Abstract
Microplastics (MPs), due to their micro size, which overlaps with the typical food size of various aquatic organisms, can be ingested and move up the food chain, accumulating in the bodies of organisms at higher trophic levels. Few studies have focused on the uptake of MPs by ciliates, which are an important element of the microbial cycle. Three different ciliate species were used in this study: Blepharisma japonicum, Euplotes sp., and Spirostomum teres, as well as polystyrene beads with diameters of 1 and 2 µm at two concentrations (106 and 107 beads × mL−1). The results of the experiments showed that MPs have a variable, species-specific effect on the population growth rate of ciliates, which is directly dependent on their concentration in the environment (P < 0.01). It was also observed that the number of MPs ingested changed over time depending on their concentration and size. On average, the highest number of ingested MPs (883.11 ± 521.47) was recorded at 60 min of exposure to a low concentration of small beads in B. japonicum. The lowest number of beads was ingested after 5 min of exposure to a low concentration of large beads in the same species. The rate of MP uptake by the ciliate species was significantly dependent on their concentration, exposure time, and size (P < 0.001). The highest clearance rate was observed in the fifth minute of the experiment in the environment with the lowest MP concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The history of plastic pollution research within the scientific community began in 1972 when the abundance of small floating particles on the surface of the Atlantic Ocean was first described (Carpenter and Smith 1972; Carpenter et al. 1972). The majority of published studies and reports on plastic pollution have focused on relatively large debris that is hazardous to marine mammals, birds, or fish (Derraik 2002; Amélineau et al. 2016). However, over the past few decades, a large body of research has focused on smaller, less visible plastic debris, known as microplastics (MPs) (Weis 2020; Issac and Kandasubramanian 2021). By definition, MPs are a diverse mixture of differently structured materials. Depending on their shape, MPs can be classified as fibres, spheroids, granules, flakes, beads, or irregularly structured particles. They range in diameter from 0.1 to 5.0 mm and can be classified as primary or secondary microplastics, with the latter being formed by the fragmentation of larger objects (EFSA 2016). Microplastics enter the environment in many ways, but the most common is through the washing of synthetic clothes and abrasion of car tyres. In addition to shopping bags and plastic fishing nets, MPs can also be found in various cosmetics, such as scrubs, hair shampoos, and facial cleansers (Boucher and Friot 2017; Guerranti et al. 2019). The complexity and diversity of MPs are immense. They originate from a variety of sources and product types composed of different polymers, which affects their morphology, and show a wide range of sizes and shapes and incorporation of different mixtures of chemical additives (Thompson et al. 2009). Given their heterogeneity, Rochman et al. (2019) argued that we should not refer to MPs as a single pollutant but rather as a diverse set of pollutants, as is done in the case of pesticides or heavy metals. The term plastics is used to describe a wide range of materials, comprising some 20 different groups, each with many grades and sub-groups (APME 2006). Plastics are incredibly versatile materials given their great adaptability, corrosion resistance, and high thermal and electrical insulation properties. They are affordable, chemical- and water-resistant, strong, durable, and lightweight (Andrady and Neal 2009; Plastics Europe 2010). Moreover, the same properties that make plastics so extraordinary have become a major environmental concern when they end up as waste (Cole et al. 2011). Plastics are an overused material due to their durability, but at the same time, their remarkably long decomposition time and resistance to degradation make them problematic to dispose of (Barnes et al. 2009; Sivan 2011; Cole et al. 2011). In addition, as marine and freshwater contaminants, MPs are widely dispersed in aquatic environments due to their buoyancy and durability properties (Lusher 2015). As observed by Lobelle and Cunliffe (2011), these physicochemical properties of MPs can change rapidly due to the development of microbial films on the surface of submerged particles, which can eventually lead to the sinking of fragments and their accumulation on the bottom of water bodies (Barnes et al. 2009). In addition, waterborne pollutants with hydrophobic properties, most of which are toxic and persistent in the environment, can be adsorbed onto plastic waste, which can enhance their persistence, and be transported with them (Teuten et al. 2009; Thompson et al. 2009; Cole et al. 2011; Engler 2012; Fries et al. 2013; Velzeboer et al. 2014). Although MPs pose a number of threats to aquatic ecosystems, the greatest hazard is their small size, which allows them to enter the aquatic food web at very low trophic levels and diffuse to higher levels or even to terrestrial organisms (Wright et al. 2013; Besseling et al. 2014), as small plastic fragments are easier to assimilate into biological processes due to their favourable surface-to-volume ratio (Zarfl and Matthies 2010; Jeong et al. 2016). The ingestion and accumulation of MPs by many different organisms leads to their accumulation in the food chain, with particular emphasis on organisms at the highest trophic levels (Vandermeersch et al. 2015). Microplastics are available for consumption by a wide range of different aquatic organisms due to their small size, which overlaps with the size range of typical prey (Galloway et al. 2017). They have been found in the guts of zooplankton, invertebrates, molluscs, fish, and other larger animals, including those intended for human consumption and those that play important ecological roles (Browne et al. 2008; Lusher 2015; Galloway et al. 2017; Stienbarger et al. 2021). In addition, ingestion of MPs can have serious consequences, such as clogging of the digestive tract, resulting in impaired food intake and digestion (Cole et al. 2013; Setälä et al. 2014; Canniff and Hoang 2018). Furthermore, most laboratory experiments confirm the adverse effects of MPs on the consumption, nutrition, fertility, growth, development, and lifespan of the organisms tested (Lee et al. 2013; Cole et al. 2013, 2015). However, information on the consumption of MPs and their potential accumulation in naturally occurring populations is still lacking (Lusher et al. 2013).
Diverse groups of zooplankton exhibit a variety of feeding strategies, including consumption of suspended organic matter, filter feeding, detritivory, and predation, which can vary with species, life stage, and prey availability (Strickler 1982; Wirtz 2012). The ingestion of MPs by pelagic filter-feeding zooplankton is well documented, but freshwater ciliates are a prominent group of zooplankton that has been understudied (Bulannga and Schmidt 2022). Heterotrophic protists play an integral role in the aquatic ecosystem in the microbial cycle, as they feed on bacteria and other types of microorganisms such as algae, other protists, and even rotifers (Foissner et al. 1991, 1992, 1999). Microplastic particles, measuring approximately 1–2 µm in size, can be ingested unintentionally or accidentally by ciliates and transferred to higher trophic levels, when protists are ingested by crustacean zooplankton and other small organisms, thus linking all parts of the food web (Cole et al. 2013). However, some filter-, suspension-, and detritus-feeding ciliates living in water and sediments have been observed to intentionally ingest MPs in laboratory experiments (Nugroho and Fyda 2020; Nałęcz-Jawecki et al. 2021; Bulannga and Schmidt 2022). Some ciliates that exhibit the above consumption modes may feed on bacteria, suspended organic matter, or small algae but may also graze on fine inorganic particles (Bernard and Rassoulzadegan 1990; Pierce and Turner 1992). It is therefore of great importance to investigate the ability to ingest plastic microparticles and its effects among zooplankton, including protozoa, which are essential to the aquatic food web.
The primary aim of this study was to describe the effects of MP ingestion on the viability of freshwater ciliates. A secondary aim was to determine the ability of these organisms to ingest MPs and whether there are differences in the number of particles ingested among species at different times of exposure, MP sizes, and concentrations. The specific research objectives were (1) to determine whether the population growth rate is affected by microplastics, (2) to calculate the number of ingested microbeads, and (3) to evaluate the clearance rate in different experimental settings.
Materials and methods
Culture and maintenance of ciliated protozoa
The ciliated protozoa used in this study were obtained from permanent cultures maintained at the Aquatic Ecosystems Laboratory at the Institute of Environmental Sciences Jagiellonian University, Poland. From these stock cultures, several clones of Blepharisma japonicum Suzuki, 1954, Spirostomum teres Claparède and Lachmann, 1858, and Euplotes sp. were established using a Zeiss Stemii 2000 stereomicroscope and fine glass micropipettes in 24 well tissue plates (TPP, Switzerland) filled with 500 µL of spring water (Żywiec Zdrój, Poland) as medium. A drop of unidentified bacteria growing on the same medium and wheat grain for B. japonicum and S. teres and a drop of a dense culture of the flagellate Chilomonas sp. for Euplotes were added as food. The clones were kept in a SANYO MLR-350 climate chamber at 20 ± 1 °C in the dark. After a few days, the most numerous clones were transferred to Petri dishes of 5 cm in diameter filled with 5 ml of medium. Wheat grains were added as a nutrient source for the bacteria. All clones were kept in the same climate chamber under the same conditions and monitored twice a week. Once a high number of individuals had been reached, at 24 h before the start of the experiment, the ciliates were transferred to clean medium to remove any residual culture. Individuals belonging to the same clone were then randomly selected for each experiment.
Microplastic particles
Spherical, carboxylate-modified polystyrene latex beads with yellow-green fluorescence of 2 µm (large) and 1 µm (small) diameter, supplied by Sigma-Aldrich (Poland), and stored in the dark at 4 °C were used in the experiments. The maximum excitation wavelength of the microbeads was λex ~ 441 nm, and the maximum emission was λem ~ 486 nm, which is a fluorescence spectrum similar to that of fluorescein isothiocyanate (FITC) (λex ~ 491 nm, λem ~ 516 nm). For experiments, 10 µL of microbead suspension was transferred to 240 µL of deionised water to dilute the original concentration of microbeads. To determine the exact concentration of microbeads in a well, the beads were counted in a Bürker chamber, and their concentration was determined. The low concentration was obtained by pipetting 1 µL of small-bead suspension or 3 µL of large-bead suspension. The high concentration was obtained by pipetting 5 µL of small-bead suspension and 35 µL of large-bead suspension into a well filled with 400 µL of water. The final concentrations were 106 and 107 beads per mL for the low and high concentrations, respectively.
Influence of MPs on the population growth rate
Population growth rates (PGRs) were expressed as the increase in the number of individuals per day of both control and MP-exposed individuals and were measured in 24-well tissue culture plates. Three cells of each ciliate species were placed in 200 µL of Żywiec Zdrój spring water, and food was provided ad libitum. An appropriate amount of microbeads of each size representing the control, low, and high concentrations was then added. Each experimental setup consisted of 3 replicates. Ciliates were counted in the tissue culture plate after 24, 48, and 72 h of incubation. Tissue culture plates containing ciliates were stored in the dark at room temperature during the experiment. Population growth rates were calculated using the equation described by Fyda and Wiackowski (1998) and modified to fit the data obtained: \(\mu =\frac{\left({\text{ln}}\left(N+1\right)-{\text{ln}}\left({N}_{0}+1\right)\right) }{x}\), where N0 = 3, N is the final number of cells and x is the subsequent day of exposure to MPs.
Uptake of plastic microbeads
The experiments were performed in 24-well tissue culture plates. Each well was filled with 400 µL of Żywiec Zdrój spring water, to which 50 individuals randomly taken from monoclonal cultures and one of the two concentrations of microsphere sizes were added. Each experiment consisted of 4 replicates. Then, after 5, 30, or 60 min in different setups, the experiment was terminated by adding an equal amount of 4% formaldehyde for B. japonicum and Euplotes sp. or methylcellulose for S. teres. In brief, 7–11 randomly selected protozoa were removed from each well with a glass micropipette, each of which was treated as a replicate for subsequent microscopic analysis. The cells were then placed on a microscope slide and covered with a coverslip. Each slide was briefly observed under a bright field microscope to identify individuals for subsequent analysis.
Samples were examined using a Nikon Eclipse E-80 epifluorescence microscope. Photographs of randomly selected individuals of B. japonicum, Euplotes sp., and S. teres for further analysis were taken using a Nikon DS-Ri2 DO camera and the NIH program at 400 × magnification. The images were then used to calculate the amount of microplastic beads ingested by each ciliate species using ImageJ software. To calculate the actual number of microspheres absorbed, it was assumed that the MP particles were round in a 2-dimensional visualization. Calculations were made using a scale where 100 µm at 400 × magnification corresponds to 544 pixels in ImageJ software. ImageJ tools were then used to determine the area occupied by the food vacuole filled with the collected MPs, to calculate the area of a MP sphere, and to calculate the number of spheres ingested by the ciliates by dividing the area of the vacuole by the area of a microsphere.
Clearance rate
To determine the clearance rate (C) per individual in each experiment at different concentrations and times, the calculation described by Peters (1984) was followed: \(C =\frac{B}{\left(S\cdot T\right)} ,\) where B = beads counted inside the cell, S = concentration, and T = time of exposure of the cells to the bead suspension. The clearance rate for individuals was expressed as the number of beads ingested × concentrations−1 × time−1.
Statistical analysis
A three-factor factorial model and ANOVA were used to test whether MP concentration, exposure time, and size (fixed factors) influenced the number of ingested microbeads (dependent variable). The interaction between the variables was also taken into account in the model. Separate analyses were performed for each ciliate species.
The effects of MP concentration, size, and exposure time (fixed factors) on clearance rate (dependent variable) were tested using three-way ANOVA. A new model was fitted independently for each species. Each model included an interaction between the variables.
Three-way ANOVA was adapted to test whether the concentration of MPs in the water, their size, and exposure time, considered fixed factors, had an effect on the PGR, which was considered the dependent variable. Discrete models were fitted for each ciliate species, and all models took into account the interaction between concentration, size, and time. Interactions that did not show statistical significance were later removed from the models.
In addition, to test whether the size of the ciliate cell influenced the uptake and clearance rate of MPs, a linear model was fitted with cell area as the independent variable and bead uptake and clearance rate as the dependent variables. Statistical analyses and plotting were performed in R Studio (R Core Team, 2020). A P value = 0.05 was considered a significant difference between groups in all tests performed.
Results
Influence of MPs on the population growth rate
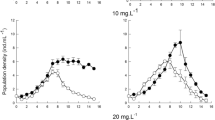
The PGR varied among species and different experimental conditions. In the control, the population growth rate of B. japonicum was observed to be negative on each day of the experiment. A similar result was observed in the experiment with S. teres and large particles at low concentrations. In contrast, for B. japonicum in the experiment with small particles, the population growth rate was positive. Similar to Blepharisma, an even more pronounced result was observed for Euplotes sp., with PGR values reaching µ = 0.2–0.4 in the experiment with small plastic particles (Fig. 1e, f).
The growth rate of B. japonicum was influenced by both the concentration and size of the microbeads (Table 1). A high PGR was observed at low concentration of small microbeads (Fig. 1e). A post hoc test showed that there was no difference between the low and high concentrations, but a highly significant difference (P ≤ 0.0001) was observed between the remaining treatments. The effect of size was most pronounced at lower PGR levels in the large bead treatment (Fig. 2c, f).
The PGR of Euplotes sp. was significantly dependent on exposure time, microbead concentration and size, as well as on the interaction between exposure time and microbead size (Table 1). The results show a difference in the PGR between the treatments with two different MP sizes, with higher PGR values observed after exposure to smaller microbeads. The post hoc Tukey test was performed to assess between which exposure times the PGR differed. The results show a significant difference between 24 and 48 h (P = 0.0005) and between 24 and 72 h (P = 0.0015). There was no significant difference between 48 and 72 h of exposure. Due to interaction effects, this is only applicable when considering the size of the MPs. In addition, the same post hoc test was performed to determine differences between concentrations. In this case, no difference was found between the control and low concentrations, but significant differences (P < 0.0001) were found between the control and high concentrations and between the low concentration and high concentrations (Fig. 2b, e, h).
For S. teres, the PGR was significantly dependent on concentration and the interaction between concentration and particle size (Table 1). The post hoc Tukey test showed significant differences between the control and low concentrations (P = 0.0026) and between the low and high concentrations (P = 0.0015) (Fig. 2h). Because of the interaction involved, it is crucial to consider the effect of concentration in relation to microparticle size. At low concentrations, there seems to be no difference between the PGR observed for both microparticle sizes. Conversely, at high concentrations, the PGR was lower in the treatment with smaller beads (Fig. 2i).
Plastic microbead uptake
The results of the experiment showed that ciliates readily ingested microplastic particles (Fig. 3). In the case of Euplotes sp. and S. teres, both ciliate species showed positive ingestion of microbeads in all experimental setups. In contrast, no beads were found in the food vacuoles of some individuals of B. japonicum at low concentrations of large microbeads after 5 min of exposure. The maximum number of ingested particles varied among species. For Euplotes sp., the highest number of MPs was achieved at the high concentration at 60-min exposure time with small beads, reaching 280.83 ± 150.42 beads per individual.
For B. japonicum and S. teres, MP uptake peaked at low concentrations of smaller beads at 60 min of exposure time, reaching 779.28 ± 371.10 and 883.11 ± 521.47 beads, respectively (Fig. 4d).
In B. japonicum, the number of microbeads ingested was significantly influenced by the variables individually as well as by their first-order interactions (Table 2). Grazing was greater for beads of smaller size as well as for beads at high concentrations. For the low concentration, the number of ingested beads increased with exposure time, whereas for the high concentration, the number of ingested MPs peaked at 30 min and decreased thereafter. Moreover, a similar effect can be observed for large MPs, where ingestion peaked at 30 min decrease thereafter. For small beads, uptake increased with increasing exposure time (Fig. 5). Furthermore, the Tukey test showed that ingestion at the 5-min exposure time was significantly different from ingestion at 30 and 60 min (P < 0.0001).
The uptake of MPs by Euplotes sp. was mainly determined by exposure time, sphere size, and their interaction. The effect of concentration was only significant in the interaction with exposure time (Table 2). The number of beads ingested was generally greater for small beads than for large beads at each concentration. In the experiment with smaller microbeads, the number of MPs ingested increased with exposure time, but for exposure to large microbeads, ingestion did not increase with longer times. A similar result is observed for the effect of concentration, with the number of ingested beads increasing with longer exposure times (Fig. 5). In addition, a post hoc Tukey test was performed to test which exposure times differed from each other. The results show that the number of ingested MPs differed between all exposure times.
The grazing of S. teres on MPs was dependent on time, concentration and size of MPs, the interaction of concentration and size of MPs and most importantly the interaction of all three factors (Table 2). The results show an increasing number of microspheres ingested with increasing exposure time, with the highest number of beads ingested at 60 min. In addition, a post hoc test showed that there was a significant difference in the number of MPs ingested at each exposure time. Finally, small beads were preferred by this ciliate and ingested in greater numbers than large beads (Fig. 5b).
Clearance rate
Microplastics were cleared at the highest rate by S. teres at low concentrations at 5 min of exposure time for small and large particles, reaching 4.95 × 10−5 and 8.06 × 10−6, respectively (Fig. 6b, d). Euplotes sp. achieved the second highest clearance rate at 5 min of exposure at low concentrations, reaching 1.92 × 10−5 for small beads and 6.1 × 10−6 for large beads. The lowest clearance rate was observed for B. japonicum under the same conditions and reached 1.12 × 10−5 for small beads and 2.1 × 10−6 for large beads (Fig. 6b, d).
In B. japonicum, the clearance rate was significantly dependent on all three tested factors as well as their first-order interactions (Table 3). At the 5th minute of exposure, the clearance rate was highest for both concentrations. For the high concentration, the clearance rate gradually decreased with exposure time, whereas for the low concentration, the clearance rate was lowest in the 30th min and then increased in the 60th min. The post hoc test showed that the clearance rate at each exposure time was significantly different from each other (P = 0.0001). For large beads, the clearance rate was higher than for small beads (Fig. 6).
The clearance rate of Euplotes sp. was significantly influenced by exposure time, concentration, and size of the MPs. In addition, the interaction between time and concentration and time and particle size was also significant (Table 3). At lower particle concentrations, the clearance rate was highest at the 5th minute of exposure and then decreased at the 30th and 60th min. The same effect was observed at the high concentration. Post hoc tests showed no differences between the 30th and 60th min of exposure (P = 0.08). For small microbeads, the clearance rate decreased from the 5th to the 30th min and then increased at the 60th min. However, for large particles, the clearance rate decreased progressively (Fig. 6).
The clearance rate of S. teres was determined by the concentration and size of the microparticles as well as by the exposure time. In addition, the interaction of concentration and size as well as the second level interaction of time, concentration and size were also significant (Table 3). The clearance rate observed in the experiment with small beads was higher than that observed in the experiment with large beads in this species. A similar result was observed for concentration, with a higher clearance rate at high than low concentration. Finally, in each experimental condition, the clearance rate decreased with increasing exposure time, although the post hoc test showed no significant differences between the 30th and 60th minute of exposure (Fig. 7a–c).
The results show that the area of the ciliate cell correlates with the clearance rate for both Euplotes sp. and S. teres (Fig. 8a). In S. teres, larger organisms were found to graze at a higher rate than smaller individuals. In the case of Euplotes sp., the results show the opposite direction to S. teres, with smaller cells grazing more effectively. For B. japonicum, no significant relationship was found between grazing rate and cell area. Cell area was also significantly positively correlated with the number of beads ingested by each ciliate species (Fig. 8b).
Discussion
Population growth rate
This study was designed to determine the effect of MPs on the population growth rate of three ciliates: Blepharisma japonicum, Spirostomum teres, and Euplotes sp. For each experiment, two different sizes (1 µm and 2 µm) and concentrations (106 and 107 beads per mL) of MPs as well as three different time points were used to obtain a thorough picture of the influence of these factors on the viability of the ciliates studied. To date, the literature on the effects of MPs on ciliates remains scarce, and very limited sources are available on the topic of ciliate population growth rates.
To the best of the authors’ knowledge, no studies have investigated the growth rate of B. japonicum populations in response to MPs. The results showed that for B. japonicum, both MP size and concentration had a significant effect on the growth rate. Interestingly, the population growth rates in the experiment with small beads reached higher values (0.05) than in the experiment with larger beads, in which the population growth rate was negative and measured approximately − 0.05. The second major finding of the experiment was that the population growth rate in the control experiment was negative and much lower than that in the experiments with added microbeads at both concentrations. Although there is no literature on B. japonicum, a similar hormesis-like effect can be observed in different species. As observed by Chen et al. (2021), MPs had a positive effect on the growth of marine microalgae when their density was high and concentration per cell was lower. In addition, in a review of the hormetic effects of MPs, Sun et al. (2021) described the positive influence of MPs on the consumption, growth, reproduction and survival of aquatic organisms. It may seem intuitive that MPs, which have no nutritional value and can potentially cause blockage of the digestive tract and impaired feeding, would be harmful to ciliates, but as Paracelsus stated “the dose makes the poison,” and as highlighted in a review by Agathokleous et al. (2021), there may be no adverse effects on organisms below the toxicological threshold, as can be observed in B. japonicum.
Despite the interest in the effects of MPs on the genus Spirostomum, no studies have considered the effects of MPs on the growth rate of S. teres. As already mentioned in the results, the growth rate of the S. teres population was dependent on the concentration and its relationship with the size of the microparticles. The same population growth rate was observed for the control and high concentration of MPs, suggesting that there is no visible effect of exposure to a high concentration of MPs. These results are consistent with those of Chojnacka et al. (2023), who studied the joint effects of MPs and antidepressants on the ciliate Spirostomum ambiguum and found that MPs in the environment are unlikely to pose a threat to protozoa. Furthermore, Davarpanah and Guilhermino (2015) measured the specific mean growth rate of the green alga Tetraselmis chuii and found no significant effects of MPs. In contrast, in the present study, the population growth rate was significantly lower at low concentrations of beads compared to both the control and high concentrations, suggesting a negative effect of MPs on the viability of these species, in contrast to the previous findings of Nałęcz-Jawecki et al. (2021), who described a possible negative effect of nanoparticles on vacuole formation in S. ambiguum only when the toxicity threshold was exceeded. These conflicting results may be due to differences in particle concentrations, type of MPs or different ciliate species used in the two studies.
Several studies have found that MPs are harmful to aquatic organisms (Lee et al. 2013; Cole et al. 2013, 2015; Setälä et al. 2014; Canniff and Hoang 2018). In a study of the small planktonic crustacean Daphnia magna, the authors found that long-term exposure to MPs had negative effects on their reproduction and population growth rates, especially at elevated water temperature and light intensity (Guilhermino et al. 2021). These results are consistent with our findings that the population growth rate of Euplotes sp. decreased from the third day of the experiment and with increasing concentrations of MPs, although it should be noted that the experiments were conducted on different time scales. Furthermore, in a study on Euplotes vannus, the consumption of MPs significantly reduced the richness and carbon biomass of the populations (Wang et al. 2022). Furthermore, data presented by the same authors show different patterns in the detrimental effects of different microparticle sizes, with smaller particles being more detrimental, while our results indicate that larger particles had more severe effects on the population growth rate of Euplotes sp. Finally, in a study by Shore et al. (2021), MPs were found to reduce the body length and survival of the marine copepod Acartia tonsa, with estimates of over 15% reduction in population growth.
Plastic microbead uptake
The secondary aim of this study was to determine the maximum ingestion capacity of MPs by the three ciliate species studied. As in the previous experiment, two different sizes and concentrations of MPs and three different time points were used to determine the detailed differences in the ingestion of MPs by these species. Previous work in this area has focused primarily on the ingestion of natural foods, and it has not yet been established how many microspheres can be ingested by the species studied in this experiment.
Of all the possible interactions between MPs and aquatic organisms, ingestion is one of the most likely. The microscopic size range of MPs is the reason why they could be ingested by a wide range of biota in aquatic ecosystems. Sometimes organisms are unable to discriminate between natural prey and artificial food (Moore et al. 2001). Furthermore, when MPs are present at high concentrations compared to natural food, organisms may graze on them unintentionally and ingest them accidentally or simply fail to prevent ingestion (Moore 2008; Lusher 2015). During feeding in filtering ciliates, food particles are concentrated by the filtering apparatus and then digested in a food vacuole. In this study, it was observed that ingested MPs were not digested by ciliates and were expelled in the form of compacted masses after approximately one hour. The egestion of MPs in the form of packets filled with smaller particles has also been observed in previous studies (Mueller et al. 1965; Cole et al. 2013). The concentration of food in the environment affects both of the above processes, since both require unrestricted access to food. In filtering ciliates, the concentration of food is the limiting factor if the filtering apparatus cannot collect particles as fast as food vacuoles can be formed. However, if food is ingested at a faster rate than food vacuole formation and lysosomal digestion can handle it, then concentration can be considered a non-limiting factor (Zubkov and Sleigh 1995). In Euplotes sp., the number of microspheres ingested depended on the time of exposure and the size of the MPs, as well as the interactions of time with size and concentration. The ingestion of MPs increased with time in almost all experimental setups, except for exposure to low concentrations of large beads, where the highest number was reached at 30 min. In addition, in the B. japonicum experiment, the number of ingested beads increased with time only at the low concentration, whereas at the high concentration, the number of ingested beads peaked at 30 min for both MP sizes. Similar results, where equilibrium is reached after some exposure time and then the number of ingested particles decreases, have been obtained in experiments with other ciliate species (Nugroho and Fyda 2020). A reasonable explanation for this result is that, as explained by Zubkov and Sleigh (1995), particle concentration was not a limiting factor. However, the number of beads ingested by S. teres increased with increasing exposure time for both concentrations and sizes of MPs. Most likely, these results could be explained by a limit to the capacity of the ciliate food vacuole that must not be exceeded, as in the case of B. japonicum and Euplotes sp. However, in the case of S. teres, the spheres did not reach a critical point as ingestion continued to increase.
All three ciliate species tested are bacterivorous but can also feed on flagellates, algae and other ciliates (Foissner et al. (1991, 1992, 1999). The diet of B. japonicum includes a variety of smaller organisms among them bacteria, algae, rotifers, and other ciliates, even individuals of the same species, so prey size varies from 0.5 to 139 µm (Giese 1938). On the other hand, Spirostomum teres ingests particles ranging from approximately 0.5 to 100 µm (Finlay and Esteban 1998; Fernandes and da Silva-Neto 2013; Boscaro et al. 2014). Euplotes generally feeds on prey from 0.5 to 2 µm but is capable of ingesting larger particles up to 10 µm (Wilks and Sleigh 1998, 2008). For filtering organisms, a clear relationship was found between their morphology and particle size. The minimum particle size is mainly determined by the shape and size of the filtering apparatus. The maximum size of ingested particles is determined by the morphology of the mouthparts (Scherer et al. 2018). Therefore, differences in ingestion may be due to differences in the mechanical properties of the ciliate cytostome, which captures suitable prey and differs in structure in the studied species.
Particle size preference
Another interesting aspect that emerged from the analysis is that the number of particles ingested is significantly related to both the size of the ciliate and the size of the MPs, with smaller beads being ingested in much greater quantities than the larger beads used in this experiment. Despite the fact that ciliates in general show food selectivity under natural conditions, the sizes of plastic microbeads used in the experiment overlap with the usual prey sizes of each ciliate. Furthermore, previous studies have not addressed particle size preference in B. japonicum and S. teres, but we have obtained satisfactory results showing that particles of 1 µm in diameter are preferred over particles of 2 µm in both species. The same results were obtained in the experiment with Euplotes sp. Our data showed a different trend from that observed by Wilks and Sleigh (1998), who studied the ingestion of microspheres as a function of their size and concentration in the ciliate Euplotes mutabilis. In their work, the preference was shifted towards larger particles and confirmed the results of Gonzalez et al. (1990), who showed the preference of ciliates for larger bacteria.
Clearance rate
During the experiment, we measured the clearance rates of the three investigated species at two different concentrations, three time points, and two particle sizes. The analysis confirmed that the clearance rate was significantly dependent on the factors studied and their interactions. Børsheim (1984) used monodisperse fluorescent latex particles with diameters of 0.57 and 1.04 µm to measure the clearance rates of bacteria-sized particles by two ciliates from a Norwegian lake. The clearance rates of these particles ranged from 0.23 to 1.26 µL ind−1 h−1 in the case of Epistylis rotans and from 0.26 to 0.90 µL ind−1 h−1 for Strombidium sp. Clearance rates varied with food content and temperature.
Our results show that for each species, the clearance rate was highest at the lowest concentration and shortest exposure time. The clearance rate generally decreased with increasing exposure time for both concentrations but was higher for smaller beads for all species. However, for B. japonicum and Euplotes sp. in the experiment with the low concentration of small beads, the clearance rate was lowest at the 30th minute of incubation. To date, research and reports confirm that the consumption rate of natural food is consistent with estimates of the consumption rate of synthetic particles and that only a limited preference for real cells can be observed compared to inert microspheres (Mueller et al. 1965; Fenchel 1980; Wilks and Sleigh 2008; Weisse et al. 2021).
These results are consistent with those of Peters (1984), who found that the clearance rate of planktonic organisms gradually decreased after peaking, even though food concentrations increased. Similar conclusions were drawn by Nugroho and Fyda (2020) in their experiment with the ciliate Paramecium aurelia. Furthermore, our results support Fenchel’s (1980) hypothesis that ciliates have different food preferences that may affect particle ingestion and that their clearance rate decreases as they ingest larger particles. However, for the species used in this experiment, the 1- and 2-µm-diameter microbeads used are an optimal size. Slightly different results were reported by Wilks and Sleigh (2008), who observed that smaller particles, approximately 1 µm in diameter, were ingested in large numbers but less efficiently than larger particles.
However, patterns of MP ingestion by filter-feeding ciliates should not be generalised, as laboratory conditions differ significantly from natural ones, where MPs are not perfectly spherical, absorb chemicals from the environment, harbour bacterial biofilms and can aggregate with each other. For example, Pajdak-Stós et al. (2023) showed that the rotifer Lecane inermis preferentially ingests microplastics embedded in biofilm, and that MP aggregation is significantly enhanced by the presence of biofilm, and further enhanced in the presence of rotifers. Moreover, the presence of microplastics does not affect growth and fecundity of the rotifers. This confirms that there are complex and intricate relationships between organisms in the environment that influence the interaction between ciliates and MPs (Scherer et al. 2018).
Interesting results confirming the relationship between preferred food particle size and the size of planktonic ciliates were found by Kivi and Setälä (1995). In their experiments, the authors observed that each of the nine species tested had a specific particle size preference pattern. For example, the optimal particle size varied from 1.4 µm for Strombidium sp., which is 20 µm in size, to 9.8 µm for Strobilidium sp., which is 40 µm in size. The authors conclude that most ciliate species were able to effectively ingest nanoflagellate-sized prey, but only two of the ciliates studied showed effective grazing on the smallest particles, suggesting a possible ability to utilise bacteria-sized prey (Kivi and Setälä 1995).
Interestingly, it was also observed that the grazing rates of Euplotes sp. and S. teres were significantly correlated with their cell size. In the case of Euplotes sp., the larger the cell, the lower the grazing rate, whereas the relationship between ingestion rate and S. teres cell size was positive. These contradictory results may be due to differences in the structure of the oral apparatus as well as differences in locomotion. The clearance rate was not significantly dependent on the cell size of B. japonicum. These results differ slightly from those of Fenchel (1980), who reported that the clearance rates of Euplotes or Blepharisma were dependent on cell volume.
Conclusions
Our results suggest that MPs are readily ingested by ciliates and could pose a threat to aquatic organisms such as some ciliate species and other zooplankton taxa and that these effects are pronounced when MP concentrations exceed those found in the natural environment, which are e.g. 22.0 ± 0.4 particles m−3 on average along the Biobío river basin located in Chile (Correa-Araneda et al. 2022) or 100,000 particles m−3 in the Dutch river delta and Amsterdam canals (Leslie et al. 2017). In addition, the ingestion of MPs depends on exposure time, but longer times do not always result in higher ingestion of MP particles. Furthermore, ingestion was also influenced by the diameter of the particles and the size of the ciliate. In our study, ciliates showed a preference for particles with a diameter of 1 µm over 2 µm. Additionally, the highest clearance rates were observed at the lowest concentrations and exposure times. The grazing rate was higher for smaller particles than for larger MPs and generally decreased with increasing incubation time.
This work provides new insights into the study of zooplankton communities and their very important members, ciliates. We have obtained satisfactory results showing that MPs are a serious problem that needs to be investigated in more detail. These results are a further step towards understanding the complex nature of MP pollution in the environment.
Data availability
Data available under reasonable request.
References
Agathokleous E, Iavicoli I, Barceló D, Calabrese EJ (2021) Micro/nanoplastics effects on organisms: A review focusing on ‘dose.’ J Hazard Mater 417:126084. https://doi.org/10.1016/j.jhazmat.2021.126084
Amélineau F, Bonnet D, Heitz O, Mortreux V, Harding AMA, Karnovsky N, Walkusz W, Fort J, Grémillet D (2016) Microplastic pollution in the Greenland Sea: background levels and selective contamination of planktivorous diving seabirds. Environ Pollut 219:1131–1139. https://doi.org/10.1016/j.envpol.2016.09.017
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci 364:1977–1984. https://doi.org/10.1098/rstb.2008.0304
APME (2006) An analysis of plastics production, demand and recovery in Europe. Association of Plastics Manufacturers, Brussels
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond B Biol Sci 364:1985–1998. https://doi.org/10.1098/rstb.2008.0205
Bernard C, Rassoulzadegan F (1990) Bacteria or microflagellates as a major food source for marine ciliates: possible implications for the microzooplankton. Mar Ecol Prog Ser 64:147–155. https://doi.org/10.3354/meps064147
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 20:12336–12343. https://doi.org/10.1021/es503001d
Boscaro V, Carducci D, Barbieri G, Senra MVX, Andreoli I, Erra F, Petroni G, Verni F, Fokin SI (2014) Focusing on genera to improve species identification: revised systematics of the ciliate Spirostomum. Protist 165(4):527–541. https://doi.org/10.1016/j.protis.2014.05.004
Boucher J, Friot D (2017) Primary microplastics in the oceans: A global evaluation of sources. IUCN, Gland 43. https://doi.org/10.2305/IUCN.CH.2017.01.en
Børsheim KY (1984) Clearance rates of bacteria-sized particles by freshwater ciliates, measured with monodisperse fluorescent latex beads. Oecologia 63:286–288. https://doi.org/10.1007/BF00379891
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031. https://doi.org/10.1021/es800249a
Bulannga RB, Schmidt S (2022) Uptake and accumulation of microplastic particles by two freshwater ciliates isolated from a local river in South Africa. Environ Res 204:112123. https://doi.org/10.1016/j.envres.2021.112123
Canniff PM, Hoang TC (2018) Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci Total Environ 633:500–507. https://doi.org/10.1016/j.scitotenv.2018.03.176
Carpenter EJ, Anderson SJ, Harvey GR, Miklas HP, Peck BB (1972) Polystyrene spherules in coastal waters. Science 178(4062):749–750. https://doi.org/10.1126/science.178.4062.749
Carpenter EJ, Smith KL (1972) Plastics on the Sargasso sea surface. Science 175(4027):1240–1241. https://doi.org/10.1126/science.175.4027.1240
Chen Z, Li L, Hao L, Hong Y, Wang W (2021) Hormesis-like growth and photosynthetic physiology of marine diatom Phaeodactylum tricornutum Bohlin exposed to polystyrene microplastics. Front Environ Sci Eng 16(2). https://doi.org/10.1007/s11783-021-1436-0
Chojnacka J, Drobniewska A, Lenga W, Misztal J, Wawryniuk M, Nałęcz-Jawecki G (2023) The mutual effect of microparticles and antidepressants on the protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 15(3):552. https://doi.org/10.3390/w15030552
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47(12):6646–6655. https://doi.org/10.1021/es400663f
Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS (2015) The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol 49(2):1130–1137. https://doi.org/10.1021/es504525u
Correa-Araneda F, Pérez J, Tonin AM, Esse C, Boyero L, Díaz ME, Figueroa R, Santander-Massa R, Cornejo A, Link O, Jorquera E, Urbina MA (2022) Microplastic concentration, distribution and dynamics along one of the largest Mediterranean-climate rivers: a whole watershed approach. Environ Res 209:112808. https://doi.org/10.1016/j.envres.2022.112808
Davarpanah E, Guilhermino L (2015) Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar Coast Shelf Sci 167:269–275. https://doi.org/10.1016/j.ecss.2015.07.023
Derraik JGB (2002) The pollution of the marine environment by plastic debris: A review. Mar Pollut Bull 44(9):842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2016) Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA Journal, 14(6). https://doi.org/10.2903/j.efsa.2016.4501
Engler RE (2012) The complex interaction between marine debris and toxic chemicals in the ocean. Environ Sci Technol 46(22):12302–12315. https://doi.org/10.1021/es3027105
Fenchel T (1980) Suspension feeding in, ciliated protozoa: structure and function of feeding organelles. Arch Protistenkd 123(3):239–260. https://doi.org/10.1016/S0003-9365(80)80009-1
Fernandes N, da Silva-Neto I (2013) Morphology and 18S rDNA gene sequence of Spirostomum minus and Spirostomum teres (Ciliophora: Heterotrichea) from Rio de Janeiro, Brazil. Zoologia 30:72–79. https://doi.org/10.1590/S1984-46702013000100009
Finlay BJ, Esteban GF (1998) Freshwater protozoa: biodiversity and ecological function. Biodivers Conserv 7(9):1163–1186. https://doi.org/10.1023/A:1008879616066
Foissner W, Blatterer H, Berger H, Kohmann F (1991) Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. In: Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, München, Germany
Foissner W, Berger H, Kohmann F (1992) Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems Band II: Peritrichia, Heterotrichida, Odontostomatida. In: Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, München, Germany
Foissner W, Berger H, Schaumburg J (1999) Identification and ecology of limnetic plankton ciliates. In Informationsberichte des Bayer, Landesamtes für Wasserwirtschaft, München, Germany
Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15(10):1949. https://doi.org/10.1039/c3em00214d
Fyda J, Wiackowski K (1998) Benefits and costs of predator-induced morphological changes in the ciliate Colpidium kleini (Protozoa, Ciliophora). Eur J Protistol 34:118–123. https://doi.org/10.1016/S0932-4739(98)80021-7
Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5):0116. https://doi.org/10.1038/s41559-017-0116
Giese AC (1938) Cannibalism and gigantism in Blepharisma. Trans Am Microsc Soc 57(3):245. https://doi.org/10.2307/3222693
Gonzalez JM, Sherr EB, Sherr BF (1990) Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol 56(3):583–589. https://doi.org/10.1128/aem.56.3.583-589.1990
Guerranti C, Martellini T, Perra G, Scopetani C, Cincinelli A (2019) Microplastics in cosmetics: environmental issues and needs for global bans. Environ Toxicol Pharmacol 68:75–79. https://doi.org/10.1016/j.etap.2019.03.007
Guilhermino L, Martins A, Cunha S, Fernandes JO (2021) Long-term adverse effects of microplastics on Daphnia magna reproduction and population growth rate at increased water temperature and light intensity: combined effects of stressors and interactions. Sci Total Environ 784:147082. https://doi.org/10.1016/j.scitotenv.2021.147082
Issac MN, Kandasubramanian B (2021) Effect of microplastics in water and aquatic systems. Environ Sci Pollut Res 28(16):19544–19562. https://doi.org/10.1007/s11356-021-13184-2
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Zhou B, Souissi S, Lee SJ, Lee JS (2016) Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ Sci Technol 50(16):8849–8857. https://doi.org/10.1021/acs.est.6b01441
Kivi K, Setälä O (1995) Simultaneous measurement of food particle selection and clearance rates of planktonic oligotrich ciliates (Ciliophora: Oligotrichina). Marine Ecology Progress Series Oldendorf 119(1):125–137
Lee KW, Shim WJ, Kwon OY, Kang JH (2013) Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ Sci Technol 47(19):11278–11283. https://doi.org/10.1021/es401932b
Leslie HA, Brandsma SH, van Velzen MJM, Vethaak AD (2017) microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ Int 101:133–142. https://doi.org/10.1016/j.envint.2017.01.018
Lobelle D, Cunliffe M (2011) Early microbial biofilm formation on marine plastic debris. Mar Pollut Bull 62(1):197–200. https://doi.org/10.1016/j.marpolbul.2010.10.013
Lusher A (2015) Microplastics in the marine environment: distribution, interactions and effects. In: Bergmann M, Gutow L, Klages M (eds) Marine Anthropogenic Litter. Springer, Cham. https://doi.org/10.1007/978-3-319-16510-3_10
Lusher AL, McHugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1–2):94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Moore CJ (2008) Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res 108(2):131–139. https://doi.org/10.1016/j.envres.2008.07.025
Moore CJ, Moore SL, Leecaster MK, Weisberg SB (2001) a comparison of plastic and plankton in the North Pacific Central Gyre. Mar Pollut Bull 42(12):1297–1300. https://doi.org/10.1016/S0025-326X(01)00114-X
Mueller M, Röhlich P, Törö I (1965) Studies on feeding and digestion in protozoa VII Ingestion of polystyrene latex particles and its early effect on acid phosphatase in Paramecium multimicronucleatum and Tetrahymena pyriformis. J Protozool 12(1):27–34. https://doi.org/10.1111/j.1550-7408.1965.tb01807.x
Nałęcz-Jawecki G, Chojnacka J, Wawryniuk M, Drobniewska A (2021) Influence of nano- and small microplastics on ciliated protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 13(20):2857. https://doi.org/10.3390/w13202857
Nugroho F, Fyda J (2020) Uptake of plastic microbeads by ciliate Paramecium aurelia. Sci Tech Innov 9(2):1–9. https://doi.org/10.13140/RG.2.2.16735.69289
Pajdak-Stós A, Fiałkowska E, Hajdyła F, Fiałkowski W (2023) The potential of Lecane rotifers in microplastics removal. Sci Total Environ 899. https://doi.org/10.1016/j.scitotenv.2023.165662
Peters R (1984) Methods for the study of feeding, filtering and assimilation by zooplankton. In: Downing J (ed) A manual for the assessment of secondary production in fresh waters, 2nd edn. Blackwell Scientific Publications, Oxford, pp 336–395
Pierce R, Turner J (1992) Ecology of plankton ciliates in marine food webs. Rev Aquat Sci 6(2):139–181
Plastics Europe (2010) Plastics—the facts 2010. Plastics Europe. https://plasticseurope.org/knowledge-hub/plastics-the-facts-2010. Accesed 21 May 2023
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rochman CM, Brookson C et al (2019) Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem 38(4):703–711. https://doi.org/10.1002/etc.4371
Scherer C, Weber A, Lambert S, Wagner M (2018) Interactions of microplastics with freshwater biota. In: Wagner M, Lambert S (eds.) Freshwater microplastics. The Handbook of Environmental Chemistry, Springer, Cham, 153–180. https://doi.org/10.1007/978-3-319-61615-5_8
Setälä O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83. https://doi.org/10.1016/j.envpol.2013.10.013
Shore EA, deMayo JA, Pespeni MH (2021) Microplastics reduce net population growth and fecal pellet sinking rates for the marine copepod. Acartia Tonsa Environ Pollut 284:17379. https://doi.org/10.1016/j.envpol.2021.117379
Sivan A (2011) New perspectives in plastic biodegradation. Curr Opin Biotechnol 22(3):422–426. https://doi.org/10.1016/j.copbio.2011.01.013
Stienbarger CD, Joseph J, Athey SN, Monteleone B, Andrady AL, Watanabe WO, Seaton P, Taylor AR, Brander SM (2021) Direct ingestion, trophic transfer, and physiological effects of microplastics in the early life stages of Centropristis striata, a commercially and recreationally valuable fishery species. Environ Pollut 285:117653. https://doi.org/10.1016/j.envpol.2021.117653
Strickler JR (1982) Calanoid copepods, feeding currents, and the role of gravity. Science 218:4568. https://doi.org/10.1126/science.218.4568.158
Sun T, Zhan J, Li F, Ji C, Wu H (2021) Effect of microplastics on aquatic biota: a hormetic perspective. Environ Pollut 285:117206. https://doi.org/10.1016/j.envpol.2021.117206
Teuten E, Saquing J, Knappe D, Barlaz M, Jonsson S, Björn A, Rowland S, Thompson R, Galloway T, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet P, Tana T, Prudente M, Boonyathumanond R, Zakaria M, Akkhavong K, Takada H (2009) Transport and release of chemicals from plastic to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci 364:2027–2045. https://doi.org/10.1098/rstb.2008.0284
Thompson RC, Swan SH, Moore CJ, vom Saal FS (2009) Our plastic age. Philos Trans R Soc Lond B Biol Sci 364(1526):1973–1976. https://doi.org/10.1098/rstb.2009.0054
Vandermeersch G, Van Cauwenberghe L, Janssen CR, Marques A, Granby K, Fait G, Kotterman MJJ, Diogène J, Bekaert K, Robbens J, Devriese L (2015) A critical view on microplastic quantification in aquatic organisms. Environ Res 143:46–55. https://doi.org/10.1016/j.envres.2015.07.016
Velzeboer I, Kwadijk CJAF, Koelmans AA (2014) Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ Sci Technol 48(9):4869–4876. https://doi.org/10.1021/es405721v
Wang Y, Liu M, Geng X, Zhang Y, Jia R, Zhang Y, Wang X, Jiang Y (2022) The combined effects of microplastics and the heavy metal cadmium on the marine periphytic ciliate Euplotes vannus. Environ Pollut 308:119663. https://doi.org/10.1016/j.envpol.2022.119663
Weis JS (2020) Aquatic microplastic research—a critique and suggestions for the future. Water 12(5):1475. https://doi.org/10.3390/w12051475
Weisse T, Jezberova J, Moser M (2021) Picoplankton feeding by the ciliate Vorticella similis in comparison to other peritrichs emphasizes their significance in the water purification process. Ecol Indic 121:106992. https://doi.org/10.1016/j.ecolind.2020.106992
Wilks SA, Sleigh MA (1998) Grazing rates in Euplotes mutabilis: relationship between particle size and concentration. Microb Ecol 36(2):165–174. https://doi.org/10.1007/s002489900103
Wilks SA, Sleigh MA (2008) Feeding by Euplotes mutabilis (Ciliophora, Hypotrichida). Denisia 23:383–388
Wirtz K (2012) Who is eating whom? Morphology and feeding type determine the size relation between planktonic predators and their ideal prey. Mar Ecol Prog Ser 445:1–12. https://doi.org/10.3354/meps09502
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: A review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Zarfl C, Matthies M (2010) Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar Pollut Bull 10:1810–1814. https://doi.org/10.1016/j.marpolbul.2010.05.026
Zubkov MV, Sleigh MA (1995) Ingestion and assimilation by marine protists fed on bacteria labeled with radioactive thymidine and leucine estimated without separating predator and prey. Microb Ecol 30:157–170. https://doi.org/10.1007/BF00172571
Acknowledgements
We thank the two anonymous reviewers for their valuable comments on an earlier version of the manuscript.
Funding
This work was funded by the BioS Priority Research Area of the Excellence Initiative—Research University programme at Jagiellonian University in Kraków.
Author information
Authors and Affiliations
Contributions
Janusz Fyda was mainly responsible for the idea of the study. Together with Martyna Budziak, they developed and implemented the methodology and carried out the experiments. Martyna Budziak wrote the first draft of the manuscript. Janusz Fyda read and revised the manuscript, and both authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable, research does not report on or involve the use of any animal or human data or tissue.
Consent to participate
Not applicable.
Consent for publication
The authors consent for publication of the manuscript in Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Budziak, M., Fyda, J. Effect of microplastic particles on the population growth rate and clearance rate of selected ciliates (Protista, Ciliophora). Environ Sci Pollut Res 31, 6907–6921 (2024). https://doi.org/10.1007/s11356-023-31635-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31635-w