Fabric is manufactured by successive processing of raw materials (cellulose, protein, and synthetic fibers) to yarn to greige fabric to the fabric product.
Fabric production involves dry and wet processes. The wet processes require a large quantity of water and generate highly polluted wastewater (Yaseen and Scholz 2019). Water consumption can range from 30 to 150 L/kg of cloth according to the type of fiber being processed, with the effluent containing 200–600 mg/L BOD, 1,000–1,600 mg/L total solids, and 30–50 mg/L TSS (Azanaw et al. 2022). Cotton is the main raw material used for fabric production, accounting for 60% of the earnings of the industry (Velusamy et al. 2021). Wet processing of cotton includes sizing, desizing, scouring, bleaching, mercerization, dyeing, printing, and finishing (Holkar et al. 2016). Wet processes generate wastewater with varying characteristics resulting from the differences in the raw materials and process used. Table 3 shows the characteristics of wastewaters from different wet processes (Correia et al. 1994; Kant 2012; Sarayu and Sandhya 2012; Holkar et al. 2016; Azanaw et al. 2022).
Wastewater from the dyeing and printing process is diverse because of the numerous dyes used and variations in the dyeing process. Dyes can be classified according to their chemical structure (such as azo, nitro, anthraquinone, cyanine, and carbonyl) or the type of fiber being dyed (cellulose, protein, and synthetic) and application (such as direct, acid, basic, cationic, direct, reactive, and mordant) (Correia et al. 1994; Mustroph 2014). Synthetic dyes have been widely used for their color range, brightness of color, and fastness (Kant 2012). Azo dyes are the most commonly used synthetic dyes because of a low production cost, color variety, and fastness (Piaskowski et al. 2018; Al-Tohamy et al. 2022), accounting for 60%–70% of the synthetic dye industry (Slama et al. 2021). Untreated dye wastewater is harmful to aquatic and terrestrial life as well as the human skin, liver, nervous system, kidney, and reproductive system. (Al-Tohamy et al. 2022). Untreated dye effluents block the transmission of sunlight in water bodies and inhibit photosynthesis, leading to oxygen depletion and low biodegradability by aerobic microorganisms and disruption of the aquatic ecosystem (Slama et al. 2021). Heavy metals, such as lead, chromium, cadmium, and copper, as well as metals may appear in wastewater because of the use of metal-complex dyes (Khan et al. 2022). These heavy metals are toxic and harmful to aquatic life and human health, causing health problems, such as cancer and cardiovascular disease (Khan et al. 2022). To prevent these problems and meet effluent standards, textile wastewater must be treated before being released into the environment or reused. Physical, biological, and chemical methods are used to treat textile wastewater.
Physical treatment
Physical treatment methods, such as adsorption, membrane filtration, and ion exchange, can remove 85%–99% of dyes from effluent (Samsami et al. 2020). Adsorption using materials such as clay, zeolite, and activated carbon has been proposed as an efficient and low-cost method for the removal of heavy metals and dyes from wastewater (Velusamy et al. 2021). Membrane processes, such as microfiltration (MF), UF, nanofiltration (NF), and RO, for the removal of dyes, salts, and other auxiliary chemicals from textile wastewater have been investigated mostly in lab-scale studies and some pilot and full-scale studies, where over 95% removals of COD, turbidity, and color have been attained (Keskin et al. 2021). In particular, good performance has been reported using hybrid systems, such as AS followed by UF and RO (Keskin et al. 2021). Ion exchange using synthetic and natural resins for dye removal has been studied at the lab-scale but has a high cost and limited applicability to dyes (Khan et al. 2023; Singh et al. 2022).
Biological treatment
Biological treatment of textile effluents includes aerobic, anaerobic, and combined processes using bacteria, fungi, and algae (Holkar et al. 2016; Bhatia et al. 2017). A wide variety of dyes can be degraded by using a combination of microorganisms that are compatible with and capable of degrading dyes and their intermediates. Biological treatment is low-cost, eco-friendly, and generates low quantities of sludge compared to other methods (Bhatia et al. 2017; Samsami et al. 2020). Generally, anaerobic treatment can be used to treat high-COD effluents and remove color, whereas aerobic treatment can be used to treat low-COD effluents. Rongrong et al. (2011) developed a lab-scale hybrid anaerobic baffled reactor for treating desizing effluents containing polyvinyl alcohol (PVA), achieving 42.0% COD removal while collecting methane-containing biogas, which can be used for other purposes. In the industrial treatment of textile wastewater, aerobic and anaerobic AS or MBRs are combined with technologies such as coagulation–flocculation, RO, and ozonation (Paździor et al. 2019). Compared to AS, MBRs can achieve higher biomass loadings by using supports on which biofilms can grow, and the presence of various microorganism species enables efficient removal of dyes, COD, BOD, TSS, phosphorus, and heavy metals (You et al. 2007).
Chemical treatment
Coagulation–flocculation has been used in the textile industry as a cost-effective method for color removal from textile wastewater despite excessive sludge generation (Verma et al. 2012). Coagulation–flocculation may be used after secondary biological treatment and before tertiary membrane filtration to prevent fouling by removing colloids, TSS, and color (Aragaw and Bogale 2023). Studies have been performed on using AOPs, such as ozone-based processes, H2O2, photocatalysis, and Fenton oxidation, to remove refractory pollutants from textile wastewater (Paździor et al. 2019). Bilińska et al. (2016) performed a comparative analysis on the removal of Reactive Black 5 using O3, UV/O3, O3/H2O2, O3/UV/H2O2, and H2O2/UV. The ozone-based processes—O3 and O3/H2O2—were cost-effective and could be used as a pretreatment before biological treatment to remove color and improve biodegradability. Heterogeneous photocatalysis has been investigated for the treatment of dye wastewater using semiconductor photocatalysts (TiO2 and ZnO, in particular) for their good photocatalytic activity and availability (Donkadokula et al. 2020).
Treatment of industrial wastewater
Industrial wastewater is treated using physical, biological, and chemical techniques. Each treatment method has advantages and disadvantages regarding factors such as the efficacy of removing specific pollutants, the treatment volume, ease of use, cost, energy usage, and chemical consumption. In many cases, these technologies are combined to achieve efficient removal of multiple types of pollutants, while reducing the total treatment cost.
Physical methods
Physical methods involve the removal of contaminants by exploiting physical and mechanical properties for separation (Pirzadeh 2022). These methods include screening, comminution, grit removal, sedimentation, DAF, adsorption, ion exchange, membrane filtration, evaporation, distillation, and membrane distillation.
Screening, comminution, and grit removal
Screening, comminution, and grit removal are used as pretreatment methods before primary clarification. The main purpose of these methods is to protect downstream equipment and improve the effectiveness of subsequent treatment stages by removing or grinding large solids and debris.
During screening, large solids and debris are removed by passing wastewater through a series of screens or mesh filters to prevent damage to downstream pipes and equipment. Various types of screens, including coarse and fine, are used according to the size and characteristics of the solids and debris to be removed. Coarse screens, such as bar screens, usually have openings with sizes of 6-mm or larger (U.S. EPA 2003). Fine screens usually have openings of between 1.5 and 6 mm in size (U.S. EPA 2003). To remove finer solids, very fine screens with openings of between 0.2 and 5 mm can be used, and microscreens with openings of between 0.001 and 0.3 mm can be used to further treat the secondary effluent, which may still contain fine solids (Prabu et al. 2011). Typically, coarse screens are used near the inlet to capture large solids, followed by using fine screens to capture small particles. The debris captured on the screens is removed manually or mechanically and disposed of in landfills, incinerated, or ground and returned to the wastewater stream (Prabu et al. 2011). The collected solids typically contain various materials, such as plastic, paper, rags, food, and feces from human activity (Szostkova et al. 2012). Screening is used in the textile industry to remove large solids (such as yarn, lint, fibers, and rags) (Azanaw et al. 2022) and in the meat processing industry to remove bones and meat debris (Philipp et al. 2021).
Comminution can be used as an alternative to screening to reduce the size of solids in wastewater by grinding or shredding (Deluise et al. 2005). Comminutors consist of a screen and a rotating drum with slots and cutting teeth to shred solid materials that accumulate on the screen (McLeary 2004). Comminution itself does not remove solids from wastewater. The crushed solids are removed in a subsequent grit chamber and sedimentation tank (Ahmed et al. 2021b). Considering that the ground particles are not removed and can damage the downstream equipment, comminutors are not commonly installed in newer WWTPs (McLeary 2004).
Grit chambers are used to remove heavy particles, such as sand and gravel, from wastewater to prevent damage to downstream equipment, such as pumps and pipes. There are several types of grit-chamber configurations, such as aerated, vortex, and horizontal flows as well as hydrocyclones (U.S. EPA 2003). To remove solids efficiently, the type of grit chamber used is determined by many factors, such as the particle characteristics, settling velocity, space availability, maintenance requirements, energy consumption, and cost. The variable particle density warrants direct measurement of the settling velocity (Plana et al. 2020).
Sedimentation
Sedimentation is a simple and common method for removing TSS from wastewater, which reduces BOD and COD (Jover-Smet et al. 2017). Primary sedimentation tanks, also known as primary settling tanks and primary clarifiers, are placed after screening and grit removal and before secondary biological treatment in conventional WWTPs. In sedimentation tanks, the wastewater velocity is reduced to cause TSS, organic matter, and other particles to settle to the bottom of the tank and form a layer of sludge. The clarified effluent is collected near the top of the tank around the wastewater surface.
Sedimentation tanks can be rectangular or circular. Rectangular tanks have a lower construction cost and can have a longer retention time but are less effective for treating wastewater with high TSS, whereas circular tanks have a lower maintenance cost and easier sludge collection but a shorter retention time. Short-circuiting is more likely to occur in circular tanks than in rectangular tanks (Hirom and Devi 2022). The efficiency of sedimentation tanks is determined by many parameters, such as the particle characteristics, settling velocity, tank dimensions, and wastewater and flow characteristics (Ferdowsi et al. 2022). Considering the complexity of the settling characteristics of suspended particles, experimental data have been used to develop empirical models for sedimentation tanks (Christoulas 1998; Martínez-González et al. 2009; Jover-Smet et al. 2017). As a result of advances in computational technology, computational fluid dynamics (CFD) has recently been used to model, design, and simulate sedimentation tanks, which has improved our understanding of tank hydrodynamics and thereby, tank design (Hirom and Devi 2022). The sedimented solids are mechanically removed by equipment, such as sludge scrapers and sludge pumps.
Secondary sedimentation tanks are also used in conventional treatment plants after secondary biological treatment, such as AS. The functions of secondary sedimentation tanks are settling sludge containing microorganisms to produce a clear effluent and thickening sludge for subsequent recirculation and storage (Patziger et al. 2012). Secondary sedimentation tanks have also been designed using empirical models (Gao and Stenstrom 2018). Computational fluid dynamics models have also been developed for secondary sedimentation tanks. These models have provided insights for tank design, such as the effect of the structure and position of the inlet on turbulence (de Almeida et al. 2020; Gao and Stenstrom 2018).
Sedimentation effectively clarifies wastewater by removing TSS and BOD5 at ratios of 50%–70% and 25%–40%, respectively (Jover-Smet et al. 2017). Sedimentation tanks can have complex hydrodynamics but are relatively low-cost and simple to design and operate. However, the disadvantages of these tanks, such as long retention times for settling fine particles, may lead to large tank volumes. Very fine particles and dissolved content are difficult to remove using sedimentation tanks.
Dissolved air flotation
Dissolved air flotation is used to clarify wastewater, where small air bubbles are used to remove TSS, BOD, COD, oils, and grease. Bubbles are generated by dissolving and saturating air under pressure into water and releasing the air into a flotation tank. The generated air bubbles attach to particles, which consequently float to the surface. The floating particles are removed by a skimming device and disposed. Flocculants and coagulants, such as polymers, ferric chloride, and aluminum sulfate, are often added to the wastewater to aggregate suspended particles, oils, and grease (Musa and Idrus 2021). Dissolved air flotation is used to treat many industrial wastewaters, such as those produced by the pulp and paper (Miranda et al. 2009), petrochemical (Yu et al. 2017), mineral processing (Rajapakse et al. 2022), and food industries (Shrivastava et al. 2022). For example, DAF can remove 70%–80% BOD and 30%–90% COD from slaughterhouse wastewater (Musa and Idrus 2021). For papermill wastewaters, 80%–90% TSS removal can be achieved by removing particles, such as fines, fillers, and ink (Miranda et al. 2009). The effectiveness of DAF depends on factors such as the bubble size distribution, gas–liquid mass transfer, hydrodynamics, wastewater characteristics, and tank geometry (Rajapakse et al. 2022).
Dissolved air flotation offers advantages over sedimentation, such as a shorter retention time, smaller space requirements, and faster removal of small and low-density particles (Rodrigues and Rubio 2007; Crini and Lichtfouse 2019). However, there are challenges associated with DAF, such as high operation and maintenance costs resulting from the energy requirements and maintenance costs for equipment (Yu et al. 2017; Musa and Idrus 2021). The addition of coagulants and flocculants may incur additional costs for DAF.
Ion exchange
The ion-exchange process consists of using a resin to remove dissolved ions and pollutants from water through exchange with similarly charged ions. Ion-exchange resins are solid materials made of a polymer matrix with functional groups attached by covalent bonds (Carolin et al. 2017). Some common polymer matrices include polystyrene, polyacrylic, phenolic, and polyalkylamine resins (de Dardel and Arden 2008). The resins are porous and have a large specific surface area for effective ion exchange. Conventional ion-exchange resins are bead-shaped with typical diameters between 0.04 and more than 1 mm (Fink 2013).
Ion-exchange resins can be largely categorized into cationic and anionic types. Cation-exchange resins are commonly used in softening applications to replace magnesium and calcium ions with sodium ions (Samer 2015). At the industrial scale, wastewater is passed through a column packed with an ion-exchange resin. The saturated resin can be regenerated by flushing the column with a sodium solution (Samer 2015). Anion-exchange resins can be used to exchange negatively charged ions, such as nitrate, sulfate, chloride, and bicarbonate (Gomaa et al. 2021). Anion-exchange resins can be regenerated by treatment with a basic solution, such as a sodium hydroxide solution or an ammonium hydroxide solution. Cationic and anionic resins can be classified by their functional groups, including the strongly acidic cation (SAC) with a sulfonic group, weakly acidic cation (WAC) with a carboxylic group, strongly basic anion (SBA) with a quaternary ammonium group, and weakly basic anion (WBA) with primary, secondary, or tertiary amino groups, and chelating with functional groups, such as iminodiacetic and phosphinic groups (Chen et al. 2021). The resin is selected depending on criteria such as the target ions to be removed, the wastewater characteristics, economic feasibility, and the desired water quality.
Ion exchange has been used for numerous purposes, such as water softening, demineralization, deionization, deacidification, removal of impurities (such as nitrates and heavy metals), decoloring, separation, and dehydration (de Dardel and Arden 2008). Thus, ion exchange is used in many fields, such as agriculture, food processing, chemical synthesis, laboratory use, wastewater treatment, and hydrometallurgy (Ijanu et al. 2020). Studies have been performed on using ion exchange for industrial wastewater treatment, such as to treat textile-dyeing wastewater (Khan et al. 2023) and remove fluoride (Wan et al. 2021), phenol (Anku et al. 2017), and heavy metals (Barakat 2011). Ion exchange has been shown to be an effective wastewater treatment but is not widely used industrially because of drawbacks, such as high associated costs and the limited selectivity of conventional resins (Crini and Lichtfouse 2019; Khan et al. 2023).
Adsorption
Generally, adsorption refers to the change in concentration of a substance relative to those of neighboring phases at the interface between two phases, including liquid–gas, liquid–liquid, solid–liquid, and solid–gas interfaces (Dąbrowski 2001). Solid adsorbents are used to remove organic and inorganic pollutants from wastewater. There are two types of adsorption: physisorption and chemisorption. Physisorption is the adhesion of an adsorbate onto an adsorbent surface through van der Waals forces. Chemisorption involves the formation of covalent or ionic bonds between an adsorbate and an adsorbent. Physisorption is weak, nonspecific, and reversible, whereas chemisorption is strong, more specific, and often irreversible. Many factors affect the effectiveness of adsorption, such as the temperature, pH, use of stirring, contact time, adsorbent dosage, and initial concentration (Sukmana et al. 2021; Chai et al. 2021).
Adsorbents used in water treatment include natural and synthetic materials, such as activated carbon, clay, biosorbents, graphene oxide, and various nanomaterials. (Tran 2023). Among various available adsorbents, activated carbon is the most popular and is widely used for wastewater treatment because of advantages such as a high specific surface area, wide applicability to many pollutants, and regeneration ability (De Gisi et al. 2016). However, activated carbon is expensive and can be costly to regenerate (Chai et al. 2021). The nonselectivity of activated carbon must be traded off against its wide applicability. Studies have been performed on using low-cost adsorbents, such as agricultural wastes (e.g., orange peel, banana peel, and rice husks) and industrial wastes (e.g., flue ash, red mud and bagasse ash) (Rashid et al. 2021). Li et al. (2019) reviewed the key requirements and analyzed the feasibility of using bioadsorption for industrial-scale treatment of dye wastewater. Bioadsorption was determined to be a competitive technology if the adsorbent used has a good adsorption/desorption performance and is reused numerous times.
Adsorption can be implemented at various stages of industrial wastewater treatment depending on the target pollutant, concentration, and desired treated effluent quality. For example, adsorption can be combined with technologies such as ozone, DAF, and coagulation to treat papermill wastewater and remove toxic pollutants, color, and COD (Thompson et al. 2001). Adsorption may also be used for secondary or tertiary treatment of wastewater containing oil and grease, such as those from petroleum refineries and metalworks (Pintor et al. 2016).
Membrane filtration
Wastewater can be filtered through semipermeable membranes with various pore sizes to separate pollutants, such as colloids, microorganisms, organics, salts, and ions. Semipermeable membranes are selectively permeated by substances with particular sizes and charges (Breite et al. 2019). Wastewater can be treated using MF, UF, NF, and RO membranes (Keskin et al. 2021). Asymmetric membranes are used in a pressure-driven process for their high flux and mechanical stability (Strathmann 2005). The membranes can be composed of polymers (such as polyethylene, polytetrafluorethylene, and polypropylene) or inorganic materials (such as ceramics, zeolites, and silica) (Ezugbe and Rathilal 2020).
Microfiltration membranes have relatively large pore sizes of 0.1 μm or more and are often used to pretreat wastewater before UF, NF, and RO (Behroozi and Ataabadi 2021). These membranes can separate larger particles, such as TSS, colloids, and organic matter at relatively low operating pressures of 0.02 to 0.5 MPa (Zioui et al. 2023). Ultrafiltration membranes have pore sizes of 0.001 to 1 μm and are used to remove pollutants, such as TSS, organics, oils, and pigments (Ezugbe and Rathilal 2020). Nanofiltration membranes have pore sizes of 1 to 5 nm and can therefore separate pollutants with relatively low molecular weights and reject pollutants such as sugar, salt, minerals, heavy metals, oils, and dyes (Mulyanti and Susanto 2018). Reverse osmosis membranes have pore sizes of a few angstroms and can separate sodium and chloride ions, making RO a promising technology for seawater desalination (Jamaly et al. 2014). Reverse osmosis membranes have small pore sizes that enable removal of all pollutants but are susceptible to fouling, must be operated under high pressure, and are more expensive than other membranes (Nqombolo et al. 2018).
Membrane filtration is a versatile technology for industrial wastewater treatment and can be combined with other technologies to achieve sufficiently high water quality for reuse. For example, membranes have been used in combination with biological treatment in MBRs. Membrane filtration can be used to treat wastewater from many industries, including pulp and paper (Valderrama et al. 2021), textiles (Keskin et al. 2021), electroplating, and petroleum (Barakat 2011). Some advantages of membrane filtration are the production of high-quality treated water, a smaller footprint than that of conventional filtration, and the ability to be installed in existing WWTPs. Some challenges associated with membrane filtration are higher overall costs compared with those of conventional WWTPs and susceptibility to fouling (Othman et al. 2022).
Evaporation, distillation, and membrane distillation
Evaporation, distillation, and membrane distillation are thermal wastewater treatment processes that can separate and concentrate pollutants in wastewater. The evaporated wastewater may be collected by condensation.
Evaporation ponds are shallow, open-air basins that are lined with materials, such as clay and synthetic materials, to prevent wastewater seepage. Water is evaporated by solar irradiation, resulting in the concentration of contaminants, precipitation of crystalline salts, and sediment accumulation. The accumulated solids are removed regularly and disposed. Evaporation ponds have various applications, such as the treatment of oil-produced water and mine wastewater and the rejection of brine from desalination plants and other industrial wastewater (Izady et al. 2020). Advantages of evaporation ponds include straightforward application and low capital and operational costs. Some disadvantages of evaporation ponds are the environmental and health impact of the release of heavy metals, pesticides, VOCs, CO2, and CH4 (Amoatey et al. 2021). Evaporation ponds require large land usage and solar irradiation and are therefore suitable for dry and warm locations with low-cost land (Abdeljalil et al. 2022).
Other evaporation techniques consist of using equipment such as flash evaporators, condensers, and distillation columns. Wastewater is converted to water vapor using heating devices, such as heat pumps and heating elements. The water vapor is then condensed and collected as distilled water. A portion of the wastewater may be left behind that contains unevaporated salts and other solids. Evaporation techniques can be used to treat a wide variety of wastewaters and are especially useful for desalination. However, steam generation has a large energy demand. Organics that evaporate at low temperatures may enter the treated water stream. To lower the energy demand for heating and removal of organics, Yang et al. (2018) proposed a desalination system with a low-temperature heat pump. A COD removal of 97% was achieved at 48 °C, producing 3 kg of treated water in 1 h for a power consumption of 250 W. Evaporation technologies have been developed to reduce energy consumption and improve the separation efficiency. Among evaporation technologies, multieffect evaporation is used most often because of maturity and high efficiency (Lu et al. 2017). Multieffect evaporation is implemented using a series of single-effect evaporators, where the vapor generated from one evaporator is used to heat the next evaporator to reduce energy consumption. Mechanical vapor recompression is an alternative emerging technology in which generated vapor is compressed and reused to heat the feed. Mechanical vapor recompression is mainly used for desalination and has advantages such as a higher energy efficiency than multieffect evaporation, compactness, and low-temperature operation (Liang et al. 2013). Some advantages of evaporation technologies are a high recovery rate, recoverability of both high-quality water and salts, wide applicability, and no use of supplementary materials. Some disadvantages of evaporation technologies are high capital costs, high energy consumption, and complexity (Mizuno et al. 2013, 2015; Lu et al. 2017).
Membrane distillation is a thermal separation process in which a hydrophobic porous membrane is used to pass water vapor while rejecting pollutants. Membrane distillation can be divided into four types depending on the permeation side: direct contact in which both sides of the membrane surface contact vapor, air-gap in which the permeation side has an air gap, sweeping gas in which a cold inert gas is used to transfer vapor from the permeate side, and vacuum in which a vacuum is applied to the permeate side by a pump at a lower pressure than the saturation pressure of the volatile molecules (Yan et al. 2021). Membrane distillation has been considered one of the most promising technologies for the treatment of saline wastewater and can also be used to treat oily wastewater by being combined with other processes to reduce fouling (Kalla 2021). Some advantages of membrane distillation are low working pressures (to prevent fouling), high selectivity, and low sensitivity to the feed solute concentration. Some disadvantages of membrane distillation are a lower throughput than RO, pore-wetting risk, and a high energy demand (Shirazi and Dumée 2022).
Chemical methods
Chemical treatment involves the use of chemicals, such as inorganics (iron and aluminum salts) and organics (cationic, anionic, and nonionic polymers). Some examples of chemical treatment methods are coagulation–flocculation and chemical precipitation (for increasing the settleability of pollutants), chemical oxidation, and AOPs (for degradation of organics, pH adjustment, and disinfection). Chemical treatment is often combined with other biological and physical treatment processes as a pretreatment or post-treatment strategy.
Chemical precipitation
Chemical precipitation removes dissolved pollutants from wastewater as solid particles. This technique is effective for the removal of heavy metals and is widely used in industry because of low costs and facile operation (Yadav et al. 2019). Counterions are added to reduce the solubility of dissolved ions, which are then removed through precipitate formation (Zueva 2018). These precipitates must be separated out using methods such as sedimentation and filtration. Flocculants may be used to improve the settleability of precipitants (Ojovan and Lee 2014). Chemical precipitation has the advantages of low capital costs and simple operation but also has disadvantages, such as the operating costs of using chemical precipitants and sludge disposal (Wang et al. 2005).
Dissolved metals can be precipitated as hydroxides, sulfides, and carbonates. The most widely used method for hydroxide precipitation involves the addition of alkaline agents, such as calcium hydroxide (lime) or sodium hydroxide (Dahman 2017). Lime is the most cost-effective alkaline agent for wastewater treatment (Zueva 2018). The addition of lime results in the formation of metal hydroxides and calcium ions:
The optimal pH for hydroxide precipitation depends on the dissolved metal. Considering the amphoteric nature of metal hydroxides, decreasing or increasing the pH may cause precipitates to resolubilize. Thus, it is challenging to treat wastewater containing different metals because multiple steps are required to remove the metal precipitates at their optimal pHs.
Sulfide precipitation involves the addition of sulfide ions usually generated from H2S, Na2S, CaS, (NH4)2S, or NaHS (Estay et al. 2021). The sulfide ions react with metals to form metal sulfide precipitates:
Sulfide precipitation offers advantages over hydroxide precipitation, such as less soluble precipitates, faster reaction rates, and better settling, as well as disadvantages, such as the sensitivity of the reaction system to the sulfide dosage and problems associated with the usage of excess sulfide (Lewis 2010).
Carbonate precipitation is an alternate method involving the use of sodium carbonate or calcium carbonate. Sodium carbonate participates in the following reactions, producing a metal carbonate precipitate and CO2 that can attach to and float the precipitate (Zueva 2018):
Quiton et al. (2022) compared the efficacies of carbonate and hydroxide precipitation for the removal of cobalt and copper from electroplating wastewater. Compared to hydroxide precipitation, carbonate precipitation achieved a higher removal efficiency of both metals at a lower pH of approximately 7–8, but generated a larger sludge volume.
Coagulation and flocculation
Unlike chemical precipitation, coagulation and flocculation remove TSS and colloids without a phase change. Coagulation and flocculation often occur simultaneously but are different treatment processes. Coagulation refers to the destabilization of a suspension or solution, whereas flocculation refers to the agglomeration of destabilized particles into large flocs (Bratby 2016). The generated flocs are separated by sedimentation, filtration, or air flotation.
Mixing is an important factor that affects the overall process performance. Coagulation and flocculation have different optimal mixing speeds and times. Rapid mixing is employed for coagulation, whereas slow mixing is employed for flocculation (Saritha et al. 2017). Yu et al. (2011) investigated the effect of rapid and slow mixing on coagulation and flocculation, using aluminum sulfate hydrate (alum) as a coagulant and kaolin clay as a model suspension. Increasing the time for rapid mixing decreased the final floc size, whereas increasing the speed of slow mixing decreased the floc size. Rapid mixing can cause floc breakage because of high shear and change in the floc surface properties, which affects the coagulation efficiency. Other important factors that affect the coagulation and flocculation efficiencies include the pH, coagulant and flocculant dosage, temperature, and the presence of anions (such as bicarbonate or sulfate) (Ersoy et al. 2009).
Conventional chemical coagulants include alum, ferric sulfate, ferric chloride, and polyaluminum chloride, to which natural coagulants and flocculants derived from animals, plants, and microorganism have been considered as alternatives (Badawi et al. 2023). Inorganic coagulants and flocculants have disadvantages, such as pH sensitivity, sludge generation, and leaching of metal ions from sludge to groundwater, which has resulted in increasing use of polymer flocculants that can form large flocs at low dosages (Maćczak et al. 2020). Commonly used flocculants include nonionic flocculants (such as polyacrylamide), cationic flocculants (such as polydiallyldimethylammonium chloride), and anionic flocculants (such as copolymers of acrylamide and ammonium) (Dao et al. 2016).
The advantages of coagulation and flocculation include simplicity, effective removal of colloids and suspended particles, and effective settling of sludge. The disadvantages of these processes include high operating costs incurred by continuous addition of coagulants and flocculants, large sludge generation, and sludge disposal costs (Iwuozor 2019).
Solvent extraction
Solvent extraction can be used to remove and recover valuable materials from wastewater. This technique has been used commercially for materials recovery, such as in the petroleum, wool, and pharmaceutical industries (Lo and Baird 2003). The first step in solvent extraction is contacting wastewater with an immiscible solvent. The solvent selectively extracts the target compound, known as the solute, from the wastewater. Sufficient contact results in a solute-rich solvent (the extract) and a solute-depleted effluent (the raffinate). The extract and raffinate are separated, the solute is recovered from the extract, and the solvent is recycled. The raffinate is the treated wastewater, which can be further treated, discharged, or recycled depending on the demanded water quality. Contact between the solvent and the wastewater and solute extraction are achieved using various extractors, such as mixer–settlers, agitated columns, and packed columns, whereas solvent regeneration and solute recovery can be accomplished by distillation and gas stripping (Chang 2020).
Solvent extraction is widely used for the treatment and recovery of wastewater with high concentrations of phenolic compounds, such as wastewater from coal gasification (Feng et al. 2017). Yang et al. (2006) developed a solvent extraction process using methyl isobutyl ketoneas the solvent to treat wastewater from coal gasification containing 5,000 mg/L phenol. In a trial plant with a wastewater flow of 2 t/h, 93% of phenols were recovered. The recovered phenols provided economic benefits that could compensate for the operational cost of the process. The advantages of using solvent extraction to treat wastewater include selectivity for specific pollutants, recovery of valuable materials, solvent regeneration, and no sludge generation (Chang 2020). The disadvantages of this process include the investment costs associated with the use of specialized equipment and solvents and the potential environmental and health impact of solvent use.
Electrochemical methods
Electrochemical technologies involve the application of electricity through electrodes. The most studied processes include electrocoagulation (EC), electroflotation (EF), electrochemical oxidation (EO), electroreduction (ER), and electrodialysis (ED) (Sillanpää and Shestakova 2017).
Electrochemical coagulation and electrochemical flotation
In EC and EF, an electric current is applied to an anode and cathode in a reactor to produce destabilization agents, such as Al and Fe, and gas bubbles (Emamjomeh and Sivakumar 2009). Under an applied electric current, metal cations are generated at the anode and hydroxide ions and hydrogen gas are produced at the cathode. In EC, metal hydroxides are generated by the combination of metal cations and hydroxide cations. The metal hydroxides neutralize charged contaminants, and the neutralized contaminants are adsorbed by sweep coagulation, resulting in the formation of flocs (Das et al. 2022). In EF, the flocs are separated by hydrogen gas bubbles that adhere to the flocs and float the flocs to the surface or by sedimentation (Emamjomeh and Sivakumar 2009).
Similar to conventional coagulation–flocculation, EC can be used to remove contaminants from wastewater, such as TSS, TOC, oils, heavy metals, COD, color, and turbidity (Das et al. 2022). The use of EC has been investigated to treat wastewater from the dairy, textile, petroleum, pulp and paper, and pharmaceutical industries (Boinpally et al. 2023). The advantages of EC include the effective removal of colloids, no addition of chemicals, low sludge generation, and simple operation. Electrocoagulation also has disadvantages, such as periodic replacement of the sacrificial anode, electrode fouling, the possibility of metal hydroxide dissolution, and power consumption (Sivaranjani et al. 2020; Boinpally et al. 2023).
Electrochemical oxidation
Electrochemical oxidation involves both direct and indirect oxidation. During direct oxidation, pollutants adsorb onto the anode surface and an electron is directly transferred between the anode and the pollutant. During indirect oxidation, reactive species, such as reactive oxidation species and chlorine active species, are generated at the electrode surface and react with pollutants (Garcia-Segura et al. 2018). Garcia-Segura et al. (2018) investigated using EO to treat wastewaters, such as those from the petroleum, pulp and paper, and pharmaceutical industries, by removing COD, TSS, and recalcitrant organics. Electrodes, especially the anode where oxidation occurs, are key EO components that affect the cost and efficiency of the process. Thus, effort has been expended in developing anode materials with high performance, low cost, and high stability (Qiao and Xiong 2021). The advantages of EO include the ability to degrade recalcitrant pollutants, a small footprint, no addition of chemicals, and reduced secondary pollution. The disadvantages of EO are high energy consumption, high cost of some electrodes (such as those based on noble metals and diamond), challenges with mass producing some electrodes, possible corrosion and fouling of electrodes, and electrode replacement.
Electrochemical reduction
Electrochemical reduction is an emerging technology that involves either direct or indirect reduction. During direct reduction, electron transfer occurs between the cathode and the pollutant adsorbed on the cathode. During indirect reduction, the cathode reduces a mediator, and the reduced mediator reduces the pollutant (Mousset and Doudrick 2020). Electrochemical reduction has been studied for the detoxification and conversion of toxic organics into value-added materials, denitrification, removal and recovery of metals (Xue et al. 2023), and decolorization (Sala and Gutiérrez-Bouzán 2012). The advantages of ER are no chemical addition, a low footprint, metal removal and recovery, and the conversion of pollutants into value-added materials. The disadvantages of ER include the high cost of noble-metal electrodes, high energy consumption, the need to control competition with the hydrogen evolution reaction, and the possibility of some corrosion. Compared to EO, ER has been the subject of fewer studies, cannot mineralize pollutants, and has slower kinetics (Xue et al. 2023).
Electrodialysis
Electrodialysis involves the application of an electric field and ion-exchange membranes t(IEMs) to separate ions, such as dissolved salts. Electrode compartments contacting the anode and cathode are placed on the outer sides of ED units. Between the electrodes, there are alternating layers of anion exchange membranes (AEMs) and cation exchange membranes (CEMs) separated by spacers. Under an applied electrical potential, cations migrate toward the cathode by passing through the CEMs and anions migrate toward the anode by passing through the AEMs. Cations are blocked by the AEMs, and anions are blocked by the CEMs, resulting in compartments with alternating concentrated and dilute solutions (Mohammadi et al. 2021). The number of cell pairs in an ED stack depends on the scale of the units, ranging from a few cell pairs for lab-scale units to several hundreds of pairs for pilot-scale units (Mohammadi et al. 2021). The IEMs are selective and separate molecules based on their charge.
Electrodialysis has been studied to treat industrial wastewater containing salts, such as those from oil and gas extraction, the petrochemical and coal mining industries, and power plants (Gurreri et al. 2020). The use of ED has been considered for the recovery of metals, such as those from the metal deposition and electroplating industries (Arana Juve et al. 2022). The advantages of ED include high salt removal, metal removal, low susceptibility to scaling, no addition of chemicals, and low operating pressures. The disadvantages of ED are high capital costs and energy demand, membrane fouling, and the inability to remove nonionic pollutants (Zhao et al. 2019; Mir and Bicer 2021; Arana Juve et al. 2022).
Chemical oxidation
Conventional chemical oxidation involves using various oxidizing agents to degrade organic pollutants in wastewater and disinfect biologically treated wastewater. Various oxidizing agents have been used, such as permanganate, O3, H2O2, chlorine, and persulfate (Devi et al. 2016). Chlorination has been commonly used to treat wastewater before discharge or reuse. Rodríguez‐Chueca et al. (2015) compared the efficacy of using chlorination to disinfect Escherichia coli in municipal wastewater with that of using NaClO and various AOPs, including UV, H2O2/solar irradiation, and photo-Fenton oxidation. Although the optimal disinfection/cost ratio was obtained using chlorination, chlorine can generate carcinogenic halogenated byproducts that pose health and environmental risks. H2O2 is another oxidizing agent that has been used to reduce BOD, COD, and odors to further improve the quality of wastewater treated by physical or biological treatment (Ksibi 2006). Doltade et al. (2022) used O3 and H2O2 as oxidizing agents to treat wastewater from the polymer industry, achieving COD reductions of 85% and 91%, respectively, from an initial COD of approximately 1920 ppm and demonstrated the synergistic effect of combining the two oxidants.
The advantages of chemical oxidation are versatility and effectiveness in removing a wide range of organic contaminants, a relatively short contact time for treatment compared to biological methods, odor and color removal, and disinfection. The disadvantages of chemical oxidation are the environmental impact of the oxidizing agent used and intermediates produced, operation costs associated with the production, storage, transportation, and usage of oxidants, and the need to use pretreatment.
Advanced oxidation processes
Advanced oxidation processes are emerging water treatment methods that can degrade trace toxic organics in wastewater and upgrade treated wastewater for reuse. Reactive species, such as hydroxyl radicals (HO•), are generated and nonselectively oxidize organic pollutants in wastewater to nontoxic substances, such as CO2 and H2O. These methods can effectively treat wastewater containing recalcitrant organics that are difficult to remove by conventional treatment, such as pharmaceuticals, pesticides, phenols, and dyes. As a result of growing interest in water reuse and the need to meet stricter water pollution regulations, AOPs are increasingly being used to upgrade effluents by removing persistent pollutants. Advanced oxidation processes include O3/H2O2/UV, Fenton oxidation, photocatalysis, and sonolysis.
O3, H2O2, and UV
The strong oxidants—O3 and H2O2—can be used alone or in various combinations with each other and UV, such as O3/UV, H2O2/UV, O3/H2O2, and O3/H2O2/UV. During ozonation, organics can be degraded by direct oxidation (reaction with O3) or by indirect oxidation (reaction with hydroxyl radicals) (Chiang et al. 2006). The combined use of O3, H2O2, and UV has been proposed to improve the organic removal efficiency (Matsumoto et al. 2021). These methods, especially O3/H2O2/UV, have been used to treat wastewater from the textile, pharmaceutical, petroleum, and aquaculture industries by efficiently removing emerging pollutants (Angeles Amaro-Soriano et al. 2021). The advantages of these technologies are nonselective oxidation of pollutants by radical species, complete mineralization, no generation of halogenated byproducts, disinfection, simple operation, and the ability to upgrade treated wastewater for reuse. The disadvantages are the capital and operating costs of ozone generation, UV irradiation, and H2O2 usage as well as the risks posed by handling O3 and H2O2.
Fenton oxidation
During Fenton oxidation, HO• radicals are generated by using H2O2 and iron ions as a homogeneous catalyst under acidic and ambient conditions. The generally accepted mechanism is given below (Bautista et al. 2008):
Photo-Fenton oxidation, which combines Fenton oxidation with UV–vis irradiation, has been used to improve organic degradation. The generation of HO• is realized by the decomposition of H2O2 under light irradiation and the regeneration of Fe2+ through the following reactions:
Fenton and photo-Fenton oxidation have been investigated to treat wastewater from industries such as oil, textile, and pulp and paper (Machado et al. 2023). The advantages of Fenton oxidation include simplicity, effective pollutant degradation, availability of Fe2+ and H2O2, and environmental safety. The disadvantages of this method include the need for sludge disposal, pH control, and high chemical inputs (Bello et al. 2019).
Photocatalysis
Heterogeneous photocatalysis involves the use of a semiconductor photocatalyst that is activated by light irradiation. The photocatalyst absorbs photons with sufficient energy to generate electron–hole pairs that participate in reactions. Organic pollutants may be degraded directly on a photocatalyst surface or indirectly by generated HO• (Oturan and Aaron 2014).
In photocatalytic applications, TiO2 has been widely used for its chemical stability, durability, low cost, and nontoxicity (Nakata and Fujishima 2012). Alternative photocatalysts have been developed but many are impractical because of being based on expensive, rare, or toxic materials as well as fragility and chemical instability. Thus, TiO2 remains a popular choice (Loeb et al. 2019).
Studies have been performed on increasing the removal of organic pollutants by using hybrid photocatalytic technologies, such as a photocatalytic circulating-bed biofilm reactor with photocatalytic-biological carriers (Marsolek et al. 2008), photocatalytic membrane reactor consisting of a photocatalyst deposited on ceramic membranes (Lim and Goei 2016), and sonophotocatalysis (the simultaneous application of photocatalysts and ultrasound (US)) (Kakavandi et al. 2019). In other studies, photocatalytic oxidation has been improved by using a US-generated mist (Itoh and Kojima 2019; Kato et al. 2023).
Photocatalysis offers advantages of nonselective degradation and mineralization of a wide range of organics, low chemical consumption because the photocatalyst can be reused, and the option to use solar irradiation. The disadvantages of photocatalysis are the need for efficient light irradiation of the photocatalyst, operational costs, energy input of artificial light sources, photocatalyst fouling, instability and safety concerns for some photocatalysts, photocatalyst recovery for slurry reactors, and equipment costs.
Sonolysis
Sonolysis involves using US to degrade organic pollutants in wastewater. Irradiation by US results in cavitation; that is, the formation, growth, and collapse of bubbles generating hot spots of approximately 5,000 and 500 atm and shock waves (Suslick 1990). Cavitation causes thermal dissociation and the formation of radicals (e.g., HO•, O•, H•, and HO2•) that react with organic pollutants (Atalay and Ersöz 2021). The sonolysis frequency is an important parameter that determines whether physical or chemical effects are dominant. Low US frequencies of 20–80 kHz are considered to mainly cause physical effects, whereas higher US frequencies of 150–2000 kHz are considered to mainly cause chemical effects (Chatel et al. 2017). The application of multiple frequencies may simultaneously enhance the cavitational intensity and chemical and physical effects (Gogate and Patil 2016). Sonolysis has advantages of safety, eco-friendliness, no addition of chemicals, utility as a pretreatment to enhance biodegradability, and the ability to degrade recalcitrant organics. The disadvantages of sonolysis are equipment costs, high energy consumption, and conversion of cavitational energy producing chemical and physical effects, which have limited the full-scale application of this technique (Pang et al. 2011; Pirsaheb et al. 2023). Sonolysis is versatile and compatible with other treatment techniques, such as biological treatment, photocatalysis, and the use of UV, O3, and H2O2 (Savun-Hekimoğlu 2020; Pirsaheb et al. 2023).
Biological methods
Biological treatment uses microorganisms, such as bacteria, fungi, yeast, and algae, to remove pollutants from wastewater. Biological treatment of wastewater is mainly used to reduce organics but can also remove inorganic compounds, such as heavy metals (Singh et al. 2022). Biological treatment can effectively treat industrial wastewaters with high organic contents, such as those from the food, paper, and textile industries.
Biological treatment is typically categorized into aerobic and anaerobic types. Aerobic biological treatment consists of using microorganisms to convert organic pollutants into CO2, water, and biomass in the presence of oxygen, which is often supplied by mechanical aeration using air blowers and compressors. During anaerobic biological treatment, pollutants are metabolized by microorganisms in the absence of oxygen through anaerobic processes, including hydrolysis, acidogenesis, acetogenesis, and methanogenesis (Vijin Prabhu et al. 2021). As a result, biogas containing mainly CO2 and CH4 is produced (Vijin Prabhu et al. 2021). Aerobic treatment is usually used for low-strength effluents with CODs below 1000 mg/L, whereas anaerobic treatment is suitable for high-strength effluents with CODs above 4000 mg/L (Chan et al. 2009). Considering the greenhouse gas (GHG) emissions (CO2 and CH4), Cakir and Stenstrom (2005) reported that crossover points exist in the range of 300–700 mg/L ultimate BOD (BODu), depending on the aerobic treatment efficiency. They indicate that anaerobic treatment emits less GHG for wastewater with a BODu value above the crossover point while aerobic treatment emits less GHG for a BODu value below the crossover point.
Anaerobic treatment offers advantages over anaerobic treatment, including a lower energy demand, six-to-eightfold lower biomass production, a smaller reactor volume, and the production of biogas, which can be used as fuel (Ghangrekar and Behera 2013). Ranieri et al. (2021) investigated the electricity consumption of 202 WWTPs in Italy and found an electricity consumption of 1.02 kWh/m3 for aerobic treatment and 0.43 kWh/m3 for anaerobic treatment. Aerobic treatment offers advantages over anaerobic treatment, including a higher quality of treated wastewater, reduced odor (Martin et al. 2011), and nutrient removal (Aziz et al. 2019). Thus, anaerobic–aerobic systems can be used to efficiently remove organic content and increase effluent quality to meet discharge standards (Chan et al. 2009). Other combined systems include AOAO, AAO, and AAOO. Biological treatment is combined with physical and chemical treatment, typically as a secondary treatment, in WWTPs.
Aerobic digestion
Commonly used aerobic treatment methods include AS, aerated lagoons, the sequential batch reactor (SBR), trickling filter, MBR, rotating biological contactor (RBC), and aerobic MBBR.
The handling of excess sludge has been a key issue in aerobic treatment. Although sludge is most commonly disposed of in landfills, other handling methods have been proposed because of the environmental impact of sludge, to comply with environmental regulations, and the increasing costs of landfill disposal (Nguyen et al. 2022). Some alternative methods for sludge disposal include sludge thickening followed by anaerobic digestion and incineration as well as dewatering and drying followed by incineration (Hao et al. 2020). The main disposal methods used in the EU have been sludge reuse (such as in agriculture) and sludge incineration (Kelessidis and Stasinakis 2012).
Activated sludge
Activated sludge is one of the most used biological processes for treating solids and organic pollutants in wastewater (Zhang 2020; Islam and Mahdi 2022). Microorganisms suspended in aeration tanks are used to remove organic pollutants and nutrients from wastewater. Following the aeration tank, sedimentation tanks are used to separate sludge, where some of the sludge is returned to the aeration tank to maintain the concentration of microorganisms, and the rest of the sludge is removed. Depending on the water quality demand or standards, the supernatant from the sedimentation tank may either be discharged directly or undergo further treatment or disinfection before discharge. Biocarriers may be used to increase the efficiency of COD removal by AS. Jagaba et al. (2022) reported up to 88.4% removal of COD in wastewater from the pulp and paper industry using a hydraulic retention time (HRT) of 2 days and rice-straw activated carbon. The advantages of AS include low installation costs, high effluent quality, and a low footprint. The disadvantages of AS include high operating costs, sludge disposal, and sensitivity to effluent characteristics (Rezai and Allahkarami 2021).
Sequential batch reactor
The SBR is a variation on AS in which unit operations, such as aeration and sedimentation, are carried out in the same tank (Albahnasawi et al. 2023). There are five stages of SBR operation. In the filling phase, wastewater is added to the tank. In the reaction phase, pollutants are removed with or without mixing and aeration. In the settling phase, the tank acts as a clarifier without inflow or outflow. In the drawing phase, the supernatant is discharged and excess sludge is removed. The idle phase is used to switch between tanks for multiple tank systems (Singh and Srivastava 2011). Aeration can be flexibly controlled in SBRs to realize aerobic or anaerobic conditions. The advantages of the SBR include flexibility, control, a small footprint, and low costs because unit operations can be conducted in a single tank. The SBR also has disadvantages, such as complex operation, maintenance, and possible sludge discharge and blockages in aeration equipment (U.S. EPA 1999).
Trickling filter
In trickling filters, wastewater is distributed over a bed of solid media, such as rocks, gravel, or plastic. The solid media provide a surface on which microorganisms can grow and form a biofilm. Aerobic conditions are achieved by either upward or downward natural airflow, depending on the temperature and the difference in the humidity inside and outside the trickling filter. Alternately, mechanical ventilation using low-pressure fans can be used to provide consistent upward or downward airflow (Daigger and Boltz 2011). Sloughing occurs as the microbial layer thickens, and a portion of the biofilm falls off into the effluent (U.S. EPA 2000). The sloughed film is separated out in a secondary clarifier featuring the secondary sludge. Trickling filters have been employed to treat wastewater from the dairy industry, achieving 85% COD removal using a HRT of 10 days at 7–13 °C (Shahriari and Shokouhi 2015). The SBR has been used to remove various pollutants in wastewaters from mining, textile, and other industries (Dhokpande et al. 2014). Trickling filters offer advantages such as simplicity, reliability, and low power requirements as well as disadvantages of odor emission and the need for additional treatment and operator monitoring (Rezai and Allahkarami 2021).
Membrane bioreactor
Membrane bioreactors combine biological treatment and membrane filtration (MF and UF). Biological treatment removes pollutants from effluent, and the generated sludge is separated by membranes rather than by sedimentation. There are external and submerged MBRs. Tubular membranes are installed separately from the bioreactor in external MBRs, whereas hollow-fiber or flat-sheet membranes are immersed in the bioreactor in submerged MBRs (Martínez et al. 2020). Aeration is used to provide air and turbulence, which is crucial for preventing membrane fouling in submerged MBRs (Melin et al. 2006). The use of ceramic filters with enhanced resistance to fouling and anaerobic operation has been proposed to reduce the energy demands of MBRs (Judd 2008). Some advantages of MBRs include the high effluent quality produced by membranes, small sizes, and simplicity of automation (U.S. EPA 2007). In an MBR, the HRT and solids retention time (SRT) can be controlled independently, enabling higher sludge concentrations, longer sludge retention times, and the development of specialized microorganisms (Melin et al. 2006). Membrane bioreactors have disadvantages such as fouling, the need for membrane maintenance, foaming, electricity demand up to double that of CAS, and high capital and operational costs (Al-Asheh et al. 2021).
Rotating biological contactor
Rotating biological contactors use rotating cylindrical discs that are partially submerged in wastewater (Waqas et al. 2023). Microorganisms consume organic matter and form a biofilm on the disk surface. The rotating discs promote oxygen transfer to maintain aerobic conditions and provide turbulence to remove excess solids from the disc (Cortez et al. 2008). Thus, the cost of aeration is lower than that for AS. The effluent from an RBC is sent to a secondary sedimentation tank to remove TSS (Pathan et al. 2016). The advantages of RBCs include small land usage, high biomass concentrations, low energy consumption, short HRTs, and low operational and maintenance costs. The disadvantages of RBCs include low flexibility, the need for sludge removal, and sensitivity to wastewater characteristics (Mizyed 2021).
Aerobic moving bed biofilm reactor
In MBBRs, suspended plastic biofilm carriers are used to support microbial growth and the development of biofilms. The plastic carriers have a similar density to that of water and are maintained in suspension by aeration, liquid circulation, or mechanical mixing (Gzar et al. 2021). Biofilm sloughing occurs in MBBRs, as in trickling filters. Biomass from the MBBR effluent is removed using methods such as sedimentation, flotation, microscreening, and membrane filtration (Ødegaard et al. 2010). A major advantage of MBBRs is the ability to upgrade and increase the performance of existing WWTPs that use AS, eliminating the need to build new tanks (Falletti et al. 2014). Upgrading AS to MBBRs can decrease the HRT, increase the SRT, prevent clogging and channeling, and reduce capital costs (Ahmadi et al. 2011).
Aerated lagoons
Aerated lagoons consist of basins or ponds in which mechanical aeration is provided by devices, such as floating surface aerators and submerged diffusers. Aerated lagoons may be further classified into complete- and partial-mix lagoons. Complete-mix lagoons are aerated to maintain solids in suspension, which requires a high energy input. The aerators in partial-mix lagoons are designed to provide oxygen and do not provide sufficient turbulence to maintain solids in suspension (Alvarado et al. 2013). Consequently, sludge settling occurs and anaerobic zones form. Partial-mix lagoons have one-tenth the energy demand of complete-mix lagoons (U.S. EPA 2002). The advantages of aerated lagoons include lower operation and management costs than those of AS, lower sludge generation than other secondary treatment techniques, and lower land usage than those of stabilization ponds. The disadvantages of aerated lagoons are larger land usage compared to that of AS, lower nutrient removal compared to that of stabilization ponds, and energy input for aeration (U.S. EPA 2002).
Anaerobic digestion
As reviewed by van Lier et al. (2015), the development of continuous anaerobic reactors began with low-rate anaerobic reactors like the single-flow through tank designed by Karl Imhoff in 1905 and continuous stirred tank reactors (CSTR) that were prevalent until the 1960s. These low-rate reactors have similar HRTs and SRTs, and due to the low growth rate of bacteria, they require large volumes to provide sufficient biomass concentration (van Lier 2008).
To increase the biomass concentration and enhance treatment capacity, high-rate anaerobic reactors were developed, which decoupled SRT and HRT. The first high-rate anaerobic reactors to be developed were the anaerobic contact process (ACP), which has a secondary clarifier and recirculates sludge similar to AS, and the anaerobic filter (AF), which uses support materials for microbial growth (van Lier et al. 2015).
In the 1970s, Lettinga et al. (1976, 1980) developed the UASB reactor. The expanded granular sludge bed (EGSB) reactor was developed to improve the UASB reactor so that higher loadings can be applied. High-rate anaerobic sludge bed reactors, including the UASB reactor, EGSB reactor, and their derivatives, have been the most popular anaerobic treatment for industrial wastewater, holding approximately 90% of the market share due to their compactness, simple operation, and ability to operate at high volumetric loading rates and short HRTs (van Lier 2008). Sludge retention in these reactors is increased by the effective separability and settleability of biomass.
The advantages of anaerobic reactors include efficient COD removal at high organic loadings, biogas production, low energy demand and operational costs, a small footprint, lower sludge production than AS, and generation of biologically stabilized sludge. The disadvantages of anaerobic reactors include the difficulty of controlling sludge granulation, the dependence of granulation on the wastewater quality, the need for pretreatment to remove TSS, sensitivity to shock loads (Hansen and Cheong 2007), temperature sensitivity, and long startup times.
The key conditions for anaerobic systems that can treat high COD loads include high sludge concentrations and retention, sufficient contact of biomass and wastewater, a high reaction rate, effective transfer of metabolic products from biofilms, adaptation of biomass to the wastewater characteristics, and favorable conditions for microorganisms (van Lier et al. 2020).
Upflow anaerobic sludge blanket
In UASB reactors, wastewater is fed from the bottom of the reactor and flows upward through a granular sludge bed and blanket. The biogas produced from the anaerobic digestion of organics provides mixing and facilitates the formation and maintenance of sludge granules (Daud et al. 2018). The formation of sludge granules is essential for UASB reactors because these granules support biofilms and provide buoyancy and settleability, facilitating the contact of biomass with wastewater and enabling biomass retention (Abbasi and Abbasi 2012). A gas–liquid–solid separator (GLSS), usually in the shape of a funnel or triangular prism, is installed in the upper section of the reactor to separate sludge granules, biogas, and water. The separated biogas and treated wastewater are discharged from the top of the reactor. The GLSS acts as a clarifier by separating and preventing mixing of the sludge granules and the effluent. The sludge concentration is maintained by the settling of the separated sludge granules. Efforts have been made to improve the GLSS design so that the sludge retention increases in the UASB reactor. Dos Santos et al. (2016) installed parallel plates at a 45° angle above the conventional separator, doubling the treatment capacity.
Expanded granular sludge bed
The EGSB reactor is a variant of the UASB reactor with a slight bed expansion resulting from a high superficial velocity of 4–10 m/h, which is achieved by using a tall reactor or effluent recirculation (Tauseef et al. 2013). An EGSB reactor has a specifically designed GLSS to separate biogas, effluent, and sludge. Compared to UASB reactors, EGSB reactors provide better contact between the biofilm and wastewater and may use higher sludge concentrations. Other advantages of EGSB reactors include a small footprint, the ability to function at high organic and hydraulic loadings, and the ability to treat wastewater containing lipids and toxic compounds (Mao et al. 2015). These advantages have resulted in an increasing number of installments of full-scale EGSB reactors compared to the declining use of UASB reactors (van Lier et al. 2020).
Conclusions
A comprehensive overview has been provided of the characteristics of wastewater from various industries and wastewater treatment technologies, highlighting their principles, applications, advantages, and disadvantages. A wide variety of physical, biological, and chemical treatment methods exist to treat the diverse pollutants found in industrial wastewater. Each technology offers unique advantages in terms of efficiency, cost-effectiveness, selectivity, environmental compatibility, and resource recovery. Differences in wastewater characteristics, local site-specific factors, and treatment objectives make it necessary to implement wastewater treatment facilities tailored to these needs. A single technique often cannot adequately achieve the treatment objectives, motivating the combination of various techniques. However, only a few methods are used to treat industrial wastewater for technological and economic reasons (Crini and Lichtfouse 2019). Ongoing research and innovation in wastewater treatment continue to drive sustainable water usage. It is necessary to develop more advanced treatment methods to remove a wide range of pollutants in industrial wastewater to protect the environment, increase the quality of treated wastewater for recycling, reduce the resource intensity of wastewater treatment, and recover valuable resources. The complexity of wastewater management warrants the realization of technological advances and interdisciplinary collaboration to develop holistic approaches considering technological, economic, and social factors.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Abbasi T, Abbasi SA (2012) Formation and impact of granules in fostering clean energy production and wastewater treatment in upflow anaerobic sludge blanket (UASB) reactors. Renew Sustain Energy Rev 16:1696–1708. https://doi.org/10.1016/j.rser.2011.11.017
Abdel-Fatah MA (2023) Integrated Management of Industrial Wastewater in the Food Sector. Sustainability 15:16193. https://doi.org/10.3390/su152316193
Abdeljalil A, Nabil S, Rachid M (2022) Feasibility and sustainability of evaporation ponds as final basins for industrial wastewater: statistical evaluation of gross parameters. Desalin Water Treat 257:41–54. https://doi.org/10.5004/dwt.2022.28276
Abu Shmeis RM (2018) Water Chemistry and Microbiology. In: Comprehensive Analytical Chemistry. Elsevier, pp 1–56. https://doi.org/10.1016/bs.coac.2018.02.001
Abuhasel K, Kchaou M, Alquraish M et al (2021) Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 13:980. https://doi.org/10.3390/w13070980
Ahmad T, Belwal T, Li L et al (2020) Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci Technol 99:21–33. https://doi.org/10.1016/j.tifs.2020.02.017
Ahmadi M, Izanloo H, Mehr alian A et al (2011) Upgrading of Kish Island Markazi wastewater treatment plant by MBBR. J Water Reuse Desalin 1:243–249. https://doi.org/10.2166/wrd.2011.038
Ahmed J, Thakur A, Goyal A (2021a) Industrial Wastewater and Its Toxic Effects. In: Shah MP (ed) Biological Treatment of Industrial Wastewater, 1st edn. The Royal Society of Chemistry, London, pp 1–14
Ahmed SF, Mofijur M, Nuzhat S et al (2021b) Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J Hazard Mater 416:125912. https://doi.org/10.1016/j.jhazmat.2021.125912
Akpor O, Otohinoyi D, Olaolu T, Aderiye J (2014) Pollutants in wastewater effluents: impacts and remediation processes. Int J Environ Res Earth Sci 3:50–59
Alam R, Sheob M, Saeed B et al (2021) Use of Electrocoagulation for Treatment of Pharmaceutical Compounds in Water/Wastewater: A Review Exploring Opportunities and Challenges. Water 13:2105. https://doi.org/10.3390/w13152105
Al-Asheh S, Bagheri M, Aidan A (2021) Membrane bioreactor for wastewater treatment: A review. Case Stud Chem Environ Eng 4:100109. https://doi.org/10.1016/j.cscee.2021.100109
Albahnasawi A, Agir H, Cicerali MF et al (2023) Performance of aerobic sequential batch reactor in the treatment of textile wastewaters. Int J Environ Sci Technol 20:791–800. https://doi.org/10.1007/s13762-022-04014-0
Alemayehu YA, Asfaw SL, Tirfie TA (2020) Management options for coffee processing wastewater. A review. J Mater Cycles Waste Manag 22:454–469. https://doi.org/10.1007/s10163-019-00953-y
Ali MN, Fouad HA, Meky MM, Elhefny RM (2021) Pilot-scale study based on integrated fixed-film activated sludge process for cement industrial wastewater treatment. J Degrad Min Lands Manag 9:3073–3081. https://doi.org/10.15243/jdmlm.2021.091.3073
Al-Tohamy R, Ali SS, Li F et al (2022) A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf 231:113160. https://doi.org/10.1016/j.ecoenv.2021.113160
Alvarado A, Vesvikar M, Cisneros JF et al (2013) CFD study to determine the optimal configuration of aerators in a full-scale waste stabilization pond. Water Res 47:4528–4537. https://doi.org/10.1016/j.watres.2013.05.016
Amândio MST, Pereira JM, Rocha JMS et al (2022) Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy. Energies 15:4105. https://doi.org/10.3390/en15114105
Amaro-Soriano A, Hernández-Aldana F, Rivera A (2021) Photochemical treatments (UV/H2O2, UV/O3 and UV/H2O2/O3) and inverse osmosis in wastewater: Systematic review. World J Adv Res Rev 10:229–240. https://doi.org/10.30574/wjarr.2021.10.2.0231
Amoatey P, Izady A, Al-Maktoumi A et al (2021) A critical review of environmental and public health impacts from the activities of evaporation ponds. Sci Total Environ 796:149065. https://doi.org/10.1016/j.scitotenv.2021.149065
Anku WW, Mamo MA, Govender PP (2017) Phenolic compounds in water: sources, reactivity, toxicity and treatment methods. In: Soto-Hernandez M, Palma-Tenango M, del Rosario Garcia-Mateos M (eds) Phenolic compounds - natural sources, importance and applications. InTechOpen, London, pp 420–443, https://doi.org/10.5772/66927
Aprianti T, Miskah S, Selpiana et al (2018) Heavy metal ions adsorption from pulp and paper industry wastewater using zeolite/activated carbon-ceramic composite adsorbent. AIP Conf Proc 2014:020127. https://doi.org/10.1063/1.5054531
Aragaw TA, Bogale FM (2023) Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front Environ Sci 11:1142227. https://doi.org/10.3389/fenvs.2023.1142227
Arana Juve J-M, Christensen FMS, Wang Y, Wei Z (2022) Electrodialysis for metal removal and recovery: A review. Chem Eng J 435:134857. https://doi.org/10.1016/j.cej.2022.134857
Argyropoulos DDS, Crestini C, Dahlstrand C et al (2023) Kraft Lignin: A valuable, sustainable resource, opportunities and challenges. Chemsuschem 2023:e202300492. https://doi.org/10.1002/cssc.202300492
Asgharnejad H, Khorshidi Nazloo E, Madani Larijani M et al (2021) Comprehensive review of water management and wastewater treatment in food processing industries in the framework of water-food-environment nexus. Compr Rev Food Sci Food Saf 20:4779–4815. https://doi.org/10.1111/1541-4337.12782
Ashrafi O, Yerushalmi L, Haghighat F (2015) Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J Environ Manag 158:146–157. https://doi.org/10.1016/j.jenvman.2015.05.010
Asif MB, Khan Z (2016) Characterization and treatment of flour mills wastewater for reuse – a case study of Al-kausar Flour Mills, Pakistan. Desalin Water Treat 57:3881–3890. https://doi.org/10.1080/19443994.2014.990927
Atalay S, Ersöz G (2021) Hybrid application of advanced oxidation processes to dyes′ removal. In: Green Chemistry and Water Remediation: Research and Applications. Elsevier, pp 209–238. https://doi.org/10.1016/B978-0-12-817742-6.00007-4
Atasoy M, Eyice O, Cetecioglu Z (2020) A comprehensive study of volatile fatty acids production from batch reactor to anaerobic sequencing batch reactor by using cheese processing wastewater. Bioresour Technol 311:123529. https://doi.org/10.1016/j.biortech.2020.123529
Azanaw A, Birlie B, Teshome B, Jemberie M (2022) Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud Chem Environ Eng 6:100230. https://doi.org/10.1016/j.cscee.2022.100230
Azeem A, Ahmad S, Hanif A (2023) Wastewater utilization for concrete production: Prospects, challenges, and opportunities. J Build Eng 80:108078. https://doi.org/10.1016/j.jobe.2023.108078
Aziz A, Basheer F, Sengar A et al (2019) Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci Total Environ 686:681–708. https://doi.org/10.1016/j.scitotenv.2019.05.295
Badawi AK, Salama RS, Mostafa MMM (2023) Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: a review of recent developments. RSC Adv 13:19335–19355. https://doi.org/10.1039/d3ra01999c
Bajpai P (2018) Pulp Bleaching. Biermann’s Handb Pulp Pap 465–491. https://doi.org/10.1016/B978-0-12-814240-0.00019-7
Ballester-Sánchez J, Gil JV, Fernández-Espinar MT, Haros CM (2019) Quinoa wet-milling: Effect of steeping conditions on starch recovery and quality. Food Hydrocoll 89:837–843. https://doi.org/10.1016/j.foodhyd.2018.11.053
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Bauer S, Linke HJ, Wagner M (2020) Combining industrial and urban water-reuse concepts for increasing the water resources in water-scarce regions. Water Environ Res 92:1027–1041. https://doi.org/10.1002/wer.1298
Bautista P, Mohedano AF, Casas JA et al (2008) An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol 83:1323–1338. https://doi.org/10.1002/jctb.1988
Behroozi AH, Ataabadi MR (2021) Improvement in microfiltration process of oily wastewater: A comprehensive review over two decades. J Environ Chem Eng 9:104981. https://doi.org/10.1016/j.jece.2020.104981
Bello MM, Abdul Raman AA, Asghar A (2019) A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf Environ Prot 126:119–140. https://doi.org/10.1016/j.psep.2019.03.028
Bhatia D, Sharma NR, Singh J, Kanwar RS (2017) Biological methods for textile dye removal from wastewater: A review. Crit Rev Environ Sci Technol 47:1836–1876. https://doi.org/10.1080/10643389.2017.1393263
Bilińska L, Gmurek M, Ledakowicz S (2016) Comparison between industrial and simulated textile wastewater treatment by AOPs – Biodegradability, toxicity and cost assessment. Chem Eng J 306:550–559. https://doi.org/10.1016/j.cej.2016.07.100
Boinpally S, Kolla A, Kainthola J et al (2023) A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 4:26–36. https://doi.org/10.1016/j.watcyc.2023.01.001
Bortoluzzi AC, Faitão JA, Di Luccio M et al (2017) Dairy wastewater treatment using integrated membrane systems. J Environ Chem Eng 5:4819–4827. https://doi.org/10.1016/j.jece.2017.09.018
Bratby J (2016) Coagulation and Flocculation in Water and Wastewater Treatment. Water Intell Online 15:9781780407500–9781780407500. https://doi.org/10.2166/9781780407500
Breite D, Went M, Prager A et al (2019) Charge Separating Microfiltration Membrane with pH-Dependent Selectivity. Polymers (Basel) 11. https://doi.org/10.3390/POLYM11010003
Bustillo-Lecompte CF (2020) Advanced Oxidation Processes - Applications, Trends, and Prospects. IntechOpen, London. https://doi.org/10.5772/intechopen.85681
Bustillo-Lecompte CF, Mehrvar M (2015) Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: a review on trends and advances. J Environ Manag 161:287–302. https://doi.org/10.1016/j.jenvman.2015.07.008
Buyukkamaci N, Koken E (2010) Economic evaluation of alternative wastewater treatment plant options for pulp and paper industry. Sci Total Environ 408:6070–6078. https://doi.org/10.1016/j.scitotenv.2010.08.045
Cakir FY, Stenstrom MK (2005) Greenhouse gas production: A comparison between aerobic and anaerobic wastewater treatment technology. Water Res 39:4197–4203. https://doi.org/10.1016/j.watres.2005.07.042
Carolin CF, Kumar PS, Saravanan A et al (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5:2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chai WS, Cheun JY, Kumar PS et al (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589. https://doi.org/10.1016/j.jclepro.2021.126589
Chan YJ, Chong MF, Law CL, Hassell DG (2009) A review on anaerobic-aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18. https://doi.org/10.1016/j.cej.2009.06.041
Chang SH (2020) Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—a review. Environ Sci Pollut Res 27:32371–32388. https://doi.org/10.1007/s11356-020-09639-7
Chatel G, Valange S, Behling R, Colmenares JC (2017) A Combined Approach using Sonochemistry and Photocatalysis: How to Apply Sonophotocatalysis for Biomass Conversion? ChemCatChem 9:2615–2621. https://doi.org/10.1002/cctc.201700297
Chen W, Hong J, Xu C (2015) Pollutants generated by cement production in China, their impacts, and the potential for environmental improvement. J Clean Prod 103:61–69. https://doi.org/10.1016/j.jclepro.2014.04.048
Chen YG, Sofińska-Chmiel W, Lv GY et al (2021) Application of modern research methods for the physicochemical characterization of ion exchangers. Materials (basel) 14:7067. https://doi.org/10.3390/ma14227067
Chen JP, Yang L, Bai R (2005) Bakery waste treatment. In: Waldron K (ed) Handbook of waste management and Co-product recovery in food processing. Elsevier, pp 611–648. https://doi.org/10.1533/9781845692520.5.611
Cheremisinoff NP, Rosenfeld PE (2010) Sources of air emissions from pulp and paper mills. In: Handbook of Pollution Prevention and Cleaner Production. Elsevier, pp 179–259. https://doi.org/10.1016/B978-0-08-096446-1.10006-1
Chiang YP, Liang YY, Chang CN, Chao AC (2006) Differentiating ozone direct and indirect reactions on decomposition of humic substances. Chemosphere 65:2395–2400. https://doi.org/10.1016/J.CHEMOSPHERE.2006.04.080
Choudhury AR, Singh N, Veeraraghavan A et al (2023) Ascertaining and Optimizing the Water Footprint and Sludge Management Practice in Steel Industries. Water 15:2177. https://doi.org/10.3390/w15122177
Christoulas D (1998) An empirical model for primary sedimentation of sewage. Environ Int 24:925–934. https://doi.org/10.1016/S0160-4120(98)00076-2
Chu L, Wang J, Dong J et al (2012) Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 86:409–414. https://doi.org/10.1016/j.chemosphere.2011.09.007
Colla V, Branca TA, Rosito F et al (2016) Sustainable Reverse Osmosis application for wastewater treatment in the steel industry. J Clean Prod 130:103–115. https://doi.org/10.1016/j.jclepro.2015.09.025
Colla V, Matino I, Branca T et al (2017) Efficient Use of Water Resources in the Steel Industry. Water 9:874. https://doi.org/10.3390/w9110874
Correia VM, Stephenson T, Judd SJ (1994) Characterisation of textile wastewaters - a review. Environ Technol 15:917–929. https://doi.org/10.1080/09593339409385500
Cortez S, Teixeira P, Oliveira R, Mota M (2008) Rotating biological contactors: A review on main factors affecting performance. Rev Environ Sci Biotechnol 7:155–172. https://doi.org/10.1007/s11157-008-9127-x
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Dąbrowski A (2001) Adsorption — from theory to practice. Adv Colloid Interface Sci 93:135–224. https://doi.org/10.1016/S0001-8686(00)00082-8
Dadi D, Mengistie E, Terefe G et al (2018) Assessment of the effluent quality of wet coffee processing wastewater and its influence on downstream water quality. Ecohydrol Hydrobiol 18:201–211. https://doi.org/10.1016/j.ecohyd.2017.10.007
Dahman Y (2017) Nanopolymers. In: Nanotechnology and Functional Materials for Engineers. Elsevier, pp 121–144. https://doi.org/10.1016/B978-0-323-51256-5.00006-X
Daigger GT, Boltz JP (2011) Trickling Filter and Trickling Filter-Suspended Growth Process Design and Operation: A State-of-the-Art Review. Water Environ Res 83:388–404. https://doi.org/10.2175/106143010X12681059117210
Dao VH, Cameron NR, Saito K (2016) Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym Chem 7:11–25. https://doi.org/10.1039/c5py01572c
de Dardel F, Arden TV (2008) Ion Exchangers. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley. https://doi.org/10.1002/14356007.a14_393.pub2
Das P, Mondal GC, Singh S et al (2018) Effluent Treatment Technologies in the Iron and Steel Industry - A State of the Art Review. Water Environ Res 90:395–408. https://doi.org/10.2175/106143017X15131012152951
Das PP, Sharma M, Purkait MK (2022) Recent progress on electrocoagulation process for wastewater treatment: A review. Sep Purif Technol 292:121058. https://doi.org/10.1016/j.seppur.2022.121058
Daud MK, Rizvi H, Akram MF et al (2018) Review of upflow anaerobic sludge blanket reactor technology: Effect of different parameters and developments for domestic wastewater treatment. J Chem 2018. https://doi.org/10.1155/2018/1596319
Dauthy ME (1995) Fruit and Vegetable Processing. FAO, Rome
de Almeida BA, Almeida M, Martins S et al (2016) Recycling liquid effluents in a ceramic industry. Boletín La Soc Española Cerámica y Vidr 55:95–104. https://doi.org/10.1016/j.bsecv.2016.04.004
de Almeida RA, de Rezende RVP, Mataczinski AK et al (2020) Three-dimensional simulation of a secondary circular settling tank: flow pattern and sedimentation process. Braz J Chem Eng 37:333–350. https://doi.org/10.1007/s43153-020-00030-0
de Santana MM, Zanoelo EF, Benincá C, Freire FB (2018) Electrochemical treatment of wastewater from a bakery industry: Experimental and modeling study. Process Saf Environ Prot 116:685–692. https://doi.org/10.1016/j.psep.2018.04.001
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
De Paula HM, De Oliveira Ilha MS, Andrade LS (2014) Concrete plant wastewater treatment process by coagulation combining aluminum sulfate and Moringa oleifera powder. J Clean Prod 76:125–130. https://doi.org/10.1016/j.jclepro.2014.04.031
Delcour JA, Bruneel C, Derde LJ et al (2010) Fate of Starch in Food Processing: From Raw Materials to Final Food Products. Annu Rev Food Sci Technol 1:87–111. https://doi.org/10.1146/annurev.food.102308.124211
Deluise F, Wang LK, Chang S-Y, Hung Y-T (2005) Screening and Comminution. In: Wang LK, Hung Y-T, Shammas NK (eds) Physicochemical Treatment Processes. Humana Press, Totowa, pp 1–19
Devi P, Das U, Dalai AK (2016) In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci Total Environ 571:643–657. https://doi.org/10.1016/j.scitotenv.2016.07.032
Dhokpande SR, Kulkarni SJ, Kaware JP (2014) A review on research on application of trickling filters in removal of various pollutants from effluent. Int J Eng Sci Res Technol 3:359–365
Dincer I, Zamfirescu C (2014) Fossil Fuels and Alternatives. In: Advanced Power Generation Systems, Elsevier, pp 95–141. https://doi.org/10.1016/B978-0-12-383860-5.00003-1
Dinçer AR, Kargı F (2000) Characterization and biological treatment of ceramic industry wastewater. Bioprocess Eng 23:0209–0212. https://doi.org/10.1007/s004490000207
Diya’uddeen BH, Daud WMAW, Abdul Aziz AR (2011) Treatment technologies for petroleum refinery effluents: A review. Process Saf Environ Prot 89:95–105. https://doi.org/10.1016/j.psep.2010.11.003
Dkhissi O, El Hakmaoui A, Souabi S et al (2018) Treatment of vegetable oil refinery wastewater by coagulation-flocculation process using the cactus as a bio-flocculant. J Mater Environ Sci 9:18–25. https://doi.org/10.26872/jmes.2018.9.1.3
Do TX, Lim Y, Lee J, Lee W (2016) Techno-economic analysis of petrochemical complex retrofitted with simulated moving-bed for olefins and aromatics production. Chem Eng Res Des 106:222–241. https://doi.org/10.1016/j.cherd.2015.12.020
Doltade SB, Yadav YJ, Jadhav NL (2022) Industrial wastewater treatment using oxidative integrated approach. S Afr J Chem Eng 40:100–106. https://doi.org/10.1016/j.sajce.2022.02.004
Donkadokula NY, Kola AK, Naz I, Saroj D (2020) A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Rev Environ Sci Biotechnol 19:543–560. https://doi.org/10.1016/j.jece.2017.05.029
Dos Santos SL, Chaves SRM, van Haandel A (2016) Influence of phase separator design on the performance of UASB reactors treating municipal wastewater. Water SA 42:176–182. https://doi.org/10.4314/wsa.v42i2.01
Ekolu SO, Dawneerangen A (2010) Evaluation of recycled water recovered from a ready-mix concrete plant for reuse in concrete. J S Afr Inst Civ Eng 52:77–82
Emamjomeh MM, Sivakumar M (2009) Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J Environ Manag 90:1663–1679. https://doi.org/10.1016/j.jenvman.2008.12.011
Ersoy B, Tosun I, Günay A, Dikmen S (2009) Turbidity Removal from Wastewaters of Natural Stone Processing by Coagulation/Flocculation Methods. CLEAN Soil Air Water 37:225–232. https://doi.org/10.1002/clen.200800209
Eskelinen K, Särkkä H, Kurniawan TA, Sillanpää MET (2010) Removal of recalcitrant contaminants from bleaching effluents in pulp and paper mills using ultrasonic irradiation and Fenton-like oxidation, electrochemical treatment, and/or chemical precipitation: A comparative study. Desalination 255:179–187. https://doi.org/10.1016/j.desal.2009.12.024
Esmaeeli A, Sarrafzadeh MH, Zeighami S et al (2023) A Comprehensive Review on Pulp and Paper Industries Wastewater Treatment Advances. Ind Eng Chem Res 62:8119–8145. https://doi.org/10.1021/ACS.IECR.2C04393/ASSET/IMAGES/LARGE/IE2C04393_0014.JPEG
Estay H, Barros L, Troncoso E (2021) Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals 11:1385. https://doi.org/10.3390/min11121385
Eurostat (2023) Annual detailed enterprise statistics for industry (NACE Rev. 2, B-E). In: Eurostat. https://ec.europa.eu/eurostat/databrowser/view/sbs_na_ind_r2__custom_9380628/bookmark/table?lang=en&bookmarkId=b8cae444-7e6c-4faf-a562-233d91c7e9e1. Accessed 1 Aug 2024
Ezugbe EO, Rathilal S (2020) Membrane technologies in wastewater treatment: A review. Membranes (Basel) 10. https://doi.org/10.3390/membranes10050089
Fakhru’l-Razi A, Pendashteh A, Abdullah LC et al (2009) Review of technologies for oil and gas produced water treatment. J Hazard Mater 170:530–551. https://doi.org/10.1016/j.jhazmat.2009.05.044
Falletti L, Conte L, Maestri A (2014) Upgrading of a wastewater treatment plant with a hybrid moving bed biofilm reactor (MBBR). AIMS Environ Sci 1:45–52. https://doi.org/10.3934/environsci.2014.2.45
FAO (2024) FAOSTAT. In: Food Agric. Organ. United Nations. https://www.fao.org/faostat. Accessed 6 Feb 2024
Feng Y, Song H, Xiao M et al (2017) Development of Phenols Recovery process from coal gasification wastewater with mesityl oxide as a novel extractant. J Clean Prod 166:1314–1322. https://doi.org/10.1016/j.jclepro.2017.08.119
Feng N, Wang G, Kang X et al (2022a) Treatment of organic pollutants in coke plant wastewater by micro-nanometer catalytic ozonation, A/A/O and reverse osmosis membrane. Water Sci Technol 86:1629–1641. https://doi.org/10.2166/wst.2022.292
Feng X, Long R, Wang L et al (2022b) A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep Purif Technol 284:120099. https://doi.org/10.1016/j.seppur.2021.120099
Ferdowsi A, Valikhan-Anaraki M, Farzin S, Mousavi S-F (2022) A new combination approach for optimal design of sedimentation tanks based on hydrodynamic simulation model and machine learning algorithms. Phys Chem Earth Parts A/B/C 127:103201. https://doi.org/10.1016/j.pce.2022.103201
Fink JK (2013) Phenol/Formaldehyde Resins. React Polym Fundam Appl 155–177. https://doi.org/10.1016/B978-1-4557-3149-7.00004-8
Finnegan W, Yan M, Holden NM, Goggins J (2018) A review of environmental life cycle assessment studies examining cheese production. Int J Life Cycle Assess 23:1773–1787. https://doi.org/10.1007/s11367-017-1407-7
Fito J, Tefera N, Van Hulle SWH (2019) Sugarcane biorefineries wastewater: bioremediation technologies for environmental sustainability. Chem Biol Technol Agric 6:1–13. https://doi.org/10.1186/s40538-019-0144-5
Fornarelli R, Bahri PA, Moheimani N (2017) Utilization of microalgae to purify waste streams and production of value added products. AMPC. https://www.ampc.com.au/getmedia/45586025-7b89-4f92-8e36-36f4679dfcd7/AMPC_UtilizationOfMicroAlgaeToPurifyWasteStreams_FinalReport.pdf?ext=.pdf. Accessed 17 Jan 2024
Freeda Gnana Rani D, Arunkumar K, Sivakumar SR (2005) Physico-chemical analysis of waste water from cement units. J Ind Pollut Control 21:337–340
Gadipelly C, Pérez-González A, Yadav GD et al (2014) Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind Eng Chem Res 53:11571–11592. https://doi.org/10.1021/ie501210j
Gao H, Stenstrom MK (2018) Evaluation of three turbulence models in predicting the steady state hydrodynamics of a secondary sedimentation tank. Water Res 143:445–456. https://doi.org/10.1016/j.watres.2018.06.067
Garcia-Segura S, Ocon JD, Chong MN (2018) Electrochemical oxidation remediation of real wastewater effluents — A review. Process Saf Environ Prot 113:48–67. https://doi.org/10.1016/j.psep.2017.09.014
Garg R, Singh SK (2022) Treatment technologies for sustainable management of wastewater from iron and steel industry — a review. Environ Sci Pollut Res 29:75203–75222. https://doi.org/10.1007/s11356-022-23051-3
Ghangrekar MM, Behera M (2013) 3.5 Suspended Growth Treatment Processes. In: Comprehensive Water Quality and Purification. Elsevier, pp 74–89. https://doi.org/10.1016/B978-0-12-382182-9.00087-6
Ghimire N, Wang S (2019) Biological Treatment of Petrochemical Wastewater. In: Petroleum Chemicals - Recent Insight. IntechOpen. https://doi.org/10.5772/intechopen.79655
Gogate PR, Patil PN (2016) Sonochemical Reactors. Top Curr Chem 374:1–27. https://doi.org/10.1007/S41061-016-0064-9
Gomaa HE, Alotaibi AA, Gomaa FA et al (2021) Integrated ion exchange-based system for nitrate and sulfate removal from water of different matrices: Analysis and optimization using response surface methodology and Taguchi experimental design techniques. Process Saf Environ Prot 153:500–517. https://doi.org/10.1016/J.PSEP.2021.07.045
Gurreri L, Tamburini A, Cipollina A, Micale G (2020) Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives. Membranes (basel) 10:1–93. https://doi.org/10.3390/membranes10070146
Gzar HA, Al-Rekabi WS, Shuhaieb ZK (2021) Applicaion of Moving Bed Biofilm Reactor (MBBR) for Treatment of Industrial Wastewater: A mini Review. J Phys Conf Ser 1973. https://doi.org/10.1088/1742-6596/1973/1/012024
Häder D-P (2018) Ecotoxicological monitoring of wastewater. In: Häder D-P, Erzinger GS (eds) Bioassays. Elsevier, pp 369–386. https://doi.org/10.1016/B978-0-12-811861-0.00018-8
Hall GM (1997) Fish Processing Technology. Springer US, Boston. https://doi.org/10.1007/978-1-4613-1113-3
Hansen CL, Cheong DY (2007) Fermentation, biogas and biohydrogen production from solid food processing. In: Waldron K (ed) Handbook of waste management and co-product recovery in food processing. Elsevier, pp 611–648. https://doi.org/10.1533/9781845692520.5.611
Hao X, Chen Q, van Loosdrecht MCM et al (2020) Sustainable disposal of excess sludge: Incineration without anaerobic digestion. Water Res 170. https://doi.org/10.1016/j.watres.2019.115298
Hermosilla D, Merayo N, Gascó A, Blanco Á (2014) The application of advanced oxidation technologies to the treatment of effluents from the pulp and paper industry: A review. Environ Sci Pollut Res 22:168–191. https://doi.org/10.1007/s11356-014-3516-1
Hirom K, Devi TT (2022) Application of Computational Fluid Dynamics in Sedimentation Tank Design and Its Recent Developments: a Review. Water Air Soil Pollut 233:22. https://doi.org/10.1007/s11270-021-05458-9
Hitzmann B, Sievert D, Hoseney RC, Delcour JA (2022) Bread and Other Baked Products. In: Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley & Sons, Ltd, pp 1–58. https://doi.org/10.1002/14356007.a04_331.pub3
Holkar CR, Jadhav AJ, Pinjari DV et al (2016) A critical review on textile wastewater treatments: Possible approaches. J Environ Manag 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Huang XF, Ling J, Xu JC et al (2011) Advanced treatment of wastewater from an iron and steel enterprise by a constructed wetland/ultrafiltration/reverse osmosis process. Desalination 269:41–49. https://doi.org/10.1016/j.desal.2010.10.040
Ijanu EM, Kamaruddin MA, Norashiddin FA (2020) Coffee processing wastewater treatment: a critical review on current treatment technologies with a proposed alternative. Appl Water Sci 10:11. https://doi.org/10.1007/s13201-019-1091-9
Ipeaiyeda AR, Obaje GM (2017) Impact of cement effluent on water quality of rivers: A case study of Onyi river at Obajana, Nigeria. Cogent Environ Sci 3. https://doi.org/10.1080/23311843.2017.1319102
Islam MD, Mahdi MM (2022) Evaluation of micro-pollutants removal from industrial wastewater using conventional and advanced biological treatment processes. In: Biodegradation and Detoxification of Micropollutants in Industrial Wastewater. Elsevier, pp 1–26. https://doi.org/10.1016/B978-0-323-88507-2.00011-7
Itoh T, Kojima Y (2019) Synergistic effects of ultrasound and ultraviolet light irradiation on oxidation reaction using photocatalyst. J Chem Eng Japan 52:877–881. https://doi.org/10.1252/JCEJ.18WE077
Iwuozor KO (2019) Prospects and Challenges of Using Coagulation-Flocculation method in the treatment of Effluents. Adv J Chem A 2:105–127. https://doi.org/10.29088/SAMI/AJCA.2019.2.105127
Izady A, Nikoo MR, Bakhtiari PH et al (2020) Risk-based Stochastic Optimization of Evaporation Ponds as a Cost-Effective and Environmentally-Friendly Solution for the Disposal of Oil-Produced Water. J Water Process Eng 38:101607. https://doi.org/10.1016/j.jwpe.2020.101607
Jagaba AH, Kutty SRM, Baloo L et al (2022) Combined treatment of domestic and pulp and paper industry wastewater in a rice straw embedded activated sludge bioreactor to achieve sustainable development goals. Case Stud Chem Environ Eng 6:100261. https://doi.org/10.1016/j.cscee.2022.100261
Jamaly S, Darwish NN, Ahmed I, Hasan SW (2014) A short review on reverse osmosis pretreatment technologies. Desalination 354:30–38. https://doi.org/10.1016/j.desal.2014.09.017
Jones ER, van Vliet MTH, Qadir M, Bierkens MFP (2021) Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst Sci Data 13:237–254. https://doi.org/10.5194/essd-13-237-2021
Jover-Smet M, Martín-Pascual J, Trapote A (2017) Model of Suspended Solids Removal in the Primary Sedimentation Tanks for the Treatment of Urban Wastewater. Water 9:448. https://doi.org/10.3390/w9060448
Judd S (2008) The status of membrane bioreactor technology. Trends Biotechnol 26:109–116. https://doi.org/10.1016/j.tibtech.2007.11.005
Kajla S, Nagi GK, Kumari R (2021) Microorganisms employed in the removal of contaminants from wastewater of iron and steel industries. Rend Lincei Sci Fis e Nat 32:257–272. https://doi.org/10.1007/s12210-021-00982-6
Kakavandi B, Bahari N, Rezaei Kalantary R, Dehghani Fard E (2019) Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason Sonochem 55:75–85. https://doi.org/10.1016/j.ultsonch.2019.02.026
Kalla S (2021) Use of membrane distillation for oily wastewater treatment - A review. J Environ Chem Eng 9:104641. https://doi.org/10.1016/j.jece.2020.104641
Kant R (2012) Textile dyeing industry an environmental hazard. Nat Sci 04:22–26. https://doi.org/10.4236/ns.2012.41004
Kapatel DV, Rotliwala YC, Patel HJ (2022) Co-pyrolysis based activated Bio-char: Characterization and its utilization for secondary treated pulp and paper industry wastewater. Mater Today Proc 57:1724–1729. https://doi.org/10.1016/J.MATPR.2021.12.361
Kato S, Sakai Y, Sato Y, Kansha Y (2023) Enhancement of Wastewater Treatment Using Mist and Photocatalyst. Chem Eng Technol 46:1185–1190. https://doi.org/10.1002/ceat.202200524
Kaur N (2021) Different treatment techniques of dairy wastewater. Groundw Sustain Dev 14:100640. https://doi.org/10.1016/j.gsd.2021.100640
Kaur B, Garg RK, Singh AP (2020) Treatment of Wastewater from Pulp and Paper Mill using Coagulation and Flocculation. J Environ Treat Tech 9:158–163. https://doi.org/10.47277/JETT/9(1)163
Kearney J (2010) Food consumption trends and drivers. Philos Trans R Soc B Biol Sci 365:2793–2807. https://doi.org/10.1098/rstb.2010.0149
Keerthana S, Sekar S, Kumar SD et al (2020) Scenedesmus pecsensis cultivation in rice mill effluent using commercial scale nutrient sources. Bioresour Technol Reports 9:100379. https://doi.org/10.1016/j.biteb.2019.100379
Kelessidis A, Stasinakis AS (2012) Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag 32:1186–1195. https://doi.org/10.1016/j.wasman.2012.01.012
Keskin B, Ersahin ME, Ozgun H, Koyuncu I (2021) Pilot and full-scale applications of membrane processes for textile wastewater treatment: A critical review. J Water Process Eng 42:102172. https://doi.org/10.1016/j.jwpe.2021.102172
Khakimova N, Maravić N, Davidović P et al (2022) Sugar Beet Processing Wastewater Treatment by Microalgae through Biosorption. Water 14:860. https://doi.org/10.3390/w14060860
Khan AA, Gul J, Naqvi SR et al (2022) Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 306:135565. https://doi.org/10.1016/j.chemosphere.2022.135565
Khan MD, Singh A, Khan MZ et al (2023) Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J Water Process Eng 53:103579. https://doi.org/10.1016/j.jwpe.2023.103579
Ksibi M (2006) Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem Eng J 119:161–165. https://doi.org/10.1016/j.cej.2006.03.022
Kumar MS, Gopinath A, Ranjith N et al (2023) Treatment of Integrated Steel Plant Wastewater Using Conventional and Advanced Techniques. CLEAN Soil Air Water 51:1–19. https://doi.org/10.1002/clen.202100169
Kwarciak-Kozłowska A, Włodarczyk R (2020) Efficiency assessment of coke industry wastewater treatment during advanced oxidation process with biochar adsorption. Desalin Water Treat 199:441–450. https://doi.org/10.5004/dwt.2020.26335
Lawal J, Anaun TE (2022) An Overview of Characterization and Treatment Methods of Wastewater from Iron and Steel Industries. Achievers J Sci Res 4:152–163
Lettinga G, van der Ben J, van der Sar J (1976) Anaërobe Zuivering Van Het Afvalwater Van De Bietsuikerindustrie. H2O 9:38–43
Lettinga G, van Velsen AFM, Hobma SW et al (1980) Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol Bioeng 22:699–734. https://doi.org/10.1002/bit.260220402
Lewis AEE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234. https://doi.org/10.1016/j.hydromet.2010.06.010
Li W, Mu B, Yang Y (2019) Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour Technol 277:157–170. https://doi.org/10.1016/j.biortech.2019.01.002
Liang L, Han D, Ma R, Peng T (2013) Treatment of high-concentration wastewater using double-effect mechanical vapor recompression. Desalination 314:139–146. https://doi.org/10.1016/j.desal.2013.01.016
Liang S, Du Y, Liu Q et al (2023) Wastewater reuse and recycling of the steel industry in China: history, current situation, and future perspectives. Water Reuse 13:162–179. https://doi.org/10.2166/wrd.2023.072
Libutti A, Gatta G, Gagliardi A et al (2018) Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric Water Manag 196:1–14. https://doi.org/10.1016/j.agwat.2017.10.015
Lim T-T, Goei R (2016) CHAPTER 5. Combined Photocatalysis–Separation Processes for Water Treatment Using Hybrid Photocatalytic Membrane Reactors. In: RSC Energy and Environment Series. The Royal Society of Chemistry, pp 130–156. https://doi.org/10.1039/9781782627104-00130
Lin P-H, Su Y-C, Chen C-L, Tsao I-Y (2023) Characterization of fouled ultrafiltration membranes from a full-scale wastewater reclamation plant in iron and steel industry. Int J Environ Sci Technol 20:11501–11512. https://doi.org/10.1007/s13762-022-04751-2
Liu S, Ma Q, Wang B et al (2014) Advanced treatment of refractory organic pollutants in petrochemical industrial wastewater by bioactive enhanced ponds and wetland system. Ecotoxicology 23:689–698. https://doi.org/10.1007/s10646-014-1215-9
Lo TC, Baird MHI (2003) Solvent extraction. In: Meyers RA (ed) Encyclopedia of physical science and technology, 3rd edn. Elsevier, pp 341–362. https://doi.org/10.1016/B0-12-227410-5/00713-4
Loeb SK, Alvarez PJJ, Brame JA et al (2019) The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ Sci Technol 53:2937–2947. https://doi.org/10.1021/acs.est.8b05041
Loubes MA, Barrera GN, Tolaba MP (2016) High-impact wet-milling: Effects of steeping conditions on rice starch attributes. Starch/staerke 68:1095–1102. https://doi.org/10.1002/star.201600092
Lu H, Wang J, Wang T et al (2017) Crystallization techniques in wastewater treatment: An overview of applications. Chemosphere 173:474–484. https://doi.org/10.1016/j.chemosphere.2017.01.070
Ma Q, Qu Y, Shen W et al (2015) Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour Technol 179:436–443. https://doi.org/10.1016/j.biortech.2014.12.041
Maćczak P, Kaczmarek H, Ziegler-Borowska M (2020) Recent achievements in polymer bio-based flocculants for water treatment. Materials (Basel) 13. https://doi.org/10.3390/ma13183951
Machado F, Teixeira ACSC, Ruotolo LAM (2023) Critical review of Fenton and photo-Fenton wastewater treatment processes over the last two decades. Int J Environ Sci Technol 20:13995–14032. https://doi.org/10.1007/s13762-023-05015-3
Manago BL, De Sousa M, Vidal C, Beber De Souza J et al (2018) Dissolved Air Flotation for Fiber Removal from Clear Water. Floresta e Ambient 25:20160124. https://doi.org/10.1590/2179-8087.012416
Manchisi J, Matinde E, Rowson NA et al (2020) Ironmaking and Steelmaking Slags as Sustainable Adsorbents for Industrial Effluents and Wastewater Treatment: A Critical Review of Properties, Performance, Challenges and Opportunities. Sustainability 12:2118. https://doi.org/10.3390/su12052118
Manzocco L, Ignat A, Anese M et al (2015) Efficient management of the water resource in the fresh-cut industry: Current status and perspectives. Trends Food Sci Technol 46:286–294. https://doi.org/10.1016/j.tifs.2015.09.003
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Mark J, Strange R (1993) The Food Industries, 1st edn. Chapman and Hall/CRC, New York. https://doi.org/10.1201/9781003059851
Marsolek MD, Torres CI, Hausner M, Rittmann BE (2008) Intimate coupling of photocatalysis and biodegradation in a photocatalytic circulating-bed biofilm reactor. Biotechnol Bioeng 101:83–92. https://doi.org/10.1002/bit.21889
Martin I, Pidou M, Soares A et al (2011) Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ Technol 32:921–932. https://doi.org/10.1080/09593330.2011.565806
Martínez R, Ruiz MO, Ramos C et al (2020) Comparison of external and submerged membranes used in anaerobic membrane bioreactors: Fouling related issues and biological activity. Biochem Eng J 159:107558. https://doi.org/10.1016/j.bej.2020.107558
Martínez-González G, Loría-Molina H, Taboada-López D et al (2009) Approximate Method for Designing a Primary Settling Tank for Wastewater Treatment. Ind Eng Chem Res 48:7842–7846. https://doi.org/10.1021/ie801869b
Matsumoto M, Wada Y, Matsumiya S, Onoe K (2021) Enhanced generation of active oxygen species induced by O3 fine bubble injection under H2O2 addition and UV irradiation. Ozone Sci Eng 43:562–570. https://doi.org/10.1080/01919512.2021.1873735
McLeary KS (2004) Wastewater treatment processes and water reuse. Water Encyclopedia. John Wiley & Sons, Ltd, pp 814–819. https://doi.org/10.1002/047147844x.ww321
Melin T, Jefferson B, Bixio D et al (2006) Membrane bioreactor technology for wastewater treatment and reuse. Desalination 187:271–282. https://doi.org/10.1016/j.desal.2005.04.086
Meme FK, Nwadukwe FO (2016) Impact assessment of cement factory waste water on the heavy metal contents of a typical low-latitude stream in North Central Nigeria. Chem Mater Res 8:1–7
Mierzwiński D, Nosal P, Szczepanik A et al (2021) Concept of Flocks Fragmentation and Averaging Method for the Application of Electrocoagulation in Process for Coke Oven Wastewater Treatment. Materials (Basel) 14:6307. https://doi.org/10.3390/ma14216307
Mir N, Bicer Y (2021) Integration of electrodialysis with renewable energy sources for sustainable freshwater production: A review. J Environ Manag 289:112496. https://doi.org/10.1016/j.jenvman.2021.112496
Miranda R, Negro C, Blanco A (2009) Internal treatment of process waters in paper production by dissolved air flotation with newly developed chemicals. 1. Laboratory tests. Ind Eng Chem Res 48:2199–2205. https://doi.org/10.1021/IE801047H/ASSET/IMAGES/LARGE/IE-2008-01047H_0008.JPEG
Mizuno H, Kansha Y, Kishimoto A, Tsutsumi A (2013) Thermal seawater desalination based on self-heat recuperation. Clean Technol Environ Policy 15:765–769. https://doi.org/10.1007/s10098-012-0539-5
Mizuno H, Kansha Y, Ishizuka M, Tsutsumi A (2015) Agglomeration behavior in fluidized-bed evaporator for thermal seawater desalination. Appl Therm Eng 89:1096–1103. https://doi.org/10.1016/j.applthermaleng.2015.06.011
Mizyed AG (2021) Review on application of rotating biological contactor in removal of various pollutants from effluent. Tech Biochem 2:41–61
Moghaddam A, Khayatan D, Esmaeili Fard Barzegar P et al (2023) Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: a comprehensive overview. Int J Environ Sci Technol 20:5659–5696. https://doi.org/10.1007/s13762-023-04880-2
Mohammadi R, Tang W, Sillanpää M (2021) A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 498:114626. https://doi.org/10.1016/j.desal.2020.114626
Mousset E, Doudrick K (2020) A review of electrochemical reduction processes to treat oxidized contaminants in water. Curr Opin Electrochem 22:221–227. https://doi.org/10.1016/j.coelec.2020.07.008
Mulyanti R, Susanto H (2018) Wastewater treatment by nanofiltration membranes. IOP Conf Ser Earth Environ Sci 142:012017. https://doi.org/10.1088/1755-1315/142/1/012017
Murthy PS, Madhava Naidu M (2012) Sustainable management of coffee industry by-products and value addition—A review. Resour Conserv Recycl 66:45–58. https://doi.org/10.1016/j.resconrec.2012.06.005
Musa MA, Idrus S (2021) Physical and biological treatment technologies of slaughterhouse wastewater: a review. Sustainability 13:4656. https://doi.org/10.3390/su13094656
Mustroph H (2014) Dyes, General Survey. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–38
Mwaka SN (2017) Performance evaluation of constructed wetlands and conventional wastewater treatment systems in selected Kenyan tea factories. Int J Sci Technoledge 5:7–13
Naidoo S, Olaniran AO (2013) Treated wastewater effluent as a source of microbial pollution of surface water resources. Int J Environ Res Public Health 11:249–270. https://doi.org/10.3390/ijerph110100249
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol C Photochem Rev 13:169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Nguyen TC, Loganathan P, Nguyen TV et al (2018) Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ Sci Pollut Res 25:20430–20438. https://doi.org/10.1007/s11356-017-9610-4
Nguyen MD, Thomas M, Surapaneni A et al (2022) Beneficial reuse of water treatment sludge in the context of circular economy. Environ Technol Innov 28:102651. https://doi.org/10.1016/j.eti.2022.102651
Nong G, Xing D, Li Y et al (2020) Recycle cooking wood chips with the residue liquid removed out of lignin by calcification for increasing pulp yield and reducing waste water discharge. J Clean Prod 277:124028. https://doi.org/10.1016/j.jclepro.2020.124028
Nouhou Moussa AW, Sawadogo B, Konate Y et al (2023) Critical state of the art of sugarcane industry wastewater treatment technologies and perspectives for sustainability. Membranes (Basel) 13. https://doi.org/10.3390/membranes13080709
Nqombolo A, Mpupa A, Moutloali RM, Nomngongo PN (2018) Wastewater treatment using membrane technology. In: Yonar T (ed) Wastewater and water quality. IntechOpen. https://doi.org/10.5772/intechopen.76624
Nweke C, Igbokwe P, Nwabanne J (2014) Kinetics of batch anaerobic digestion of vegetable oil wastewater. Open J Water Pollut Treat 2014:1–10. https://doi.org/10.15764/WPT.2014.02001
Ødegaard H, Cimbritz M, Christensson M, Dahl CP (2010) Separation of Biomass From Moving Bed Biofilm Reactors (MBBRs). Proc Water Environ Fed 2010:212–233. https://doi.org/10.2175/193864710798208368
OECD/FAO (2021) OECD-FAO agricultural outlook 2021-2030. OECD Publishing, Paris. https://doi.org/10.1787/19428846-en
Ojovan MI, Lee WE (2014) Treatment of radioactive wastes. In: Ojovan MI, Lee WE (eds) An introduction to nuclear waste immobilisation, 2nd edn. Elsevier, pp 171–203. https://doi.org/10.1016/B978-0-08-099392-8.00014-0
Oki T, Kanae S (2006) Global hydrological cycles and world water resources. Science 313:1068–1072. https://doi.org/10.1126/science.1128845
Othman NH, Alias NH, Fuzil NS et al (2022) A review on the use of membrane technology systems in developing countries. Membranes (Basel) 12. https://doi.org/10.3390/membranes12010030
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641. https://doi.org/10.1080/10643389.2013.829765
Ozyonar F, Karagozoglu B (2015) Treatment of pretreated coke wastewater by electrocoagulation and electrochemical peroxidation processes. Sep Purif Technol 150:268–277. https://doi.org/10.1016/j.seppur.2015.07.011
Pang YL, Abdullah AZ, Bhatia S (2011) Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 277:1–14. https://doi.org/10.1016/j.desal.2011.04.049
Patel K, Patel N, Vaghamshi N et al (2021) Trends and strategies in the effluent treatment of pulp and paper industries: A review highlighting reactor options. Curr Res Microb Sci 2:100077. https://doi.org/10.1016/j.crmicr.2021.100077
Pathan AA, Memon NA, Shaikh P (2016) Effect of sedimentation on treated greywater through rotating biological contactor. Int J Eng Technol 8:1501–1505
Patziger M, Kainz H, Hunze M, Józsa J (2012) Influence of secondary settling tank performance on suspended solids mass balance in activated sludge systems. Water Res 46:2415–2424. https://doi.org/10.1016/j.watres.2012.02.007
Paździor K, Bilińska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J 376:120597. https://doi.org/10.1016/j.cej.2018.12.057
Perera KDAS, Ranathunga RGSA, Keshani YHN et al (2020) Cement Industry in Sri Lanka. J Res Technol Eng 1:16–27
Philipp M, Masmoudi Jabri K, Wellmann J et al (2021) Slaughterhouse Wastewater Treatment: A Review on Recycling and Reuse Possibilities. Water 13:3175. https://doi.org/10.3390/w13223175
Piaskowski K, Świderska-Dąbrowska R, Zarzycki PK (2018) Dye removal from water and wastewater using various physical, chemical, and biological processes. J AOAC Int 101:1371–1384. https://doi.org/10.5740/jaoacint.18-0051
Pintor AMA, Vilar VJP, Botelho CMS, Boaventura RAR (2016) Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem Eng J 297:229–255. https://doi.org/10.1016/j.cej.2016.03.121
Pirsaheb M, Moradi N, Hossini H (2023) Sonochemical processes for antibiotics removal from water and wastewater: A systematic review. Chem Eng Res Des 189:401–439. https://doi.org/10.1016/j.cherd.2022.11.019
Pirzadeh B (2022) Physical Wastewater Treatment. In: Wastewater Treatment. IntechOpen. https://doi.org/10.5772/intechopen.104324
Plana Q, Pauléat A, Gadbois A et al (2020) Characterizing the settleability of grit particles. Water Environ Res 92:731–739. https://doi.org/10.1002/wer.1268
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater—a review. Sci Total Environ 333:37–58. https://doi.org/10.1016/j.scitotenv.2004.05.017
Prabu SL, Suriyaprakash TNK, Kumar JA (2011) Wastewater treatment technologies: A review. Pharma times 43:9–13
Prakash O, Parmesh S, Chaudhari K et al (2015) The characteristics, effects, and treatment of wastewater in sugarcane industry. Water Qual Expo Heal 7:435–444. https://doi.org/10.1007/s12403-015-0158-6
Pratiwi D, Sumiarsa D, Oktavia D, Sunardi S (2023) Water quality influences self-purification in the Cihawuk and Majalaya segments upstream of the Citarum river, West Java, Indonesia. Water 15:2998. https://doi.org/10.3390/w15162998
Puchlik M, Struk-Sokołowska J (2017) Comparison of the composition of wastewater from fruit and vegetables as well as dairy industry. E3S Web Conf 17:00077. https://doi.org/10.1051/e3sconf/20171700077
Puchlik M (2018) Effectiveness of wastewater treatment from the fruit and vegetable industry in the vertical flow-type constructed wetlands. E3S Web Conf 44. https://doi.org/10.1051/e3sconf/20184400149
Pujiastuti C, Yulisningtyas E, Pareira I (2021) Ceramic industry wastewater treatment by chemical coagulation process. J Res Technol 7:217–226. https://doi.org/10.55732/jrt.v7i2.411
Qadir M, Drechsel P, Jiménez Cisneros B et al (2020) Global and regional potential of wastewater as a water, nutrient and energy source. Nat Resour Forum 44:40–51. https://doi.org/10.1111/1477-8947.12187
Qiao J, Xiong Y (2021) Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. J Water Process Eng 44:102308. https://doi.org/10.1016/j.jwpe.2021.102308
Quach-Cu J, Herrera-Lynch B, Marciniak C et al (2018) The effect of primary, secondary, and tertiary wastewater treatment processes on Antibiotic Resistance Gene (ARG) concentrations in solid and dissolved wastewater fractions. Water 10:37. https://doi.org/10.3390/w10010037
Quiton KGNGN, Huang Y-H, Lu M-C (2022) Recovery of cobalt and copper from single- and co-contaminated simulated electroplating wastewater via carbonate and hydroxide precipitation. Sustain Environ Res 32:31. https://doi.org/10.1186/s42834-022-00140-z
Radelyuk I, Tussupova K, Zhapargazinova K et al (2019) Pitfalls of wastewater treatment in oil refinery enterprises in Kazakhstan—A system approach. Sustainability 11:1618. https://doi.org/10.3390/su11061618
Rajapakse N, Zargar M, Sen T, Khiadani M (2022) Effects of influent physicochemical characteristics on air dissolution, bubble size and rise velocity in dissolved air flotation: a review. Sep Purif Technol 289:120772. https://doi.org/10.1016/j.seppur.2022.120772
Rajkumar K, Muthukumar M, Sivakumar R (2010) Novel approach for the treatment and recycle of wastewater from soya edible oil refinery industry—An economic perspective. Resour Conserv Recycl 54:752–758. https://doi.org/10.1016/j.resconrec.2009.12.005
Ramachandran SK, Gangasalam A (2019) Reduction of chemical oxygen demand and color from the rice mill wastewater by chitosan/2(5H)-furanone-incorporated ultrafiltration membrane system. Sep Sci Technol 54:409–425. https://doi.org/10.1080/01496395.2018.1505915
Rana RS, Singh P, Kandari V et al (2017) A review on characterization and bioremediation of pharmaceutical industries’ wastewater: an Indian perspective. Appl Water Sci 7:1–12. https://doi.org/10.1007/s13201-014-0225-3
Ranieri E, Giuliano S, Ranieri AC (2021) Energy consumption in anaerobic and aerobic based wastewater treatment plants in Italy. Water Pract Technol 16:851–863. https://doi.org/10.2166/wpt.2021.045
Ranken MD, Kill RC, Baker C (1997) Food industries manual. Springer US, Boston. https://doi.org/10.1007/978-1-4613-1129-4
Rashid R, Shafiq I, Akhter P et al (2021) A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res 28:9050–9066. https://doi.org/10.1007/s11356-021-12395-x
Rasras A, Hamdi R, Mansour S et al (2021) Study of the magnetocaloric effect in single-phase antiferromagnetic GdMnO3. J Phys Chem Solids 149:109798. https://doi.org/10.1016/j.jpcs.2020.109798
Ratnasari A (2023) Modified polymer membranes for the removal of pharmaceutical active compounds in wastewater and its mechanism-A review. Bioengineered 14. https://doi.org/10.1080/21655979.2023.2252234
Rattan S, Parande AK, Nagaraju VD, Ghiwari GK (2015) A comprehensive review on utilization of wastewater from coffee processing. Environ Sci Pollut Res 22:6461–6472. https://doi.org/10.1007/s11356-015-4079-5
Rawat A, Srivastava A, Bhatnagar A, Gupta AK (2023) Technological advancements for the treatment of steel industry wastewater: Effluent management and sustainable treatment strategies. J Clean Prod 383:135382. https://doi.org/10.1016/j.jclepro.2022.135382
Ren T, Daniëls B, Patel MK, Blok K (2009) Petrochemicals from oil, natural gas, coal and biomass: Production costs in 2030–2050. Resour Conserv Recycl 53:653–663. https://doi.org/10.1016/j.resconrec.2009.04.016
Rezai B, Allahkarami E (2021) Wastewater treatment processes—techniques, technologies, challenges faced, and alternative solutions. Soft Comput Tech Solid Waste Wastewater Manag 35–53. https://doi.org/10.1016/B978-0-12-824463-0.00004-5
Ribeiro JP, Marques CC, Portugal I, Nunes MI (2020) AOX removal from pulp and paper wastewater by Fenton and photo-Fenton processes: A real case-study. Energy Rep 6:770–775. https://doi.org/10.1016/j.egyr.2019.09.068
Rodrigues RT, Rubio J (2007) DAF-dissolved air flotation: potential applications in the mining and mineral processing industry. Int J Miner Process 82:1–13. https://doi.org/10.1007/s11356-015-4079-5
Rodríguez-Chueca J, Ormad MP, Mosteo R et al (2015) Conventional and Advanced Oxidation Processes Used in Disinfection of Treated Urban Wastewater. Water Environ Res 87:281–288. https://doi.org/10.2175/106143014x13987223590362
Rongrong L, Xujie L, Qing T et al (2011) The performance evaluation of hybrid anaerobic baffled reactor for treatment of PVA-containing desizing wastewater. Desalination 271:287–294. https://doi.org/10.1016/j.desal.2010.12.044
Sabaikai W, Sekine M, Tokumura M, Kawase Y (2014) UV light photo-Fenton degradation of polyphenols in oolong tea manufacturing wastewater. J Environ Sci Heal Part A 49:193–202. https://doi.org/10.1080/10934529.2013.838873
Saha S, Boro R, Das C (2019) Treatment of tea industry wastewater using coagulation-spinning basket membrane ultrafiltration hybrid system. J Environ Manag 244:180–188. https://doi.org/10.1016/j.jenvman.2019.05.043
Sala M, Gutiérrez-Bouzán MC (2012) Electrochemical techniques in textile processes and wastewater treatment. Int J Photoenergy 2012:1–12. https://doi.org/10.1155/2012/629103
Samal K, Mahapatra S, Hibzur Ali M (2022) Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 6:100076. https://doi.org/10.1016/j.nexus.2022.100076
Samer M (2015) Biological and chemical wastewater treatment processes. In: Wastewater Treatment Engineering. InTech. https://doi.org/10.5772/61250
Samsami S, Mohamadizaniani M, Sarrafzadeh M-H et al (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf Environ Prot 143:138–163. https://doi.org/10.1016/j.psep.2020.05.034
Sangamnere R, Misra T, Bherwani H et al (2023) A critical review of conventional and emerging wastewater treatment technologies. Sustain Water Resour Manag 9:58. https://doi.org/10.1007/s40899-023-00829-y
Sarayu K, Sandhya S (2012) Current technologies for biological treatment of textile wastewater-A review. Appl Biochem Biotechnol 167:645–661. https://doi.org/10.1007/S12010-012-9716-6/TABLES/5
Saritha V, Srinivas N, Srikanth Vuppala NV (2017) Analysis and optimization of coagulation and flocculation process. Appl Water Sci 7:451–460. https://doi.org/10.1007/s13201-014-0262-y
Savun-Hekimoğlu B (2020) A Review on sonochemistry and its environmental applications. Acoustics 2:766–775. https://doi.org/10.3390/acoustics2040042
Sehar S, Nasser HAA (2019) Wastewater treatment of food industries through constructed wetland: a review. Int J Environ Sci Technol 16:6453–6472. https://doi.org/10.1007/s13762-019-02472-7
Shahriari T, Shokouhi M (2015) Assessment of bio-trickling filter startup for treatment of industrial wastewater. Int J Environ Res 9:769–776
Sharma K, Jain U, Singhal A (2012) Treatment of waste generated from cement industry and their treatment-a review. In: ICSBE-2012 Int. Conf. Sustain. Built Environ. http://www.civil.mrt.ac.lk/web/conference/ICSBE2012/SBE-12-13.pdf. Accessed 1 Aug 2024
Sharma S, Can OT, Hammed M et al (2018) Organic pollutant removal from edible oil process wastewater using electrocoagulation. IOP Conf Ser Earth Environ Sci 142. https://doi.org/10.1088/1755-1315/142/1/012079
Sharma NK, Philip L (2016) Combined biological and photocatalytic treatment of real coke oven wastewater. Chem Eng J 295:20–28. https://doi.org/10.1016/j.cej.2016.03.031
Sharma S, Simsek H (2020) Sugar beet industry process wastewater treatment using electrochemical methods and optimization of parameters using response surface methodology. Chemosphere 238:124669. https://doi.org/10.1016/j.chemosphere.2019.124669
Shirazi MMA, Dumée LF (2022) Membrane distillation for sustainable wastewater treatment. J Water Process Eng 47:102670. https://doi.org/10.1016/j.jwpe.2022.102670
Shrivastava V, Ali I, Marjub MM et al (2022) Wastewater in the food industry: Treatment technologies and reuse potential. Chemosphere 293:133553. https://doi.org/10.1016/j.chemosphere.2022.133553
Sillanpää M, Shestakova M (2017) Equipment for electrochemical water treatment. In: Electrochemical water treatment methods. Elsevier, pp 227–263. https://doi.org/10.1016/B978-0-12-811462-9.00004-9
Singh M, Srivastava RK (2011) Sequencing batch reactor technology for biological wastewater treatment: a review. Asia-Pacific J Chem Eng 6:3–13. https://doi.org/10.1002/apj.490
Singh A, Pal DB, Mohammad A et al (2022) Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour Technol 343:126154. https://doi.org/10.1016/j.biortech.2021.126154
Sivaranjani GA, Ali N et al (2020) Applicability and new trends of different electrode materials and its combinations in electro coagulation process: A brief review. Mater Today Proc 37:377–382. https://doi.org/10.1016/j.matpr.2020.05.379
Slama H, Chenari Bouket A, Pourhassan Z et al (2021) Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl Sci 11:6255. https://doi.org/10.3390/app11146255
Strathmann H (2005) Membranes and membrane separation processes. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. https://doi.org/10.1002/14356007.a16_187.pub2
Struk-Sokolowska J, Tkaczuk J (2018) Analysis of bakery sewage treatment process options based on COD fraction changes. J Ecol Eng 19:226–235. https://doi.org/10.12911/22998993/89653
Sukmana H, Bellahsen N, Pantoja F, Hodur C (2021) Adsorption and coagulation in wastewater treatment - Review. Prog Agric Eng Sci 17:49–68. https://doi.org/10.1556/446.2021.00029
Sun F, Dai Y, Yu X (2017) Air pollution, food production and food security: A review from the perspective of food system. J Integr Agric 16:2945–2962. https://doi.org/10.1016/S2095-3119(17)61814-8
Suslick KS (1990) Sonochemistry. Science 247:1439–1445. https://doi.org/10.1126/science.247.4949.1439
Suvio P, van Hoorn A, Szabo M, Ekdahl Å (2012) Water management for sustainable steel industry. Ironmak Steelmak 39:263–269. https://doi.org/10.1179/030192311X13135947813898
Szostkova M, Vitez T, Marecek J, Losak T (2012) Microbial contamination of screenings from wastewater treatment plants. Polish J Environ Stud 21:1943–1947
Taulo JL, Sebitosi AB (2016) Material and energy flow analysis of the Malawian tea industry. Renew Sustain Energy Rev 56:1337–1350. https://doi.org/10.1016/j.rser.2015.11.072
Tauseef SM, Abbasi T, Abbasi SA (2013) Energy recovery from wastewaters with high-rate anaerobic digesters. Renew Sustain Energy Rev 19:704–741. https://doi.org/10.1016/j.rser.2012.11.056
Teasdale SB, Marshall S, Abbott K et al (2022) How should we judge edible oils and fats? An umbrella review of the health effects of nutrient and bioactive components found in edible oils and fats. Crit Rev Food Sci Nutr 62:5167–5182. https://doi.org/10.1080/10408398.2021.1882382
Teplická K, Sedláková Z (2023) Evaluation of the quality of the cement production process in terms of increasing the company’s performance. Processes 11. https://doi.org/10.3390/pr11030791
Thompson G, Swain J, Kay M, Forster CF (2001) The treatment of pulp and paper mill effluent: a review. Bioresour Technol 77:275–286. https://doi.org/10.1016/S0960-8524(00)00060-2
Tiwari B, Sellamuthu B, Ouarda Y et al (2017) Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol 224:1–12. https://doi.org/10.1016/j.biortech.2016.11.042
Toczyłowska-Mamińska R (2017) Limits and perspectives of pulp and paper industry wastewater treatment – A review. Renew Sustain Energy Rev 78:764–772. https://doi.org/10.1016/j.rser.2017.05.021
Tong Y, Zhang Q, Cai J et al (2018) Water consumption and wastewater discharge in China’s steel industry. Ironmak Steelmak 45:868–877. https://doi.org/10.1080/03019233.2018.1538180
Tran HN (2023) Adsorption Technology for Water and Wastewater Treatments. Water 15:2857. https://doi.org/10.3390/w15152857
U.S. EPA (1996) Ceramic products manufacturing final report. In: Emiss. Factor Doc. AP-42 Sect. 11.7. https://www3.epa.gov/ttnchie1/ap42/ch11/bgdocs/b11s07.pdf. Accessed 1 Aug 2024
U.S. EPA (1999) Wastewater technology fact sheet sequencing batch reactors. https://www3.epa.gov/npdes/pubs/sbr_new.pdf. Accessed 1 Aug 2024
U.S. EPA (2000) Wastewater technology fact sheet chemical precipitation. https://purl.fdlp.gov/GPO/LPS50744. Accessed 1 Aug 2024
U.S. EPA (2002) Wastewater technology fact sheet aerated, partial mix lagoons. https://www3.epa.gov/npdes/pubs/apartlag.pdf. Accessed 1 Aug 2024
U.S. EPA (2003) Wastewater Technology Fact Sheet Screening and Grit Removal. In: United States Environ. Prot. Agency. http://www3.epa.gov/npdes/pubs/final_sgrit_removal.pdf. Accessed 30 Oct 2023
U.S. EPA (2007) Wastewater management fact sheet: membrane biorreactors. https://www.epa.gov/sites/default/files/2019-08/documents/membrane_bioreactor_fact_sheet_p100il7g.pdf. Accessed 1 Aug 2024
UNCTAD (2016) TEA - An INFOCOMM commodity profile. https://unctad.org/system/files/official-document/INFOCOMM_cp11_Tea_en.pdf. Accessed 1 Aug 2024
United States Environmental Protection Agency (EPA) (2008) AP 42, Fifth Edition, Volume I Chapter 12: Metallurgical Industry. In: epa.gov. https://www3.epa.gov/ttnchie1/ap42/ch12/. Accessed 26 Dec 2023
Valderrama OJ, Zedda KL, Velizarov S (2021) Membrane Filtration Opportunities for the Treatment of Black Liquor in the Paper and Pulp Industry. Water 13:2270. https://doi.org/10.3390/w13162270
Valta K, Damala P, Panaretou V et al (2017) Review and assessment of waste and wastewater treatment from fruits and vegetables processing industries in Greece. Waste Biomass Valor 8:1629–1648. https://doi.org/10.1007/s12649-016-9672-4
van Lier JB (2008) High-rate anaerobic wastewater treatment: diversifying from end-of-the-pipe treatment to resource-oriented conversion techniques. Water Sci Technol 57:1137–1148. https://doi.org/10.2166/wst.2008.040
van Lier JB, van der Zee FP, Frijters CTMJ, Ersahin ME (2015) Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev Environ Sci Biotechnol 14:681–702. https://doi.org/10.1007/s11157-015-9375-5
van Lier JB, Mahmoud N, Zeeman G (2020) Anaerobic wastewater treatment. In: Chen G, Loosdrecht MCM van, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment: principles, modeling and design. IWA Publishing, pp 701–756. https://doi.org/10.2166/9781789060362_0701
Velusamy S, Roy A, Sundaram S, Mallick TK (2021) A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21:1570–1610. https://doi.org/10.1002/tcr.202000153
Venugopal V, Sasidharan A (2021) Seafood industry effluents: Environmental hazards, treatment and resource recovery. J Environ Chem Eng 9:104758. https://doi.org/10.1016/j.jece.2020.104758
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manag 93:154–168. https://doi.org/10.1016/j.jenvman.2011.09.012
Vijin Prabhu A, Sivaram AR, Prabhu N, Sundaramahalingam A (2021) A study of enhancing the biogas production in anaerobic digestion. Mater Today Proc 45:7994–7999. https://doi.org/10.1016/j.matpr.2020.12.1009
Wake H (2005) Oil refineries: a review of their ecological impacts on the aquatic environment. Estuar Coast Shelf Sci 62:131–140. https://doi.org/10.1016/j.ecss.2004.08.013
Wan K, Huang L, Yan J et al (2021) Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci Total Environ 773:145535. https://doi.org/10.1016/j.scitotenv.2021.145535
Wang LK, Vaccari DA, Li Y, Shammas NK (2005) Chemical precipitation. In: Wang LK, Hung YT, Shammas NK (eds) Physicochemical treatment processes. Humana press, Totowa, pp 141–197. https://doi.org/10.1385/1-59259-820-x:141
Wang N, Zhao Q, Xu H et al (2018) Adsorptive treatment of coking wastewater using raw coal fly ash: Adsorption kinetic, thermodynamics and regeneration by Fenton process. Chemosphere 210:624–632. https://doi.org/10.1016/j.chemosphere.2018.07.073
Wang J, Ji Y, Zhang F et al (2019) Treatment of coking wastewater using oxic-anoxic-oxic process followed by coagulation and ozonation. Carbon Resour Convers 2:151–156. https://doi.org/10.1016/j.crcon.2019.06.001
Wang W, Wang K, Hao W et al (2022) Preparation of Ti-based Yb-doped SnO2–RuO2 electrode and electrochemical oxidation treatment of coking wastewater. J Rare Earths 40:763–771. https://doi.org/10.1016/j.jre.2021.04.001
Waqas S, Harun NY, Sambudi NS et al (2023) A review of rotating biological contactors for wastewater treatment. Water 15:1913. https://doi.org/10.3390/w15101913
Warechowska M, Markowska A, Warechowski J et al (2016) Effect of tempering moisture of wheat on grinding energy, middlings and flour size distribution, and gluten and dough mixing properties. J Cereal Sci 69:306–312. https://doi.org/10.1016/j.jcs.2016.04.007
Włodarczyk-Makuła M, Wiśniowska E, Turek A, Obstój A (2016) Removal of PAHs from coking wastewater during photodegradation process. Desalin Water Treat 57:1262–1272. https://doi.org/10.1080/19443994.2014.996012
WWAP (United Nations World Water Assessment Programme) (2017) The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource. UNESCO, Paris
WWAP (United Nations World Water Assessment Programme) (2018) The United Nations World Water Development Report 2018: Nature-Based Solutions for Water. UNESCO, Paris
Xue Y, Jia Y, Liu S et al (2023) Electrochemical reduction of wastewater by non-noble metal cathodes: From terminal purification to upcycling recovery. J Hazard Mater 459:132106. https://doi.org/10.1016/j.jhazmat.2023.132106
Yadav M, Gupta R, Sharma RK (2019) Green and sustainable pathways for wastewater purification. In: Advances in Water Purification Techniques. Elsevier, pp 355–383. https://doi.org/10.1016/B978-0-12-814790-0.00014-4
Yadav SK, Kalaiyarasi R (2015) Feasibility analysis on industrial symbiosis between cement industry and tea industry. Int J Environ 4:20–34. https://doi.org/10.3126/ije.v4i3.13227
Yaghmaeian K, Rajabizadeh A, Jaberi Ansari F et al (2023) Treatment of vegetable oil industry wastewater and bioelectricity generation using microbial fuel cell via modification and surface area expansion of electrodes. J Chem Technol Biotechnol 98:978–989. https://doi.org/10.1002/jctb.7301
Yan Z, Jiang Y, Liu L et al (2021) Membrane distillation for wastewater treatment: a mini review. Water 13:3480. https://doi.org/10.3390/w13243480
Yang C, Qian Y, Zhang L, Feng J (2006) Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem Eng J 117:179–185. https://doi.org/10.1016/j.cej.2005.12.011
Yang Y, Raipala K, Holappa L (2014) Ironmaking. In: Seetharaman S (ed) Treatise on Process Metallurgy. Elsevier, Amsterdam, pp 2–88. https://doi.org/10.1385/1-59259-820-x:141
Yang J, Zhang C, Lin X et al (2018) Wastewater desalination system utilizing a low-temperature heat pump. Int J Energy Res 42:1132–1138. https://doi.org/10.1002/er.3909
Yaseen DA, Scholz M (2019) Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol 16:1193–1226. https://doi.org/10.1007/s13762-018-2130-z
Yi X, Wang Y (2017) Treatment of high salt oxidized modified starch waste water using micro-electrolysis, two-phase anaerobic aerobic and electrolysis for reuse. Appl Water Sci 7:1231–1237. https://doi.org/10.1007/s13201-016-0454-8
You SJ, Tseng DH, Ou SH, Chang WK (2007) Performance and microbial diversity of a membrane bioreactor treating real textile dyeing wastewater. Environ Technol 28:935–941. https://doi.org/10.1080/09593332808618854
Young RA, Kundrot R, Tillman DA (2003) Pulp and paper. In: Meyers RA (ed) Encyclopedia of physical science and technology, 3rd edn. Elsevier, pp 249–265. https://doi.org/10.1016/B0-12-227410-5/00619-0
Yu W, Gregory J, Campos L, Li G (2011) The role of mixing conditions on floc growth, breakage and re-growth. Chem Eng J 171:425–430. https://doi.org/10.1016/j.cej.2011.03.098
Yu L, Han M, He F (2017) A review of treating oily wastewater. Arab J Chem 10:S1913–S1922. https://doi.org/10.1016/j.arabjc.2013.07.020
Zhang R, Ma S, Li L et al (2021) Comprehensive utilization of corn starch processing by-products: A review. Grain Oil Sci Technol 4:89–107. https://doi.org/10.1016/j.gaost.2021.08.003
Zhang Z (2020) Nutrients removal in membrane bioreactors for wastewater treatment. Curr Dev Biotechnol Bioeng Adv Membr Sep Process Sustain Water Wastewater Manag - Aerob Membr Bioreact Process Technol 163–180. https://doi.org/10.1016/B978-0-12-819809-4.00008-5
Zhao D, Lee LY, Ong SL et al (2019) Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep Purif Technol 213:339–347. https://doi.org/10.1016/j.seppur.2018.12.056
Zhu X, Yang J, Huang Q, Liu T (2022) A review on pollution treatment in cement industrial areas: from prevention techniques to python-based monitoring and controlling models. Processes 10. https://doi.org/10.3390/pr10122682
Zioui D, Martins PM, Aoudjit L et al (2023) Wastewater treatment of real effluents by microfiltration using poly(vinylidene fluoride–hexafluoropropylene) membranes. Polymers (basel) 15:1–11. https://doi.org/10.3390/polym15051143
Zueva SB (2018) Current legislation and methods of treatment of wastewater coming from waste electrical and electronic equipment processing. In: Waste Electrical and Electronic Equipment Recycling. Elsevier, pp 213–240. https://doi.org/10.1016/B978-0-08-102057-9.00009-3
Acknowledgements
This work was supported by the Japan Prize Foundation and JST SPRING (Grant number JPMJSP2108).
Funding
Open Access funding provided by The University of Tokyo. This work was supported by the Japan Prize Foundation and JST SPRING (Grant number JPMJSP2108).
Author information
Authors and Affiliations
Contributions
Shoma Kato: conceptualization, literature search, writing – original draft preparation. Yasuki Kansha: conceptualization, writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kato, S., Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ Sci Pollut Res 31, 51064–51097 (2024). https://doi.org/10.1007/s11356-024-34584-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34584-0