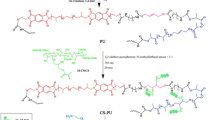
Abstract
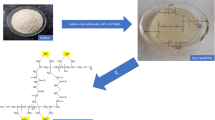
The growing concerns about water pollution have prompted researchers to explore new materials for remediating and purifying it. In recent years, there has been a focus on polysaccharides as eco-friendly polymers that exhibit high efficiency in removing chlorophenols from waste water. This study aims to develop a trifunctional polysaccharide structure using a biodegradable matrix. The chitosan/alginate-polyethyleneimine-phenyl-phosphonamidic acid (CHIT/ALG-PEIPPAA) matrix was employed for removing chlorophenols from water. The study carefully examined the impact of various physicochemical parameters such as pH, reaction time, chlorophenols concentration, temperature, and ionic strength to determine the optimal conditions for the adsorption process. Several techniques were used to confirm the morphology, physicochemical properties, structure, and functionalization of the polymer. Scanning electron microscopy (SEM) images revealed a heterogeneous morphology with agglomerates of different particle sizes, ranging from a few micrometers with irregular shapes. The FTIR spectrum and zeta potential characterization indicated the presence of hydrophilic groups and a highly positive charge (around 31.4 mV) on the surface of the CHIT/ALG-PEIPPAA adsorbent. The optimal pH for chlorophenols removal was found to be approximately 4.4. The kinetic data supported the pseudo-second-order kinetic model, which accurately described the adsorption behavior of both chlorophenol molecules. The fitting of the isotherm analysis revealed that the Langmuir model provided a better representation of the adsorption process. The maximum adsorption capacities for 4-chlorophenol and 2,4-chlorophenol were approximately 118 mg.g−1 and 249 mg.g−1, respectively. The calculated thermodynamic functions confirmed an exothermic and spontaneous adsorption process for chlorophenols, with ∆H values of -6.98 kJ.mol−1 and −2.74 kJ.mol−1 for 4-chlorophenol and 2,4-chlorophenol, respectively. The regeneration process of the CHIT/ALG-PEIPPAA adsorbent showed higher efficacy in the presence of hydrochloric acid (2.0 mol.L−1), resulting in up to 91% desorption of chlorophenols. The CHIT/ALG-PEIPPAA adsorbent demonstrated good reusability after regeneration, with only a slight decrease in extraction efficiency: 34.63% for 4-chlorophenol and 79.03% for 2,4-chlorophenol, under the same optimal conditions as the initial adsorption cycle.
Graphical abstract













Similar content being viewed by others
Data availability
All results and data generated or analyzed during this study are included in this published research article.
References
Abdulhameed AS, Jawad AH, Ridwan M, Khadiran T, Wilson LD, Yaseen ZM (2022) Chitosan/carbon-doped TiO2 composite for adsorption of two anionic dyes in solution and gaseous SO2 capture: experimental modeling and optimization. J Polym Environ 30(11):4619–4636. https://doi.org/10.1007/s10924-022-02532-z
Adewuyi A, Göpfert A, Adewuyi OA, Wolff T (2016) Adsorption of 2-chlorophenol onto the surface of underutilized seed of Adenopus breviflorus: a potential means of treating waste water. J Environ Chem Eng 4(1):664–672. https://doi.org/10.1016/j.jece.2015.12.012
Ahmed MH, Byrne JA, McLaughlin JAD, Elhissi A, Ahmed W (2013) Comparison between FTIR and XPS characterization of amino acid glycine adsorption onto diamond-like carbon (DLC) and silicon doped DLC. Appl Surf Sci 273:507–514. https://doi.org/10.1016/j.apsusc.2013.02.070
Ahmed MJ, Theydan SK (2013) Adsorption of p-chlorophenol onto Microporous activated carbon from Albizia lebbeck seed pods by one-step microwave assisted activation. J Anal Appl Pyrolysis 100:253–260. https://doi.org/10.1016/j.jaap.2013.01.008
Ahmed MJ, Hameed BH, Hummadi EH (2020) Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohydr Polym 247:116690. https://doi.org/10.1016/j.carbpol.2020.116690
Ali S, Tanweer MS, Alam M (2020) Kinetic, isothermal, thermodynamic and adsorption studies on Mentha piperita using ICP-OES. Surf Interfaces 19:100516. https://doi.org/10.1016/j.surfin.2020.100516
Aslam S, Asgher M, Khan NA, Bilal M (2021) Immobilization of Pleurotus nebrodensis WC 850 laccase on glutaraldehyde cross-linked chitosan beads for enhanced biocatalytic degradation of textile dyes. J Water Process Eng 40:101971. https://doi.org/10.1016/j.jwpe.2021.101971
Atangana E, Oberholster PJ (2020) Mathematical modeling and stimulation of thermodynamic parameters for the removal for Cr6+ from wastewater using chitosan cross linked glutaraldehyde adsorbent. Alexandria Eng J 59(4):1931–1939. https://doi.org/10.1016/j.aej.2019.12.012
Balea A, Monte MC, Fuente E, Sanchez-Salvador JL, Blanco A, Negro C (2019) Cellulose nanofibers and chitosan to remove flexographic inks from wastewaters. Environ Sci Water Res Technol 5:1558–1567. https://doi.org/10.1039/C9EW00434C
Beltrame KK, Cazetta AL, de Souza PS, Spessato L, Silva TL, Almeida VC (2018) Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol Environ Saf 147:64–71. https://doi.org/10.1016/j.ecoenv.2017.08.034
Bensalah J, Habsaoui A, Dagdag O, Lebkiri A, Ismi I, Rifi EH et al (2021) Adsorption of a cationic dye (Safranin) by artificial cationic resins AmberliteŪIRC-50: equilibrium, kinetic and thermodynamic 564 study. Chem Data Collect 35:100756. https://doi.org/10.1016/j.cdc.2021.100756
Bilal M, Jing Z, Zhao Y, Iqbal HM (2019) Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal Agri Biotechnol 19:101174. https://doi.org/10.1016/j.bcab.2019.101174
Chen C, Geng X, Huang W (2017) Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem Eng J 327:941–952. https://doi.org/10.1016/j.cej.2017.06.183
Dabrowski A, Podkościelny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon – a critical review. Chemosphere 58:1049–1070. https://doi.org/10.1016/j.chemosphere.2004.09.067
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustainable Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Elfving J, Kauppinen J, Jegoroff M, Ruuskanen V, Järvinen L, Sainio T (2021) Experimental comparison of regeneration methods for CO2 concentration from air using amine-based adsorbent. Chem Eng J 404:126337. https://doi.org/10.1016/j.cej.2020.126337
Ettala M, Koskela J, Kiesilä A (1992) Removal of chlorophenols in a municipal sewage treatment plant using activated sludge. Water Res 26(6):797–804. https://doi.org/10.1016/0043-1354(92)90011-R
Fan C, Li N, Cao X (2015) Determination of chlorophenols in honey samples using in situ ionic liquid-dispersive liquid–liquid micro extraction as a pretreatment method followed by high-performance liquid chromatography. Food Chem 174:446–451. https://doi.org/10.1016/j.foodchem.2014.11.050
Farghali RA, Sobhi M, Gaber SE, Ibrahim H, Elshehy E (2020) Adsorption of organochlorine pesticides on modified porous Al30/bentonite: kinetic and thermodynamic studies. Arab J Chem 13(8):6730 6740. https://doi.org/10.1016/j.arabjc.2020.06.027
Ferrah N, Abderrahim O, Didi MA, Villemin D (2011) Removal of copper ions from aqueous solutions by a new sorbent: polyethyleneiminemethylene phosphonic acid. Desalination 269(1-3):17–24. https://doi.org/10.1016/j.desal.2010.11.035
Ferrah N (2018) Comparative study of mercury (II) species removal onto naked and modified magnetic chitosan flakes coated ethylenediaminetetraacetic-disodium: kinetic and thermodynamic modeling. Environ Sci Pollut Res 25(25):24923–24938. https://doi.org/10.1007/s11356-018-2553-6
Ferrah N, Merghache D, Meftah S, Benbellil S (2022) A new alternative of a green polymeric matrix chitosan/alginate-polyethyleniminemethylenephosphonic acid for pharmaceutical residues adsorption. Environ Sci Pollut Res 29(9):13675–13687. https://doi.org/10.1007/s11356-021-16599-z
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24(11):4605–4609. https://doi.org/10.1007/s10570-017-1450-3
Fu J, Chen Z, Wang M, Liu S, Zhang J, Zhang J et al (2015) Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): kinetics, isotherm, thermodynamics and mechanism analysis. Chem Eng J 259:53–61. https://doi.org/10.1016/j.cej.2014.07.101
Garba ZN, Zhou W, Lawan I, Xiao W, Zhang M, Wang L et al (2019) An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review. J Environ Manag 241:59–75. https://doi.org/10.1016/j.jenvman.2019.04.004
Giagnorio M, Steffenino S, Meucci L, Zanetti MC, Tiraferri A (2018) Design and performance of a nanofiltration plant for the removal of chromium aimed at the production of safe potable water. J Environ Chem Eng 6(4):4467–4475. https://doi.org/10.1016/j.jece.2018.06.055
Hadi S, Taheri E, Amin MM, Fatehizadeh A, Aminabhavi TM (2021) Adsorption of 4-chlorophenol by magnetized activated carbon from pomegranate husk using dual stage chemical activation. Chemosphere 270:128623. https://doi.org/10.1016/j.chemosphere.2020.128623
Halsey G (1948) Physical adsorption on non-uniform surfaces. J Chem Phys 16(10):931–937. https://doi.org/10.1063/1.1746689
Hamdaoui O, Naffrechoux E (2009) Adsorption kinetics of 4-chlorophenol onto granular activated carbon in the presence of high frequency ultrasound. Ultrason Sonochem 16(1):15–22. https://doi.org/10.1016/j.ultsonch.2008.05.008
Hameed BH, Chin LH, Rengaraj S (2008) Adsorption of 4-chlorophenol onto activated carbon prepared from rattan sawdust. Desalination 225:185–198. https://doi.org/10.1016/j.desal.2007.04.095
Hamidon TS, Hussin MH (2023) Improved p-chlorophenol adsorption onto copper-modified cellulose nanocrystal-based hydrogel spheres. Int J Biol Macromol 233:123535. https://doi.org/10.1016/j.ijbiomac.2023.123535
Haroon H, Shah JA, Khan MS, Alam T, Khan R, Asad SA et al (2020) Activated carbon from a specific plant precursor biomass for hazardous Cr (VI) adsorption and recovery studies in batch and column reactors: Isotherm and kinetic modeling. J Water Process Eng 38:101577. https://doi.org/10.1016/j.jwpe.2020.101577
Işık B, Uğraşkan V (2021) Adsorption of methylene blue on sodium alginate–flax seed ash beads: isotherm, kinetic and thermodynamic studies. Int J Biol Macromol 167:1156–1167. https://doi.org/10.1016/j.ijbiomac.2020.11.070
Jawad AH, Abdulhameed AS (2020) Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf Interfaces 18:100422. https://doi.org/10.1016/j.surfin.2019.100422
Jawad AH, Abdulhameed AS, Surip SN, Alothman ZA (2023a) A new matrix of chitosan-salicylaldehyde Schiff base/algae/montmorillonite for adsorption of anionic and cationic dyes: statistical optimization and adsorption mechanism. J Polym Environ 1-15. https://doi.org/10.1007/s10924-02302853-7
Jawad AH, Hameed BH, Abdulhameed AS (2023b) Synthesis of biohybrid magnetic chitosan-polyvinyl alcohol/MgO nanocomposite blend for remazol brilliant blue R dye adsorption: solo and collective parametric optimization. Polym Bull 80(5):4927–4947. https://doi.org/10.1007/s00289-022-04294-z
Jawad AH, Abdulhameed AS, Surip SN, Alothman ZA (2023c) Hybrid multifunctional biocomposite of chitosan grafted benzaldehyde/montmorillonite/algae for effective removal of brilliant green and reactive blue 19 dyes: Optimization and adsorption mechanism. J Clean Prod 393:136334. https://doi.org/10.1016/j.jclepro.2023.136334
Jiang S, Li Z, Yang X, Li M, Wang C, Wang Z, Wu Q (2023) Sustainable and green synthesis of porous organic polymer for solid-phase extraction of four chlorophenols in water and honey. Food Chem 404:134652. https://doi.org/10.1016/j.foodchem.2022.134652
Khapre MA, Pandey S, Jugade RM (2021) Glutaraldehyde-cross-linked chitosan–alginate composite for organic dyes removal from aqueous solutions. Int J Biol Macromol 190:862–875. https://doi.org/10.1016/j.ijbiomac.2021.09.026
Kumari M, Jr-CU P, Mohan D (2015) Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J Colloid Interface Sci 442:120–132. https://doi.org/10.1016/j.jcis.2014.09.012
Kuntail J, Jain YM, Shukla M, Sinha I (2019) Adsorption mechanism of phenol, p636 chlorophenol, and p-nitrophenol on magnetite surface: a molecular dynamics study. J Mol Liq 288:111053. https://doi.org/10.1016/j.molliq.2019.111053
Li C, Chen D, Ding J, Shi Z (2020) A novel hetero-exopolysaccharide for the adsorption of methylene blue from aqueous solutions: isotherm, kinetic, and mechanism studies. J Clean Prod 265:121800. https://doi.org/10.1016/j.jclepro.2020.121800
Li D, Zhou L, Wei Z, Qin X, Ji H, Chai K (2023) β-cyclodextrin-based porous carbon with tunable pore and interface allows efficient removal of chlorophenols from aqueous solution. Appl Surf Sci 157560. https://doi.org/10.1016/j.apsusc.2023.157560
Liu Y, Men B, Hu A, You Q, Liao G, Wang D (2020a) Facile synthesis of graphene-based hyper-cross-linked porous carbon composite with superior adsorption capability for chlorophenols. J Environ Sci 90:395–407. https://doi.org/10.1016/j.jes.2019.11.018
Liu W, Wang J, Liu J, Hou F, Wu Q, Wang C, Wang Z (2020c) Preparation of phenylboronic acid based hypercrosslinked polymers for effective adsorption of chlorophenols. J Chromatogr A 1628:461470. https://doi.org/10.1016/j.chroma.2020.461470
Liu Z, Qin Q, Hu Z, Yan L, Ieong UI, Xu Y (2020b) Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: kinetics, mechanisms and influencing factors. Environ Pollut 265:114926. https://doi.org/10.1016/j.envpol.2020.114926
Ma W, Zong P, Cheng Z, Wang B, Sun Q (2014) Adsorption and bio-sorption of nickel ions and reuse for 2-chlorophenol catalytic ozonation oxidation degradation from water. J Hazard Mater 266:19–25. https://doi.org/10.1016/j.jhazmat.2013.12.007
Ma X, Wang W, Sun C, Li H, Sun J, Liu X (2021) Adsorption performance and kinetic study of hierarchical porous Fe-based MOFs for toluene removal. Sci Total Environ 793:148622. https://doi.org/10.1016/j.scitotenv.2021.148622
Magdy YM, Altaher H, ElQada E (2018) Removal of three nitrophenols from aqueous solutions by adsorption onto char ash: equilibrium and kinetic modeling. Appl Water Sci 8(1):1–15. https://doi.org/10.1007/s13201-018-0666-1
Mangrulkar PA, Kamble SP, Meshram J, Rayalu SS (2008) Adsorption of phenol and o-chlorophenol by mesoporous MCM-41. J Hazard Mater 160(2-3):414–421. https://doi.org/10.1016/j.jhazmat.2008.03.013
Mohammed BB, Yamni K, Tijani N, Alrashdi AA, Zouihri H, Dehmani Y et al (2019) Adsorptive removal of phenol using faujasite-type Y zeolite: adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J Mol Liq 296:111997. https://doi.org/10.1016/j.molliq.2019.111997
Mongioví C, Crini G, Gabrion X, Placet V, Blondeau-Patissier V, Krystianiak A et al (2022) Revealing the adsorption mechanism of copper on hemp-based materials through EDX, nano-CT, XPS, FTIR, Raman, and XANES characterization techniques. Chem Eng J Adv 10:100282. https://doi.org/10.1016/j.ceja.2022.100282
Munoz M, Kaspereit M, Etzold BJ (2016) Deducing kinetic constants for the hydrodechlorination of 4-chlorophenol using high adsorption capacity catalysts. Chem Eng J 285:228–235. https://doi.org/10.1016/j.cej.2015.10.002
Naderi K, Foroughi M, Azqhandi MHA (2022) Tetracycline capture from aqueous solutions by nanocomposite of MWCNTs reinforced with glutaraldehyde cross-linked poly (vinyl alcohol)/chitosan. Chemosphere 135124. https://doi.org/10.1016/j.chemosphere.2022.135124
Nezhadali A, Koushali SE, Divsar F (2021) Synthesis of polypyrrole–chitosane magnetic nanocomposite for the removal of carbamazepine from wastewater: adsorption isotherm and kinetic study. J Environ Chem Eng 9(4):105648. https://doi.org/10.1016/j.jece.2021.105648
Niaei HA, Rostamizadeh M (2020) Adsorption of metformin from an aqueous solution by Fe-ZSM-5 nano-adsorbent: isotherm, kinetic and thermodynamic studies. J Chem Thermodyn 142:106003. https://doi.org/10.1016/j.jct.2019.106003
Ofomaja AE, Unuabonah EI, Oladoja NA (2010) Competitive modeling for the biosorptive removal of copper and lead ions from aqueous solution by Mansonia wood sawdust. Bioresour Technol 101(11):3844–3852. https://doi.org/10.1016/j.biortech.2009.10.064
Oh WD, Lim PE, Seng CE, Sujari ANA (2011) Bioregeneration of granular activated carbon in simultaneous adsorption and biodegradation of chlorophenols. Bioresour Technol 102(20):9497–9502. https://doi.org/10.1016/j.biortech.2011.07.107
Okolo B, Park C, Keane MA (2000) Interaction of phenol and chlorophenols with activated carbon and synthetic zeolites in aqueous media. J Colloid Interface Sci 226(2):308–317. https://doi.org/10.1006/jcis.2000.6796
Păcurariu C, Mihoc G, Popa A, Muntean SG, Ianoş R (2013) Adsorption of phenol and p-chlorophenol from aqueous solutions on poly (styrene-co-divinylbenzene) functionalized materials. Chem Eng J 222:218–227. https://doi.org/10.1016/j.cej.2013.02.060
Qisse N, Alouani ME, Azzouzi LE, Fadil IE, Saufi H, Belghiti MAE et al (2020) Adsorption of Imazalil herbicide onto Moroccan agricultural soils: kinetic and isotherm adsorption studies. Ground Water Sustain Dev 11:100468. https://doi.org/10.1016/j.gsd.2020.100468
Rao JR, Viraraghavan T (2002) Biosorption of phenol from an aqueous solution by Aspergillus niger biomass. Bioresour Technol 85:165–171. https://doi.org/10.1016/S0960-8524(02)00079-2
Ren L, Zhang J, Li Y, Zhang C (2011) Preparation and evaluation of cattail fiber-based activated carbon for 2, 4-dichlorophenol and 2, 4, 6-trichlorophenol removal. Chem Eng J 168(2):553–561. https://doi.org/10.1016/j.cej.2011.01.021
Riahi K, Chaabane S, Thayer BB (2017) A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode. J Saudi Chem Soc 21:S143–S152. https://doi.org/10.1016/j.jscs.2013.11.007
Rosli K, Abdulhameed AS, Surip SN, Alothman ZA, Jawad AH (2023) An eco-friendly adsorbent of chitosan/montmorillonite/algae for removal of basic green 1 and Reactive Blue 19 dyes: Box-Behnken design optimization mechanistic study. J Polym Environ 1-18. https://doi.org/10.1007/s10924-023-02869-z
Saravanan A, Karishma S, Jeevanantham S, Jeyasri S, Kiruthika AR, Kumar PS, Yaashikaa PR (2020) Optimization and modeling of reactive yellow adsorption by surface modified Delonix regia seed: study of nonlinear isotherm and kinetic parameters. Surf Interfaces 20:100520. https://doi.org/10.1016/j.surfin.2020.100520
Sasidharan V, Sachan D, Chauhan D, Talreja N, Ashfaq M (2021) Three-dimensional (3D) polymer—metal–carbon framework for efficient removal of chemical and biological contaminants. Sci Rep 11(1):1–14. https://doi.org/10.1038/s41598-021-86661-w
Sanchez-Salvador JL, Balea A, Monte MC, Negro C, Blanco A (2021) Chitosan grafted/cross-linked with biodegradable polymers: a review. Int J Biol Macromol 178:325–343. https://doi.org/10.1016/j.ijbiomac.2021.02.200
Soomro FK, Memon SQ, Memon N, Khuhawar MY (2020) A new Schiff’s base polymer for remediation of phenol, 2-chlorophenol and 2, 4-dichlorophenol from contaminated aqueous systems. Polym Bull 77:2367–2383. https://doi.org/10.1007/s00289-019-02852-6
Srinivasan P, Bosco AJ, Kalaivizhi R, Selvi JA, Sivakumar P (2021) Adsorption isotherm and kinetic study of Direct Orange 102 dyes on TNJ activated carbon. Mater Today Proc 34:389–394. https://doi.org/10.1016/j.matpr.2020.02.198
Sze MFF, McKay G (2010) An adsorption diffusion model for removal of para725 chlorophenol by activated carbon derived from bituminous coal. Environ Pollut 158(5):1669–1674. https://doi.org/10.1016/j.envpol.2009.12.003
Taka AL, Klink MJ, Mbianda XY, Naidoo EB (2020) Chitosan nanocomposites for water treatment by fixed-bed continuous flow column adsorption: a review. Carbohydr Polym 255:117398. https://doi.org/10.1016/j.carbpol.2020.117398
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/j.watres.2017.04.014
Wahab HS, Bredow T, Aliwi SM (2009) A computational study on the adsorption and ring cleavage of para-chlorophenol on anatase TiO2 surface. Surf Sci 603(4):664–669. https://doi.org/10.1016/j.susc.2009.01.001
Wang X, Li H, Huang J (2017) Adsorption of p-chlorophenol on three amino-modified hyper-cross-linked resins. J Colloid Interface Sci 505:585–592. https://doi.org/10.1016/j.jcis.2017.06.053
Yang X, Muhammad T, Yang J, Yasen A, Chen L (2020) In-situ kinetic and thermodynamic study of 2, 4-dichlorophenoxyacetic acid adsorption on molecularly imprinted polymer based solid-phase microextraction coatings. Sens Actuators, A 313:112190. https://doi.org/10.1016/j.sna.2020.112190
Ye X, Shang S, Zhao Y, Cui S, Zhong Y, Huang L (2021) Ultra-efficient adsorption of copper ions in chitosan–montmorillonite composite aerogel at wastewater treatment. Cellulose 28(11):7201–7212. https://doi.org/10.1007/s10570-021-03976-7
Zain ZM, Abdulhameed AS, Jawad AH, Alothman ZA, Yaseen ZM (2023) A pH-sensitive surface of chitosan/sepiolite clay/algae biocomposite for the removal of malachite green and remazol brilliant blue R dyes: optimization and adsorption mechanism study. J Polym Environ 31(2):501–518. https://doi.org/10.1007/s10924-022-02614-y
Zhang XRBJ, Bai R (2003) Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J Colloid Interface Sci 264(1):3038. https://doi.org/10.1016/S0021-9797(03)00393-X
Zhang Y, Cheng Q, Wang C, Li H, Han X, Fan Z et al (2021) Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: a review. Chemosphere 279:130927. https://doi.org/10.1016/j.chemosphere.2021.130927
Zhang Z, Liu T, Wu D (2022) Facile synthesis of hydrous zirconia-impregnated chitosan beads as a filter medium for efficient removal of phosphate from water. Cellulose 29:8749–8768. https://doi.org/10.1007/s10570-022-04813-1
Zhou T, Wang Y, Li T, Li H, Yang C, Sun D et al (2021) Fabricating magnetic hydrophilic molecularly imprinted resin with enhanced adsorption and recognition performance for targeted detecting chlorophenols in environmental water. Chem Eng J 420:129904. https://doi.org/10.1016/j.cej.2021.129904
Acknowledgements
This research was supported and funded by University Center Salhi Ahmed Naama (Algeria), Laboratory of Inorganic Chemistry and Environment, Department of Chemistry (Tlemcen University), and other laboratories implicated in the above Universities.
Funding
This work was funded by University Center Salhi Ahmed Naama (Algeria). The funder had no role in study design, experimentation and analysis, decision to publish, or the preparation of the paper.
Author information
Authors and Affiliations
Contributions
Nacer FERRAH: conceptualization, project administration, writing—original draft. Djamila Merghache: methodology, formal analysis, data curation. Mustapha Chabane: writing review and editing, investigation. Abdessamed Derdour: software, validation. Riad Mansour: resources, investigation. Tayeb Nouri: resources, validation. Sid Ahmed Cheikh: methodology, experimentation. El Housseyn Zerriahen: methodology, resources. All authors contributed equally to this work, and they read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is exempt from review by any Ethics Committee, and this type of research is non-human subject study, and waived the need for informed consent. Also, this paper does not report on or involve the use of any animal or human data or tissue
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferrah, N., Merghache, D., Chabane, M. et al. Multifunctional polysaccharide structure as green adsorbent for efficient removal and preconcentration of chlorophenols from the aqueous medium: experimental and modeling approaches. Environ Sci Pollut Res 30, 93531–93545 (2023). https://doi.org/10.1007/s11356-023-28947-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28947-2